Abstract
Background and objectives
Reducing LDL cholesterol (LDL-C) with statin-based therapy reduces the risk of major atherosclerotic events among patients with CKD, including dialysis patients, but the effect of lowering LDL-C on vascular access patency is unclear.
Design, setting, participants, & measurements
The Study of Heart and Renal Protection (SHARP) randomized patients with CKD to 20 mg simvastatin plus 10 mg ezetimibe daily versus matching placebo. This study aimed to explore the effects of treatment on vascular access occlusive events, defined as any access revision procedure, access thrombosis, removal of an old dialysis access, or formation of new permanent dialysis access.
Results
Among 2353 SHARP participants who had functioning vascular access at randomization, allocation to simvastatin plus ezetimibe resulted in a 13% proportional reduction in vascular access occlusive events (355 [29.7%] for simvastatin/ezetimibe versus 388 [33.5%] for placebo; risk ratio [RR], 0.87; 95% confidence interval [95% CI], 0.75 to 1.00; P=0.05). There was no evidence that the effects of treatment differed for any of the separate components of this outcome. To test the hypothesis raised by SHARP, comparable analyses were performed using the AURORA (A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events) trial cohort. AURORA did not provide independent confirmation (vascular access occlusive events: 352 [28.9%] for rosuvastatin versus 337 [27.6%] for placebo; RR, 1.06, 95% CI, 0.91 to 1.23; P=0.44). After combining the two trials, the overall effect of reducing LDL-C with a statin-based regimen on vascular access occlusive events was not statistically significant (707 [29.3%] with any LDL-C–lowering therapy versus 725 [30.5%] with placebo; RR, 0.95, 95% CI, 0.85 to 1.05; P=0.29).
Conclusions
Exploratory analyses from SHARP suggest that lowering LDL-C with statin-based therapy may improve vascular access patency, but there was no evidence of benefit in AURORA. Taken together, the available evidence suggests that any benefits of lowering LDL-C on vascular access patency are likely to be modest.
Keywords: statins, vascular access, lipids
Introduction
Arteriovenous fistulae (AVF) are the preferred form of vascular access for dialysis, but both AVF and particularly arteriovenous (AV) grafts are prone to loss of patency (1). Because maintaining functioning vascular access is vital for hemodialysis patients and its management accounts for a significant proportion of the cost of dialysis (including around 20% of their hospital admissions) (2), strategies to reduce complication rates are needed. Although statins are regularly discussed in review articles as a medical therapy that might improve vascular access outcomes (3–6), observational cohort studies have not provided any evidence to suggest that they may do so (7–9). The Study of Heart and Renal Protection (SHARP) randomized 9270 patients with CKD to 20 mg simvastatin plus 10 mg ezetimibe daily versus matching placebo and showed that lowering LDL cholesterol (LDL-C) safely reduces the risk of major atherosclerotic events (10). This trial included 2353 patients with a functioning AVF or AV graft at the time of randomization, and therefore provides an opportunity to explore the effects of lowering LDL-C with simvastatin plus ezetimibe on vascular access patency in a large placebo-controlled randomized trial.
In a tertiary analysis of all randomized SHARP patients performed for regulatory submission, allocation to simvastatin plus ezetimibe was associated with a nonsignificantly lower risk of hemodialysis access revision (561 [12.1%] for simvastatin plus ezetimibe versus 606 [13.1%] for placebo; risk ratio [RR], 0.92; 95% confidence interval [95% CI], 0.82 to 1.03; P=0.13). This report explores the effects of treatment on vascular access patency in more detail, and assesses the results of the SHARP trial in the context of the effects of 10 mg rosuvastatin on the same outcomes in the AURORA (A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events) trial.
