Abstract
Background and objectives
Pulmonary hypertension is associated with higher mortality rates. The associations of nondialysis-dependent CKD and all-cause mortality in patients with pulmonary hypertension were studied.
Design, setting, participants, & measurements
The study population included those patients who underwent right heart catheterization for confirmation of pulmonary hypertension between 1996 and January 2011. Pulmonary hypertension was defined as the presence of mean pulmonary artery pressure≥25 mmHg at rest measured by right heart catheterization. CKD was defined as the presence of two measurements of eGFR<60 ml/min per 1.73 m2 90 days apart. The risk factors associated with CKD as well as the association between CKD and death in those patients with pulmonary hypertension using logistic regression and Cox proportional hazard models were examined.
Results
Of 1088 patients with pulmonary hypertension, 388 (36%) patients had CKD: 340 patients had stage 3 CKD, and 48 (4%) patients had stage 4 CKD. In the multivariable analysis, older age, higher hemoglobin, and higher mean right atrial pressures were independently associated with CKD. During a median follow-up of 3.2 years (interquartile range=1.5–5.6 years), 559 patients died. After adjusting for relevant covariates, presence of stage 3 CKD (hazard ratio, 1.37; 95% confidence interval, 1.14 to 1.66) and stage 4 CKD (hazard ratio, 2.69; 95% confidence interval, 1.88 to 3.86) was associated with all-cause mortality in those patients with pulmonary hypertension. When eGFR was examined as a continuous measure, a 5 ml/min per 1.73 m2 lower eGFR was associated with a 5% (95% confidence interval, 1.03 to 1.07) higher hazard for death. This higher risk with CKD was similar irrespective of demographics, left ventricular function, and pulmonary capillary wedge pressure.
Conclusion
In a clinical population referred for right heart catheterization, presence of CKD was associated with higher all-cause mortality in those patients with pulmonary hypertension. Mechanisms that may underlie these associations warrant additional studies.
Introduction
Pulmonary hypertension (PH) is a progressive, fatal pulmonary circulatory disease that accompanies left or right ventricular failure (1,2). The long-term complication that affects survival in this population is right ventricular dysfunction leading to right-sided heart failure. The interaction between PH, right ventricular failure, and kidney function has not been well studied. Several factors lead to the development and worsening of PH, and kidney dysfunction and volume overload are common occurrences in clinical practice that can lead to increased pulmonary artery pressure (PAP) (3,4). This decline in kidney function could be transiently related to hemodynamic changes during the treatment of volume overload associated with PH (5). These hemodynamic changes could lead to CKD if the insults are persistent or the underlying disease continues to worsen.
The World Health Organization classifies PH into five classes. Class I includes idiopathic pulmonary arterial hypertension (PAH), class II includes PH associated with left heart disease, class III includes PH associated with lung disease, class IV includes chronic thromboembolic disease, and class V includes PH with unclear or multifactorial etiology (6,7). The most common cause of PH is left heart disease, including left-sided systolic dysfunction caused by ischemic and other cardiomyopathies with resultant volume overload (8,9). An important and frequently underappreciated cause of PH is left-sided heart failure with a preserved ejection fraction (EF; with or without underlying diastolic dysfunction), and these conditions are often noted in CKD (10,11). In patients with PH, elevated mean right atrial pressure (RAP), decreased cardiac index, and elevated mean PAP (mPAP) were associated with a poor prognosis (3,12).
Recent data from a French registry and the United States–based Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) have shed light on prognostic markers in patients with PH (13). Specifically, in REVEAL, which included class I PH patients only, presence of renal insufficiency was associated with poor prognosis (14). Because kidney disease in itself is a significant risk factor for death (15,16), it is of relevance to examine its effects in the PH population. Therefore, we aimed to study if the presence of preexisting nondialysis-dependent CKD is an independent risk factor for death in patients with varying etiologies of PH.
Materials and Methods
Study Population
We conducted this retrospective analysis using the preexisting PH registry at our institution. Patients who had higher PAP in echocardiogram were referred for the right heart catheterization procedure for additional risk stratification and confirmation of PH. We included 1141 adult patients with diagnosis of PH based on mPAP≥25 mmHg at rest measured by right heart catheterization performed at our institution (17) between 1996 and January of 2011. Patients who were on chronic dialysis or who received a kidney transplant (n=14) at the time of right heart catheterization or patients who did not have two serum creatinine measures at the time of diagnosis (n=39) were excluded, thus leaving a total of 1088 patients for the final analysis.
Clinical and Laboratory Data
Data collection included recording of baseline demographic variables (age, sex, race, and body mass index [BMI]), comorbidites (hypertension, diabetes, and congestive heart failure), medications (including diuretics and renin-angiotensin blockers), exercise test, and laboratory measures (hemoglobin, albumin, BUN, creatinine, brain natriuretic peptide, sodium, potassium, and bicarbonate) at the time of right heart catheterization. Details relating to demographics, comorbid conditions, and laboratory data were obtained from medical records by chart review. eGFR was calculated using the simplified Modification of Diet in Renal Disease (MDRD) equation: 175×serum creatinine−1.154×(age)−0.203×(0.742 if a woman)×(1.212 if African American). CKD was defined according to the MDRD equation as the presence of two eGFR measurements<60 ml/min per 1.73 m2 90 days apart and classified into stages 3 (eGFR=30–59 ml/min per 1.73 m2) and 4 (eGFR=15–29 ml/min per 1.73 m2) CKD.