Materials and Methods
Study Design and Participants
Details of the SHARP trial design and its main results are published elsewhere (10,11). Briefly, individuals aged ≥40 years were eligible to participate if they had >1 previous measurement of serum or plasma creatinine of at least 1.7 mg/dl (150 µmol/L) in men or 1.5 mg/dl (130 µmol/L) in women, or were receiving maintenance dialysis. Patients with prior myocardial infarction or coronary revascularization were excluded. Ethical approval was obtained at all SHARP study sites before enrollment. Potentially eligible patients attended a screening visit at which written informed consent was obtained and medical history, including presence of functioning vascular access, was recorded. After 6 weeks of placebo run-in, the participants were randomized in a 4:4:1 ratio to 20 mg simvastatin plus 10 mg ezetimibe daily versus matching placebo versus 20 mg simvastatin alone. Participants who were allocated to simvastatin alone were rerandomized after 1 year to one of the two main comparison arms. After initial randomization, participants were followed-up in study clinics at 2 and 6 months and then every 6 months for at least 4 years. At each of these visits, information on vascular access procedures and all hospitalizations was specifically requested. More information about SHARP, including a copy of the trial protocol, is available at www.sharpinfo.org.
To explore the effects of allocated treatment on vascular access patency, we identified codes for serious adverse events that represented loss of patency, and defined post hoc the composite outcome “vascular access occlusive event” as any access revision procedure (percutaneous angioplasty, embolectomy, or surgical repair), any access thrombosis event, or the removal of old/formation of new permanent dialysis access (which included placement of a new AVF, AV graft, or tunnelled dialysis–catheter). Analyses were restricted to those participants known to have a functioning AVF or AV graft at randomization (i.e., preexisting/prevalent vascular access).
Because these SHARP analyses are post hoc, we explored other possible sources of trial data on the effects of statin-based treatment on vascular access patency. Of the two other large-scale statin trials among dialysis patients [Die Deutsche Diabetes Dialyze and AURORA (12,13)], only the AURORA trial recorded the necessary information to define vascular access occlusive events. The full details of the AURORA study design are published elsewhere (13). Ethical approval was obtained at all AURORA study sites before enrollment. After providing written informed consent, eligible individuals aged 50–80 years on maintenance hemodialysis were randomized 1:1 to 10 mg rosuvastatin daily versus matching placebo. Follow-up was at 3 and 6 months after randomization, and then every 6 months. Structured interviews, including documentation of any vascular access events, were completed at each visit. The SHARP definitions and analysis methods were replicated in the AURORA dataset. Both the SHARP and AURORA trials are registered with ClinicalTrials.gov (SHARP, NCT00125593 and ISRCTN54137607; AURORA, NCT00240331).
Statistical Analyses
The effects of treatment allocation on the time to first event were examined using log-rank methods to calculate two-sided P values, event RRs, and confidence intervals (CIs) (14,15), irrespective of whether participants subsequently took their allocated treatment (i.e., according to the intention-to-treat principle). A sensitivity analysis using all (i.e., not just first events) events was performed using a negative binomial model. Proportional risk reductions in different subgroups were compared using standard chi-squared tests for heterogeneity or trend. To allow for multiple testing by subdivisions, only overall summary RRs have 95% CIs; all other RRs have 99% CIs. Analyses were conducted using SAS (version 9.3; SAS Institute, Cary, NC) and R (version 2.11.1; R Project for Statistical Computing, Vienna, Austria) software.
Results
Of the 9270 SHARP participants randomized to simvastatin plus ezetimibe versus placebo, 2353 had a functioning AVF or AV graft at randomization (1196 allocated to simvastatin plus ezetimibe and 1157 allocated to placebo). Among those with a functioning AVF or AV graft, baseline characteristics were balanced between the randomized groups for all variables except sex (P for sex = 0.03; Table 1). The mean age was 59±12 years. Of the participants, 1520 (65%) were men, 525 (22%) had diabetes mellitus, and 372 (16%) were smokers. The majority of participants (2152 [91%]) were already on dialysis at randomization. The type of vascular access was an AVF in the majority (2204 [94%]), with the remainder being AV grafts (149 [6%]). A total of 729 (31%) participants were taking antiplatelet therapy at baseline and 1297 (55%) were taking an erythropoiesis-stimulating agent. Mean hemoglobin was 11.7±1.6 g/dl and mean LDL-C was 99±33 mg/dl.
Table 1.
Baseline characteristics among SHARP and AURORA participants with preexisting access at randomization, by treatment allocation
| Characteristic | SHARP | AURORA | ||
|---|---|---|---|---|
| Simvastatin Plus Ezetimibe (n=1196) | Placebo (n=1157) | Rosuvastatin (n=1219) | Placebo (n=1220) | |
| Age at randomization (yr)a | 59±12 | 59±12 | 64±9 | 64±9 |
| Men | 747 (62) | 773 (67) | 759 (62) | 794 (65) |
| Prior diabetes mellitus | 267 (22) | 258 (22) | 328 (27) | 282 (23) |
| Current smoker | 184 (15) | 188 (16) | 172 (14) | 203 (17) |
| Renal statusa | ||||
| On dialysis | 1091 (91) | 1061 (92) | 1219 (100) | 1220 (100) |
| Not on dialysis | 105 (9) | 96 (8) | ||
| Access typea | ||||
| Arteriovenous fistula | 1119 (94) | 1085 (94) | 1090 (89) | 1109 (91) |
| Arteriovenous graft | 77 (6) | 72 (6) | 129 (11) | 111 (9) |
| Comedicationa | ||||
| Antiplatelet therapy | 373 (31) | 356 (31) | 528 (43) | 513 (42) |
| Oral anticoagulant therapy | 44 (4) | 47 (4) | 75 (6) | 111 (9) |
| Erythropoiesis-stimulating agent | 660 (55) | 637 (55) | 1056 (87) | 1079 (88) |
| BP (mmHg)a | ||||
| Systolic | 139±23 | 139±23 | 137±24 | 137±24 |
| Diastolic | 77±13 | 76±13 | 76±13 | 76±13 |
| Laboratory measurements | ||||
| LDL cholesterol (mg/dl) | 98±34 | 99±33 | 101±35 | 101±35 |
| Hemoglobin (g/dl) | 11.7±1.5 | 11.7±1.7 | 11.7±1.6 | 11.7±1.6 |
Data are presented as the n (%) or mean±SD. SHARP, Study of Heart and Renal Protection; AURORA, A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events.
Variables updated at 1 year for patients in SHARP originally allocated to simvastatin alone and who were rerandomized to simvastatin plus ezetimibe or placebo.
During a median 5 years of follow-up, the average LDL-C reduction was 26 mg/dl. A first vascular access occlusive event occurred in 355 (29.7%) of 1196 participants allocated to simvastatin plus ezetimibe compared with 388 (33.5%) among 1157 participants allocated to placebo (RR, 0.87; 95% CI, 0.75 to 1.00; P=0.05; Figure 1). Similar results were obtained after adjustment for the baseline imbalance in sex (RR, 0.86; 95% CI, 0.75 to 1.00; P=0.04) and in an analysis that included first and any subsequent occlusive events (634 in the simvastatin plus ezetimibe group versus 664 in the placebo group; RR, 0.92; 95% CI, 0.78 to 1.08; P=0.26). There was insufficient statistical power to estimate the effects of simvastatin plus ezetimibe on each separate type of event, but there was no evidence that the effects of simvastatin plus ezetimibe on any type differed from the overall estimated effect on vascular access occlusive events (P for heterogeneity=0.83; Figure 1). There was also limited power to detect any possible variation in treatment effects among different types of participants and, after allowance for multiplicity of analyses, subgroup analyses did not provide strong evidence that the proportional reduction in vascular access occlusive events differed according to whether participants had an AVF or an AV graft, had a history of diabetes mellitus, were current smokers, had higher baseline LDL-C, or were taking antiplatelet therapy or erythropoiesis-stimulating agents (Figure 2).
Figure 1.