Echocardiography and Right Heart Catheterization Data
Data relating to EF and right ventricular systolic pressure from initial Doppler echocardiography on entry into the PH registry were collected. In addition, the following parameters from the right heart catheterization were also collected: central venous pressure, right ventricular systolic and diastolic pressure, right atrium mean pressure, pulmonary arterial systolic and diastolic pressure, mPAP, pulmonary capillary wedge pressure, cardiac output, and cardiac index.
Mortality Data
The primary outcome of interest was all-cause mortality, which was ascertained from our electronic medical record and linkage of our data with the Social Security Death Index. Patients were followed from their date of right heart catheterization to October 31, 2011.
Statistical Analyses
Descriptive statistics were calculated for the entire study population as well as stratified by eGFR group and expressed as mean±SD or median and interquartile range for continuous variables and n (%) for categorical variables. We compared patient characteristics across eGFR levels using ANOVAs or Kruskal–Wallis tests for continuous variables and chi-squared tests for categorical variables. To understand the relationship between PH and kidney function, we performed Spearman correlation analysis between eGFR and the PH indices of pulmonary vascular resistance, cardiac index, RAP, and 6-minute walk time. To assess the relationship between PAP/pulmonary capillary wedge pressure (PCWP) and CKD, we tested the interaction between CKD and the PCWP tertiles on PAP. Next, we performed multivariable logistic regression with eGFR<60 ml/min per 1.73 m2 as the dependent variable to identify which of the following factors were associated with lower kidney function: age, sex, race, diabetes, hypertension, EF<40%, hemoglobin, albumin, mean RAP, pulmonary arterial diastolic pressure, PCWP, medications (diuretics and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers), and PH classification. Confounding variables were selected based on their clinical relevance and previous predictors of mortality in the PH population. We used the Hosmer–Lemeshow test to evaluate the fit of the logistic regression model.
Kaplan–Meier survival plots were used to evaluate the relationship between CKD stage and survival. We also used Cox proportional hazard models to investigate the association between eGFR and mortality while adjusting for age, sex, race, diabetes, hypertension, BMI, use of diuretics and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, albumin, hemoglobin, cardiac output, mPAP, mean RAP, PCWP, EF medications, and PH classification. We ran the Cox models using eGFR as both a categorical (as stages of CKD) and a continuous variable. We used the concordance index to evaluate the calibration of the model. Among 1088 patients, the following numbers of patients had missing data: BMI, n=19; albumin, n=13; hemoglobin, n=2; cardiac output, n=52; EF, n=96; mean RAP, n=53; mPAP, n=4; pulmonary arterial diastolic pressure, n=12; PCWP, n=23; and medications, n=14. To include all patients in the models regardless of missing covariate data, we used mean value imputation along with dummy indicators for missing data.
To evaluate whether the association between CKD and mortality was modified by other factors, we tested two-way interactions between CKD and each of the following variables: age>65 years, sex, race, diabetes, EF>45, RAP>10 mmHg, PCWP>15 mmHg, and PH classification (classes I and II only). In addition, we performed a test of interaction to see if kidney disease modifies the association between the severity of PH (PAP>50 mmHg and tertiles of PAP) and death. We plotted the results of these interaction tests regardless of statistical significance. A sensitivity analysis by defining CKD using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was performed to confirm the results noted with the MDRD equation. In addition, we ran separate Cox models to study the associations between eGFR and death for those patients with classes I and II PH separately.
All data analyses were conducted using Unix SAS version 9.2 (SAS Institute, Inc., Cary, NC) and R 3.0 (The R Foundation for Statistical Computing, Vienna, Austria). The PH registry and this study were approved by the Cleveland Clinic Institutional Review Board.
Results
Patient Characteristics
Table 1 summarizes the baseline characteristics of the study cohort, which consisted of 1088 consecutive patients with PH. Mean age of the study cohort was 60±15 years; 66% of the patients were women, and 81% of the patients were white. The frequencies of hypertension and diabetes were 47% and 26%, respectively. Mean serum creatinine was 1.05±0.5 mg/dl, and eGFR was 72.2±29 ml/min per 1.73 m2. mPAP of the entire cohort was 47±14 mmHg. Among 1088 patients, 388 (36%) patients had evidence of CKD (340 [31%] patients had stage 3 CKD, and 48 [4%] patients had stage 4 CKD). Other clinical and right heart catheterization details are outlined in Tables 1 and 2.
Table 1.