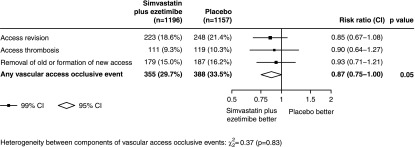
Effects of allocation to simvastatin plus ezetimibe on vascular access occlusive events among SHARP participants with preexisting access at randomization. CI, confidence interval; SHARP, Study of Heart and Renal Protection.
Figure 2.
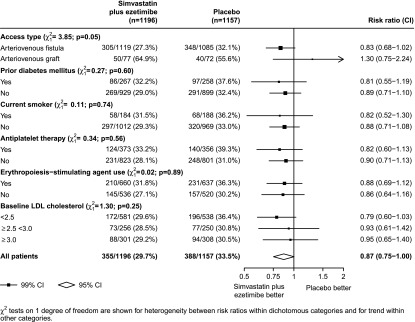
Effects of allocation to simvastatin plus ezetimibe on vascular access occlusive events in SHARP participants with preexisting access at randomization, by patient subtypes.
Because our analyses were post hoc, and the effect identified was of marginal statistical significance, the SHARP definitions and analysis methods were replicated in the AURORA dataset. Of 2776 hemodialysis patients in AURORA, 2439 had an AVF or AV graft at randomization (1219 allocated to rosuvastatin and 1220 allocated to placebo; Table 1). During a median 4.5 years of follow-up, there was no significant effect on vascular access occlusive events (352 [28.9%] in the rosuvastatin group versus 337 [27.6%] in the placebo group; RR, 1.06; 95% CI, 0.91 to 1.23; P=0.44). After combining the results of the SHARP and AURORA trials, the overall effect of reducing LDL-C with a statin-based regimen on vascular access occlusive events was not statistically significant (707 [29.3%] of participants who received any LDL-C–lowering therapy versus 725 [30.5%] participants who received placebo; RR, 0.95; 95% CI, 0.85 to 1.05; P=0.29; Figure 3). There was no good evidence that the effect of lowering LDL-C on vascular access occlusive events differed between the two studies, either overall (P for heterogeneity=0.06), or when access types were considered separately (Figure 3).
Figure 3.
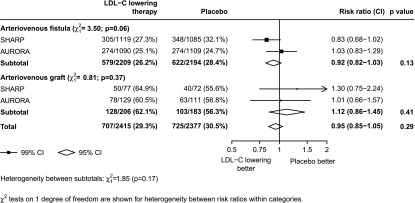
Effects of allocation to LDL-C–lowering therapy on vascular access occlusive events in SHARP and AURORA participants with preexisting access at randomization, subdivided by access type. AURORA, A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events; LDL-C, LDL cholesterol.
Discussion
Among 2353 participants in the SHARP trial who had a functioning AVF or AV graft at randomization, post hoc analyses indicated that allocation to simvastatin plus ezetimibe resulted in a 13% proportional reduction in the risk of a vascular access occlusive event. It remains unclear, however, whether this finding reflects a biologic effect or whether it is wholly or partly attributable to chance, because the finding was of only marginal statistical significance (log-rank P=0.05). The hypothesis raised by SHARP could, however, be tested because the AURORA trial collected information about events related to vascular access. Separate hypothesis-testing analysis of data on vascular access occlusive events from the AURORA trial did not confirm our findings. Taken together, the combined results of the two trials did not reach statistical significance, although the CI was wide and still remained compatible with a modest benefit. Because vascular access occlusion is common and has a major detrimental effect on patient care, even a small proportional effect of statin-based treatment could be worthwhile.
Although the available randomized trial data do not confirm a clear benefit of lowering LDL-C on vascular access patency, a small favorable effect is biologically plausible. Neointimal hyperplasia, the main reason for loss of patency of both AVF and AV grafts, is induced by a cascade of inflammatory mediators that are the consequence of injury from surgery, increased hemodynamic stress, and/or repeated needling. The venous stenoses that result are characterized by the proliferation of smooth muscle cells with a myofibroblastic phenotype, as well as their migration (with macrophages) into the vessel’s intima (16–18). Statin-based therapy might slow progression of neointimal hyperplasia by reducing vascular smooth muscle cell proliferation (19–23), smooth muscle cell migration (22), and monocyte activation (24), effects that are not known to be mediated through the LDL-C–lowering effect of hepatic hydroxymethyl glutaryl–CoA reductase inhibition.
Statin-based therapy could also have a beneficial effect on access through LDL-C dependent pathways, maintaining afferent blood flow by preventing atherosclerotic coronary (25) and peripheral arterial disease (26), which are both associated with failure of vascular access maturation (27).
After formation, successful access maturation requires vessel outward remodeling, a process that statins may also promote by increasing endothelial nitric oxide availability (23,28). However, in SHARP, it was only possible to test reliably for any benefit on preexisting (prevalent) vascular access. An unbiased test of any benefit on newly formed (incident) access would require a trial that randomized participants at the time of access formation. To maximize any LDL-C–independent effects, such a trial would need to consider using higher doses of statins than were used in either the SHARP or AURORA trials (although the clinical safety of such an approach would also need to be tested robustly).
Post hoc analysis of a subgroup of participants from a trial should be interpreted cautiously. A key strength of our analyses, however, was the availability of the AURORA data to test the hypothesis raised by SHARP.
Exploratory analyses from SHARP suggested that lowering LDL-C with statin-based therapy may improve vascular access patency in patients with preexisting access. This hypothesis was not supported by a reanalysis of AURORA. Taken together, a small benefit of statin-based therapy on vascular access patency cannot be excluded, but any such effect is of less clinical relevance than the clear benefit of lowering LDL-C on atherosclerotic events (10).
Disclosures
The SHARP trial was funded by Merck/Schering-Plough Pharmaceuticals (North Wales, PA), with additional support from the Australian National Health Medical Research Council, the British Heart Foundation, and the UK Medical Research Council. SHARP was initiated, conducted, and interpreted independently of the funders by the Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), which is part of the University of Oxford. AURORA was designed by an executive steering committee, which supervised the trial’s conduct. AURORA was conducted in collaboration with the sponsor, AstraZeneca, who collected the trial data. CTSU conducted and interpreted all of the post hoc vascular access analyses for both studies independently of the funders. CTSU has a staff policy of not accepting honoraria or consultancy fees.
Acknowledgments
The authors thank the participants in the SHARP and AURORA trials, as well as the local clinical center staff, regional and national coordinators, steering committees, and data monitoring committees.
Members of the SHARP Steering Committee are as follows: Colin Baigent, Martin J. Landray, Christina Reith, Jonathan Emberson, David C. Wheeler, Charles Tomson, Christoph Wanner, Vera Krane, Alan Cass, Jonathan Craig, Bruce Neal, Lixin Jiang, Lai Seong Hooi, Adeera Levin, Lawrence Agodoa, Mike Gaziano, Bertram Kasiske, Robert Walker, Ziad A. Massy, Bo Feldt-Rasmussen, Udom Krairittichai, Vuddidhej Ophascharoensuk, Bengt Fellström, Hallvard Holdaas, Vladimir Tesar, Andrzej Wiecek, Diederick Grobbee, Dick de Zeeuw, Carola Gronhagen-Riska, Tanaji Dasgupta, David Lewis, William Herrington, Marion Mafham, William Majoni, Karl Wallendszus, Richard Grimm, Terje Pedersen, Jonathan Tobert, Jane Armitage, Alex Baxter, Christopher Bray, Yiping Chen, Zhengming Chen, Michael Hill, Carol Knott, Sarah Parish, David Simpson, Peter Sleight, Alan Young, and Rory Collins.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10371013/-/DCSupplemental.