Characteristics of pulmonary hypertension patients stratified by eGFR
| Factor | Overall (n=1088) | eGFR≥60 ml/min per 1.73 m2 (n=700) | eGFR=30–59 ml/min per 1.73 m2 (n=340) | eGFR=15–29 ml/min per 1.73 m2 (n=48) | P Value |
|---|---|---|---|---|---|
| Age, mean±SD (yr) | 60.1±15.2 | 56.4±14.9 | 66.5±13.4 | 69.2±11.7 | <0.001a |
| Sex, no. (%) | 0.50b | ||||
| Women | 716 (65.8) | 462 (66.0) | 219 (64.4) | 35 (72.9) | |
| Men | 372 (34.2) | 238 (34.0) | 121 (35.6) | 13 (27.1) | |
| Race, no. (%) | 0.23b | ||||
| White | 877 (80.6) | 556 (79.4) | 281 (82.6) | 40 (83.3) | |
| African American | 173 (15.9) | 121 (17.3) | 44 (12.9) | 8 (16.7) | |
| Other | 38 (3.5) | 23 (3.3) | 15 (4.4) | 0 (0.0) | |
| Diabetes, no. (%) | 283 (26.0) | 152 (21.7) | 113 (33.2) | 18 (37.5) | <0.001b |
| Hypertension, no. (%) | 515 (47.3) | 287 (41.0) | 192 (56.5) | 36 (75.0) | <0.001b |
| CHF (EF<40%), no. (%)c | 71 (7.2) | 37(5.8) | 30 (9.6) | 4 (9.3) | 0.08b |
| BMI, mean±SD (kg/m2)c | 31.0±9.0 | 30.7±9.0 | 31.9±9.0 | 29.5±7.0 | 0.06a |
| WHO class of PH | <0.001b | ||||
| I (PAH) | 552 (50.7) | 374 (53.4) | 158 (46.5) | 20 (41.7) | |
| II (PVH) | 279 (25.6) | 150 (21.4) | 111 (32.6) | 18 (37.5) | |
| III (PH lung disease) | 116 (10.7) | 74 (10.6) | 39 (11.5) | 3 (6.3) | |
| IV (PH CTED) | 57 (5.2) | 47 (6.7) | 10 (2.9) | 0 (0.0) | |
| V (miscellaneous) | 84 (7.7) | 55 (7.9) | 22 (6.5) | 7 (14.6) | |
| Albumin, mean±SD (g/dl)c | 3.9±0.6 | 3.9±0.6 | 3.8±0.5 | 3.8±0.6 | 0.02a |
| Hemoglobin, mean±SD (g/dl)c | 13.6±2.3 | 13.8±2.3 | 13.3±2.2 | 11.9±1.9 | <0.001a |
| BNP, median (P25, P75)c | 183.0 (59.0, 494.0) | 130.0 (44.0, 361.0) | 288.0 (105.0, 606.0) | 640.0 (194.0, 1608.0) | <0.001d |
| BUN, mean±SD (mg/dl)c | 22.6±14.8 | 16.6±6.6 | 30.9±15.7 | 51.2±26.8 | <0.001a |
| Creatinine, mean±SD (mg/dl) | 1.05±0.47 | 0.82±0.17 | 1.3±0.29 | 2.5±0.80 | <0.001a |
| eGFR, mean±SD (ml/min per 1.73 m2) | 72.3±28.5 | 87.7±22.9 | 47.4±8.3 | 23.4±5.1 | <0.001a |
| Sodium, mean±SD (mmol/L)c | 138.9±3.7 | 139.0±3.5 | 138.8±4.0 | 138.8±3.4 | 0.66a |
| Percent pred. 6-min walk, mean±SDc | 56.7±20.9 | 57.6±20.4 | 55.1±21.7 | 50.4±24.0 | 0.13a |
| Heart rate, mean±SDc | 80.9±15.4 | 82.0±15.2 | 79.0±15.8 | 78.2±15.2 | 0.01a |
| Systolic BP, mean±SD (mmHg)c | 131.3±24.7 | 128.9±22.6 | 135.7±27.5 | 136.9±28.8 | <0.001a |
| Diastolic BP, mean±SD (mmHg)c | 76.8±14.0 | 77.2±13.6 | 76.3±15.0 | 74.0±14.3 | 0.27a |
| Mean BP, mean±SD (mmHg)c | 95.2±15.8 | 94.8±15.0 | 95.9±17.2 | 94.6±18.1 | 0.57a |
| ACEI/ARBs, no. (%)c | 335 (31.2) | 190 (27.5) | 128 (38.1) | 17 (36.2) | 0.002b |
| Diuretics, no. (%) | 716 (66.7) | 419 (60.6) | 261 (77.7) | 36 (76.6) | <0.001b |
CHF, congestive heart failure; EF, ejection fraction; BMI, body mass index; WHO, World Health Organization; PH, pulmonary hypertension; PAH, pulmonary arterial hypertension; PVH, pulmonary venous hypertension; CTED, chronic thromboembolic disease; BNP, brain natriuretic peptide; P, percentile; pred., predicted; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
ANOVA.
Pearson's chi-squared test.