References
- 1.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Arora P, Kausz AT, Obrador GT, Ruthazer R, Khan S, Jenuleson CS, Meyer KB, Pereira BJ: Hospital utilization among chronic dialysis patients. J Am Soc Nephrol 11: 740–746, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Florescu MC, Birch N: Statin therapy and hemodialysis vascular access—Were we bringing a knife to a gunfight and were hoping to win? Semin Dial 25: 700–702, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Jackson AJ, Coats P, Kingsmore DB: Pharmacotherapy to improve outcomes in vascular access surgery: A review of current treatment strategies. Nephrol Dial Transplant 27: 2005–2016, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Paraskevas KI, Mikhailidis DP, Roussas N, Giannoukas AD: Effect of antiplatelet agents, statins, and other drugs on vascular access patency rates. Angiology 63: 5–8, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Diskin CJ: Novel insights into the pathobiology of the vascular access - Do they translate into improved care? Blood Purif 29: 216–229, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Pisoni R, Barker-Finkel J, Allo M: Statin therapy is not associated with improved vascular access outcomes. Clin J Am Soc Nephrol 5: 1447–1450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saran R, Dykstra DM, Wolfe RA, Gillespie B, Held PJ, Young EW, Dialysis Outcomes and Practice Patterns Study : Association between vascular access failure and the use of specific drugs: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 40: 1255–1263, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Righetti M, Ferrario G, Serbelloni P, Milani S, Tommasi A: Some old drugs improve late primary patency rate of native arteriovenous fistulas in hemodialysis patients. Ann Vasc Surg 23: 491–497, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SHARP Collaborative Group : Study of Heart and Renal Protection (SHARP): Randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J 160: 785–794, e10, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E, German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F, AURORA Study Group : Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG: Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 34: 585–612, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG: Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer 35: 1–39, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CJ, Ko PJ, Hsu LA, Ko YS, Ko YL, Chen CF, Huang CC, Hsu TS, Lee YS, Pang JH: Highly increased cell proliferation activity in the restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: Implication in prevention of restenosis. Am J Kidney Dis 43: 74–84, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC: Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int 59: 2325–2334, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P: Biology of arteriovenous fistula failure. J Nephrol 20: 150–163, 2007 [PubMed] [Google Scholar]
- 19.Igarashi M, Takeda Y, Mori S, Ishibashi N, Komatsu E, Takahashi K, Fuse T, Yamamura M, Kubo K, Sugiyama Y, Saito Y: Suppression of neointimal thickening by a newly developed HMG-CoA reductase inhibitor, BAYw6228, and its inhibitory effect on vascular smooth muscle cell growth. Br J Pharmacol 120: 1172–1178, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Indolfi C, Cioppa A, Stabile E, Di Lorenzo E, Esposito G, Pisani A, Leccia A, Cavuto L, Stingone AM, Chieffo A, Capozzolo C, Chiariello M: Effects of hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin on smooth muscle cell proliferation in vitro and neointimal formation in vivo after vascular injury. J Am Coll Cardiol 35: 214–221, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Noa M, Más R, Mesa R: A comparative study of policosanol vs lovastatin on intimal thickening in rabbit cuffed carotid artery. Pharmacol Res 43: 31–37, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Porter KE, Naik J, Turner NA, Dickinson T, Thompson MM, London NJ: Simvastatin inhibits human saphenous vein neointima formation via inhibition of smooth muscle cell proliferation and migration. J Vasc Surg 36: 150–157, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Yang Z, Kozai T, van der Loo B, Viswambharan H, Lachat M, Turina MI, Malinski T, Lüscher TF: HMG-CoA reductase inhibition improves endothelial cell function and inhibits smooth muscle cell proliferation in human saphenous veins. J Am Coll Cardiol 36: 1691–1697, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Ferro D, Basili S, Alessandri C, Cara D, Violi F: Inhibition of tissue-factor-mediated thrombin generation by simvastatin. Atherosclerosis 149: 111–116, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R, Cholesterol Treatment Trialists’ (CTT) Collaboration : Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376: 1670–1681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heart Protection Study Collaborative Group : MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 360: 7–22, 2002. 12114036 [Google Scholar]
- 27.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang MH, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A, Paschalis A, Psarros C, Triantafyllou C, Bendall J, Casadei B, Stefanadis C, Channon KM: Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation 124: 335–345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