Data not available for all patients. Missing values: CHF (EF<40%)=96, BMI=19, albumin=13, hemoglobin=2, BNP=169, BUN=129, sodium=128, percent pred 6-min walk=347, heart rate=173, systolic BP=87, diastolic BP=92, mean BP=93, medications=14.
Kruskal–Wallis test.
Table 2.
Right heart catheterization and echocardiogram data classified by eGFR groups
| Factor (Mean±SD) | Overall (n=1088) | eGFR≥60 ml/min per 1.73 m2 (n=700) | eGFR=30–59 ml/min per 1.73 m2 (n=340) | eGFR=15–29 ml/min per 1.73 m2 (n=48) | P Value |
|---|---|---|---|---|---|
| EFa (%) | 55.7±8.7 | 56.1±8.0 | 54.9±9.7 | 54.1±9.9 | 0.07b |
| Right ventricular systolic pressurea (mmHg) | 70.6±26.3 | 70.3±27.0 | 71.6±25.6 | 68.7±21.8 | 0.71b |
| Mean right atrial pressurea (mmHg) | 12.0±7.0 | 11.4±6.8 | 12.9±7.4 | 15.1±6.9 | <0.001b |
| Pulmonary arterial systolic pressurea (mmHg) | 75.8±21.6 | 75.8±22.7 | 75.9±19.8 | 75.0±18.1 | 0.97b |
| Pulmonary arterial diastolic pressurea (mmHg) | 31.9±11.0 | 32.5±11.6 | 31.1±10.0 | 28.4±8.5 | 0.01b |
| Mean pulmonary arterial pressurea (mmHg) | 47.2±13.7 | 47.6±14.5 | 46.7±12.3 | 44.5±10.4 | 0.25b |
| Pulmonary capillary wedge pressurea (mmHg) | 16.1±8.9 | 15.3±8.5 | 17.5±9.5 | 17.3±9.2 | <0.001b |
| Fick cardiac output (L/min) | 4.8±1.8 | 4.9±1.8 | 4.8±1.8 | 5.1±1.9 | 0.47b |
| Fick cardiac index (L/min per m2) | 2.5±0.9 | 2.5±0.9 | 2.4±0.9 | 2.8±1.0 | 0.04b |
Data not available for all participants. Missing values: EF=96, right ventricular systolic pressure=142, mean right atrial pressure=53, pulmonary arterial systolic pressure=13, pulmonary arterial diastolic pressure=12, mean pulmonary arterial pressure=4, pulmonary capillary wedge pressure=23, Fick cardiac output=52, Fick cardiac index=48.
ANOVA.
Association between eGFR and Other PH Measures
We did not find a significant correlation between eGFR and pulmonary vascular resistance (ρ=0.05; 95% confidence interval [95% CI], −0.01 to 0.11), cardiac index (ρ=0.03; 95% CI, −0.03 to 0.10), or 6-minute walk time (ρ=0.03; 95% CI, −0.04 to 0.10). There was a significant but mild negative correlation between eGFR and mean RAP (ρ=−0.12; 95% CI, −0.18 to −0.06). We found an interaction between CKD and the PCWP tertiles on PAP (P=0.02). Among patients with CKD, PAP was higher in the highest tertile of PCWP, whereas this result was not seen in the non-CKD population (data not shown).
Risk Factors Associated with CKD in PH
In the multivariable logistic model, older age was associated with higher odds of having CKD (odds ratio per 10 years higher age, 1.69; 95% CI, 1.51 to 1.90) along with hypertension and higher mean RAP. However, presence of higher hemoglobin was associated with lower odds of CKD (odds ratio, 0.90; 95% CI, 0.84 to 0.96) (Table 3). Patients with classes II and IV PH had lower odds of having CKD compared with patients with class I PH. There was no evidence of lack of fit for this model (Hosmer–Lemeshow test, P=0.46).
Table 3.
Multivariable logistic regression analysis of factors associated with CKD (eGFR<60 ml/min per 1.73 m2) in those patients with PH (n=1088)
| Effect | Odds Ratio (95% Confidence Interval)a |
|---|---|
| Age (for each 10 yr higher) | 1.69 (1.51 to 1.90) |
| Women | 1.00 (0.74 to 1.36) |
| African-American race | 0.70 (0.48 to 1.04) |
| Diabetes | 1.18 (0.85 to 1.64) |
| Hypertension | 1.35 (1.01 to 1.82) |
| Systolic failure (EF<40%) | 1.78 (1.02 to 3.11) |
| Hemoglobin (for each 1 g/dl higher) | 0.90 (0.84 to 0.96) |
| Albumin (for each 1 mg/dl higher) | 0.86 (0.66 to 1.11) |
| Mean right atrial pressure (for each 1 mmHg higher) | 1.04 (1.02 to 1.07) |
| Pulmonary arterial diastolic pressure (for each 1 mmHg higher) | 1.00 (0.98 to 1.01) |
| PH class | |
| I (PAH) | Reference |
| II (PVH) | 0.63 (0.41 to 0.95) |
| III (PH lung disease) | 0.71 (0.44 to 1.15) |
| IV (PH CTED) | 0.45 (0.21 to 0.96) |
| V (miscellaneous) | 0.75 (0.43 to 1.29) |
Adjusted for age, sex, race, diabetes, hypertension, EF<40%, hemoglobin, albumin, mean right atrial pressure, pulmonary arterial diastolic pressure, pulmonary capillary wedge pressure, medications (diuretics and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers), pulmonary hypertension classification, and dummy indicators of missing data.
Mortality
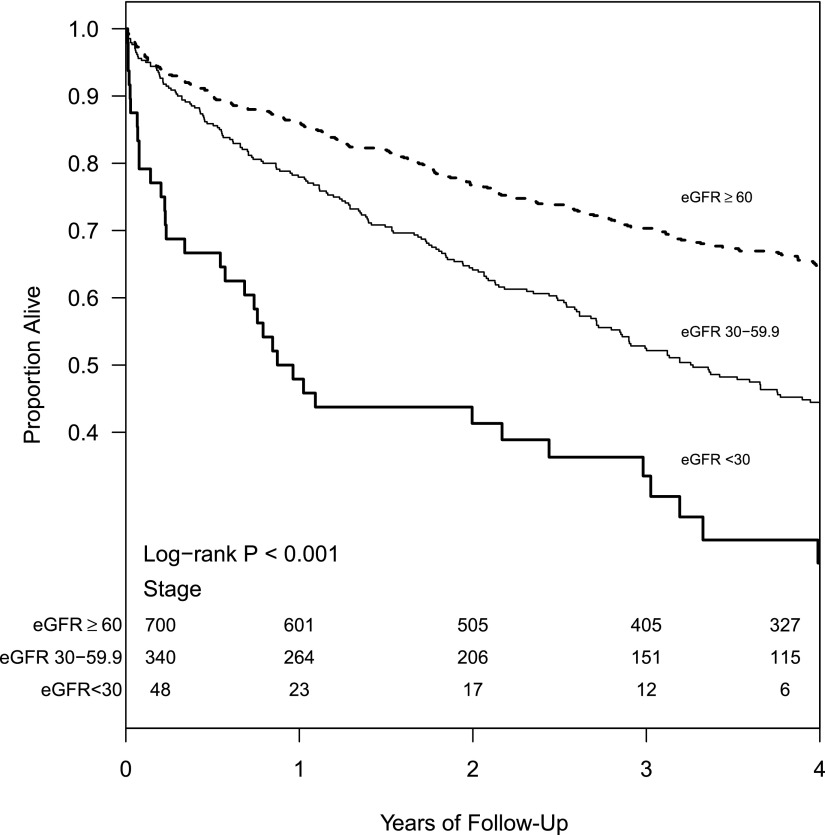
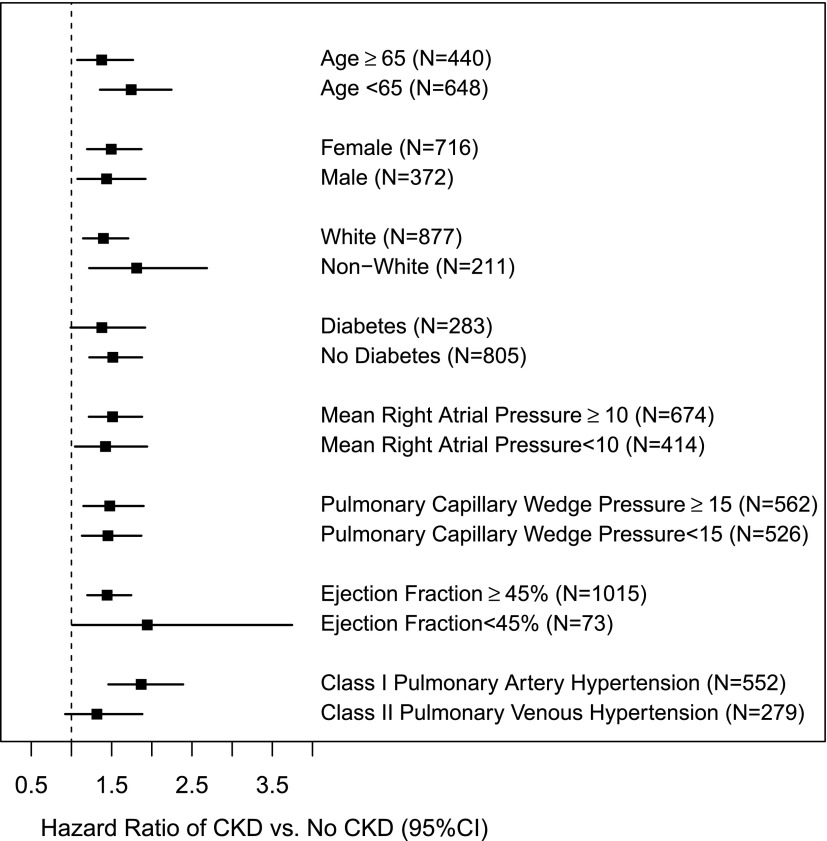
During a median follow-up of 3.2 years (interquartile range=1.5–5.6 years), 559 (51%) patients of the total cohort died. Kaplan–Meier survival estimates at 1 year were 86%, 78%, and 48% in those patients without CKD, with stage 3 CKD, and with stage 4 CKD, respectively (log rank P<0.001) (Figure 1). In the multivariable adjusted Cox proportional hazards model, presence of eGFR<60 ml/min per 1.73 m2 was independently associated with mortality (Table 4). The concordance index for this model was 0.70, suggesting that the model could predict which patients would survive longer in 70% of all eligible patient pairs. When eGFR was examined as a continuous variable, every 5 ml/min per 1.73 m2 lower eGFR was associated with a higher hazard for death (hazard ratio, 1.05; 95% CI, 1.03 to 1.07). We did not find any significant interactions between CKD (eGFR<60 ml/min per 1.73 m2) and age≥65 years, sex, race, diabetes, EF<45, PCWP≥15, mean RAP≥10, or PH class (I and II) in those patients with PH, and the results among the subgroups were similar (Figure 2). Similarly, presence of CKD did not seem to modify the associations between severity of PH and death.
Figure 1.
Survival based on eGFR in those patients with pulmonary hypertension.
Table 4.
Associations between kidney function and mortality in those patients with PH
| Variables | Unadjusted Hazard Ratio (95% Confidence Interval; n=1088) | Adjusteda Hazard Ratio (95% Confidence Interval; n=1088) |
|---|---|---|
| Categorical analysis | ||
| Kidney disease (ml/min per 1.73 m2) | ||
| eGFR≥60 (reference) | ||
| eGFR=30–59 | 1.66 (1.39 to 1.98) | 1.37 (1.14 to 1.66) |
| eGFR=15–29 | 3.94 (2.82 to 5.49) | 2.69 (1.88 to 3.86) |
| Continuous analysis | ||
| For each 5 ml/min per 1.73 m2 lower eGFR | 1.07 (1.05 to 1.09) | 1.05 (1.03 to 1.07) |
| Other variablesb | ||
| Age (for each higher year) | 1.02 (1.01 to 1.03) | |
| Women | 0.78 (0.64 to 0.95) | |
| Hemoglobin (for each 1 g/dl higher) | 0.94 (0.90 to 0.97) | |
| Albumin (for each 1 mg/dl higher) | 0.64 (0.55 to 0.74) | |
| Mean right atrial pressure (for each 1 mmHg higher) | 1.02 (1.00 to 1.03) | |
| Mean pulmonary arterial pressure (for each 1 mmHg higher) | 1.01 (1.01 to 1.02) | |
| Pulmonary capillary wedge pressure (for each 1 mmHg higher) | 0.99 (0.98 to 1.00) | |
| PH class | ||
| I (PAH) | Reference | |
| II (PVH) | 0.71 (0.54 to 0.93) | |
| III (PH lung disease) | 1.44 (1.06 to 1.94) | |
| IV (PH CTED) | 0.68 (0.42 to 1.09) | |
| V (miscellaneous) | 1.18 (0.85 to 1.63) |
Adjusted for age, sex, race, diabetes, hypertension, BMI, albumin, hemoglobin, cardiac output, mean pulmonary arterial pressure, mean right atrial pressure, pulmonary capillary wedge pressure, EF, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, diuretics, and PH classification as well as dummy indicators for missing data.
Results based on the variables included in the categorical model.
Figure 2.
Hazard ratio and 95% confidence interval (95% CI) of all-cause mortality associated with having CKD (versus no CKD) from two-way interactions with different demographic and clinical characteristics.
Other factors that were associated with higher risk for death in PH (in the multivariate model) were age, men, lower hemoglobin and albumin, higher mean RAP and PAP, lower PWCP, and class III PH compared with class I PH (Table 4).
Sensitivity Analyses
We defined CKD using CKD-EPI equation, and the results remained qualitatively similar to the results obtained with the MDRD equation (data not shown) (18). In addition, the associations between eGFR and death remained similar in those associations with classes I and II PH. Each 5 ml/min per 1.73 m2 lower eGFR was associated with a 6% higher risk for death (95% CI, 1.03 to 1.09) in those patients with class I PH versus a 6% higher risk for death (95% CI, 1.02 to 1.11) in those patients with class II PH. A sensitivity analysis by not using imputation techniques was performed, and the results were qualitatively similar.
Discussion
PH and CKD are disease states associated with poor outcomes (13,16,19). In this large cohort of patients with PH (based on right heart catheterization data), underlying CKD was highly prevalent. Older age, hypertension, and presence of elevated RAP were associated with kidney disease among PH patients. Furthermore, in PH, those patients with preexisting CKD were at higher risk for death, even after accounting for various confounding factors, including left ventricular function and PCWP.
Agarwal (20) reported that PH was prevalent among 38% of dialysis patients based on estimated pulmonary artery systolic pressure from echocardiogram and that its presence was associated with twofold higher risk for death in the dialysis population (20). Recent systematic reviews studying PH in the kidney disease population highlighted several other studies addressing the higher prevalence and consequences of PH in both hemodialysis and peritoneal dialysis populations (21–23). These reviews highlighted the lack of data relating to prevalence and effects of PH in those patients with nondialysis-dependent CKD (22). Data from the REVEAL (including PAH patients alone) reported renal insufficiency to be present in 4% of the study cohort (24). Our data, which include all classes of PH, reveal a higher proportion of CKD in those patients with PH (36% in this study versus 4% in REVEAL). Similarly, Havlucu et al. (25) reported a PH prevalence rate of 39% (assessed using Doppler echocardiogram) among those patients with nondialysis-dependent CKD. Underlying muscle mass influences serum creatinine generation (and thus, eGFR), and patients with PH might have muscle wasting from chronic heart failure, thereby underestimating the kidney function. Thus, the presence of underlying kidney disease might be higher than what is noted in our study in those patients with PH.
Shah et al. (26) reported that, among PH patients (n=500), higher serum creatinine was associated with higher death rates among those patients with RAP<10 mmHg. However, Shah et al. (26) did not notice significant associations in those patients with RAP>10 mmHg. Data from the REVEAL revealed that renal insufficiency (assessed at the time of enrollment of participants into the registry) was associated with an increased risk for death (hazard ratio, 1.90; 95% CI, 1.33 to 2.72) (14). We have now provided data from a large cohort of patients who underwent right heart catheterization with varying etiology of PH. Even after adjusting for relevant confounding variables, including RAP, higher risk for death persisted for those patients with CKD, and this risk seemed to increase as the kidney function declined. Our subgroup analysis results further reinforce that the effects are consistent, irrespective of age, sex, and presence of other comorbidities. These results support the use of renal insufficiency in the REVEAL PAH risk score calculator (14) and consider it as a risk factor for death in those patients with other classes of PH. Future studies might examine whether categorization as moderate versus advanced kidney disease in such risk assessment tools might help us predict outcomes more precisely.
The observed associations in our study could be explained by the higher prevalence of diastolic dysfunction and volume overload (resultant pulmonary congestion) in those patients with mild to moderate CKD (10). Although there were no correlations noted between other parameters, such as pulmonary vascular resistance, cardiac index, and 6-minute walk test, and kidney function, a mild correlation was observed with PCWP. In CKD, higher PAP was noted in those patients with higher PCWP. In addition, previous studies have speculated on the potential influence of uremic toxins, bone–mineral disorder, and endothelial dysfunction on outcomes among those PH patients who are on dialysis (23,27,28). Particularly, these reports note that nitric oxide levels are reduced along with an attenuated release of nitric oxide in those patients on hemodialysis with PH. This result often leads to increased pulmonary vascular tone, which in turn, can promote arterial stiffness and adverse consequences. Whether such mechanistic pathways do prevail in those patients with earlier stages of CKD (thereby rendering higher mortality rates) is unknown and could be explored in CKD cohorts with longitudinal data relating to PH and left ventricular function.
CKD patients sustain pulmonary venous hypertension (class II PH) rather than PAH (class I PH) because of the higher prevalence of left heart disease resulting from coronary artery disease or diastolic dysfunction. Although the mean PCWP was higher in those patients with eGFR<60 ml/min per 1.73 m2 than those patients with preserved kidney function, the risk for death seemed to be similar for those patients with PCWP greater than and less than 15 mmHg (reflective of underlying left heart disease) among patients with PH. These results also show that the presence of CKD might have similar effects on those patients with PAH and other forms of PH.
Our study has many strengths, including the use of the PH definition based on right heart catheterization data along with the availability of other hemodynamic parameters, appropriate definitions to categorize CKD, inclusion of patients with different categories of PH, and the ascertainment of left ventricular ejection fraction data from Doppler echocardiogram. However, this analysis does have some limitations, which include selection bias (because our PH registry includes patients who were referred to our tertiary care practice for additional intervention based on previous suspicion of PH) and ascertainment bias (because of the retrospective nature of the study). Because of the lack of blood and urine samples, we could not explore the mechanisms that might have contributed to these higher rates of death in those patients with CKD. Furthermore, this study (similar to any observational study) was adjusted for potential confounders, but we lacked details relating to socioeconomic data, functional status of the patients, and left ventricular mass index, which are factors that may contribute to mortality in PH (29,30). The Kidney Disease Outcomes Quality Initiative definition was used to define stages 3 and 4 CKD for this study’s purposes, and patients who could have had underlying longstanding cardiorenal syndrome (for >3 months) could not be excluded. We also lacked urinary protein excretion details for the study population. Despite the models showing goodness of fit, the analysis is subject to residual confounding.
In summary, CKD was widely prevalent in those patients with PH, and the lower levels of kidney function are associated with an increased risk of death. Future studies should explore the mechanisms that might underlie these associations because they will eventually help us identify some potential therapeutic interventions for this high-risk population.
Disclosures
None.
Acknowledgments
S.D.N. is supported by a career development award from the National Center for Research Resources and National Center for Advancing Translational Sciences/National Institutes of Health Grant TR000440. J.D.S. is supported by the National Institutes of Health Grants MD00265, DK085185, and DK094112, and the Genzyme Corporation. R.D. is supported by the National Institutes of Health Grants HL107147, HL081064, HL103453, HL109250, and RR026231.
Results of this study were presented as an abstract at the Annual American Society of Nephrology Meeting held November 10, 2011, in Philadelphia, PA.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Present address: Dr. Edgard Wehbe, Wichita Nephrology Group, University of Kansas School of Medicine, Wichita, Kansas.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Lindner JR, Moliterno DJ, Mukherjee D, Pohost GM, Rosenson RS, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ, ACCF/AHA : ACCF/AHA 2009 expert consensus document on pulmonary hypertension: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 119: 2250–2294, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Heresi GA, Dweik RA: Pulmonary hypertension: Evaluation and management. Compr Ther 33: 150–161, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Batal O, Khatib OF, Dweik RA, Hammel JP, McCarthy K, Minai OA: Comparison of baseline predictors of prognosis in pulmonary arterial hypertension in patients surviving ≤2 years and those surviving ≥5 years after baseline right-sided cardiac catheterization. Am J Cardiol 109: 1514–1520, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Di Lullo L, Floccari F, Rivera R, Barbera V, Granata A, Otranto G, Mudoni A, Malaguti M, Santoboni A, Ronco C: Pulmonary hypertension and right heart failure in chronic kidney disease: New challenge for 21st-century cardionephrologists. Cardiorenal Med 3: 96–103, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haddad F, Fuh E, Peterson T, Skhiri M, Kudelko KT, De Jesus Perez V, Winkelmayer WC, Doyle RL, Chertow GM, Zamanian RT: Incidence, correlates, and consequences of acute kidney injury in patients with pulmonary arterial hypertension hospitalized with acute right-side heart failure. J Card Fail 17: 533–539, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Waxman AB, Zamanian RT: Pulmonary arterial hypertension: New insights into the optimal role of current and emerging prostacyclin therapies. Am J Cardiol 111[5 Suppl]: 1A–16A, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Fishman AP: Clinical classification of pulmonary hypertension. Clin Chest Med 22: 385–391, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Guazzi M, Borlaug BA: Pulmonary hypertension due to left heart disease. Circulation 126: 975–990, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Hansdottir S, Groskreutz DJ, Gehlbach BK: WHO’s in second?: A practical review of World Health Organization group 2 pulmonary hypertension. Chest 144: 638–650, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM: Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol 53: 1119–1126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard LS: Prognostic factors in pulmonary arterial hypertension: Assessing the course of the disease. Eur Respir Rev 20: 236–242, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humbert M, Sitbon O, Yaïci A, Montani D, O’Callaghan DS, Jaïs X, Parent F, Savale L, Natali D, Günther S, Chaouat A, Chabot F, Cordier JF, Habib G, Gressin V, Jing ZC, Souza R, Simonneau G, French Pulmonary Arterial Hypertension Network : Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 36: 549–555, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD: Predicting survival in pulmonary arterial hypertension: Insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation 122: 164–172, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A: Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 54[Suppl]: S55–S66, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL: Pulmonary pressures and death in heart failure: A community study. J Am Coll Cardiol 59: 222–231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal R: Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant 27: 3908–3914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sise ME, Courtwright AM, Channick RN: Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int 84: 682–692, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, Wiecek A, Martinez-Castelao A, Covic A, Goldsmith D, Suleymanlar G, Lindholm B, Parati G, Sicari R, Gargani L, Mallamaci F, London G, Zoccali C: Pulmonary hypertension in CKD. Am J Kidney Dis 61: 612–622, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Kawar B, Ellam T, Jackson C, Kiely DG: Pulmonary hypertension in renal disease: Epidemiology, potential mechanisms and implications. Am J Nephrol 37: 281–290, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD: Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest 137: 376–387, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Havlucu Y, Kursat S, Ekmekci C, Celik P, Serter S, Bayturan O, Dinc G: Pulmonary hypertension in patients with chronic renal failure. Respiration 74: 503–510, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Shah SJ, Thenappan T, Rich S, Tian L, Archer SL, Gomberg-Maitland M: Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation 117: 2475–2483, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Amin M, Fawzy A, Hamid MA, Elhendy A: Pulmonary hypertension in patients with chronic renal failure: Role of parathyroid hormone and pulmonary artery calcifications. Chest 124: 2093–2097, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Abdelwhab S, Elshinnawy S: Pulmonary hypertension in chronic renal failure patients. Am J Nephrol 28: 990–997, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Wu WH, Yang L, Peng FH, Yao J, Zou LL, Liu D, Jiang X, Li J, Gao L, Qu JM, Kawut SM, Jing ZC: Lower socioeconomic status is associated with worse outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med 187: 303–310, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD: The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 141: 354–362, 2012 [DOI] [PubMed] [Google Scholar]