Abstract
Objective:
To determine the efficacy of medical marijuana in several neurologic conditions.
Methods:
We performed a systematic review of medical marijuana (1948–November 2013) to address treatment of symptoms of multiple sclerosis (MS), epilepsy, and movement disorders. We graded the studies according to the American Academy of Neurology classification scheme for therapeutic articles.
Results:
Thirty-four studies met inclusion criteria; 8 were rated as Class I.
Conclusions:
The following were studied in patients with MS: (1) Spasticity: oral cannabis extract (OCE) is effective, and nabiximols and tetrahydrocannabinol (THC) are probably effective, for reducing patient-centered measures; it is possible both OCE and THC are effective for reducing both patient-centered and objective measures at 1 year. (2) Central pain or painful spasms (including spasticity-related pain, excluding neuropathic pain): OCE is effective; THC and nabiximols are probably effective. (3) Urinary dysfunction: nabiximols is probably effective for reducing bladder voids/day; THC and OCE are probably ineffective for reducing bladder complaints. (4) Tremor: THC and OCE are probably ineffective; nabiximols is possibly ineffective. (5) Other neurologic conditions: OCE is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson disease. Oral cannabinoids are of unknown efficacy in non–chorea-related symptoms of Huntington disease, Tourette syndrome, cervical dystonia, and epilepsy. The risks and benefits of medical marijuana should be weighed carefully. Risk of serious adverse psychopathologic effects was nearly 1%. Comparative effectiveness of medical marijuana vs other therapies is unknown for these indications.
Marijuana contains approximately 60 pharmacologically active compounds (“cannabinoids”). Δ-9-Tetrahydrocannabinol (THC) was isolated in 1964 and the nonpsychoactive cannabidiol (CBD) in 1963; the ratio in botanical and pharmaceutical preparations determines therapeutic vs psychoactive effects, with the latter emerging when THC is higher in concentration. The presence of cannabinoid receptors in the brain led to discovery of endogenous ligands (endocannabinoids) such as anandamide and 2-arachidonylglycerol. The endocannabinoid system is widely distributed in the brain and spinal cord,1,2 with CB-1 receptors concentrated in the hippocampus, association cortices, basal ganglia, cerebellum, spinal cord (especially dorsal root ganglia), and peripheral nerves, including presynaptic sympathetic nerve terminals (and are notably absent from thalamus and brainstem). CB-2 receptors are found in the periphery, including lymph tissue, and in lower concentrations in some brain regions, including the periaqueductal gray. Activation through G-coupled membrane proteins causes physiologic responses expected from these regions, including feelings of well-being or psychosis (depending on the “dose” of THC), impaired memory and cognitive processing, slowed locomotor function, as well as antinociceptive,3 antiemetic, antispasticity, and sleep-promoting effects. Receptor activation inhibits adenylate cyclase, converting cyclic adenosine monophosphate to adenosine triphosphate, and inhibits release of multiple neurotransmitters, including acetylcholine, dopamine, and glutamate, when neuronal excitation is present.4 Indirect effects on opiate, serotonin, NMDA, and γ-aminobutyric acid receptors allow endocannibinoids to modulate other networks.3 The concentration of THC present in formulations and the ratio of THC to CBD, which limits THC's psychoactive effects, play a role in therapeutic effects of cannabis products. Table 1 presents the cannabinoid formulations examined here. A variety of formulations was used, with differing amounts of THC and CBD: some were pills, one was a mucosal spray, and some were vaporized or smoked.
Table 1.
Cannabinoid formulations
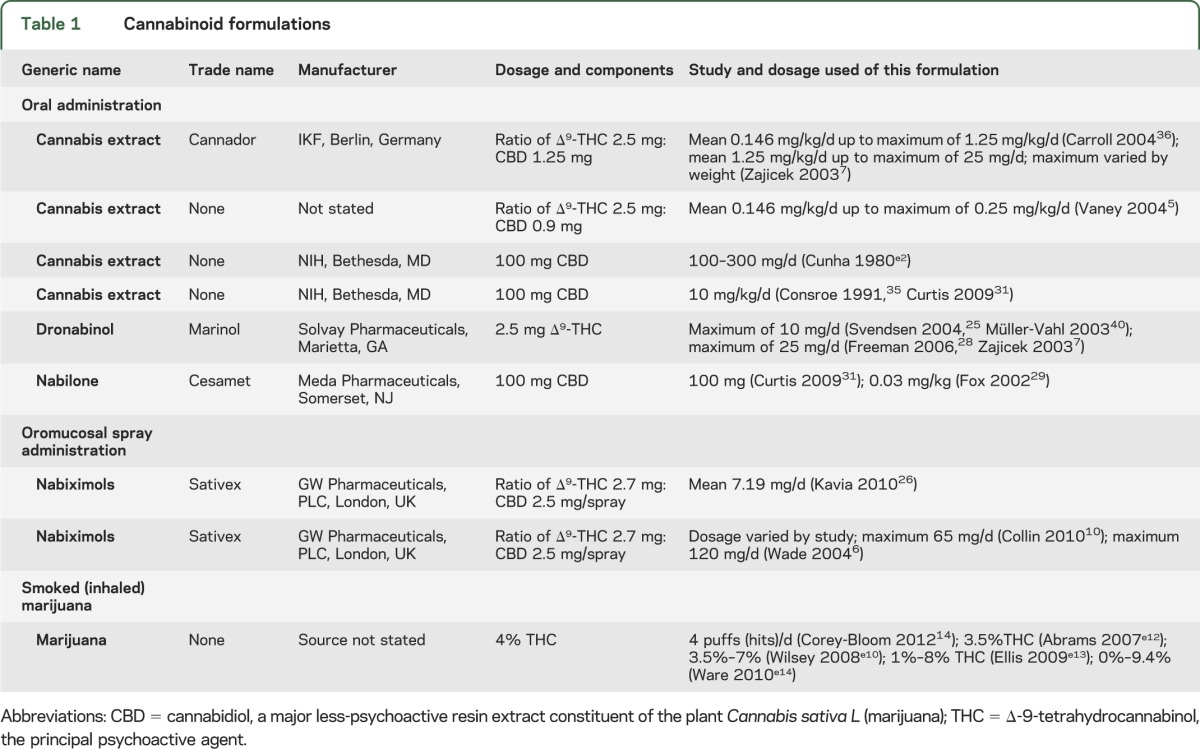
This evidence-based systematic review seeks to answer questions regarding safety and efficacy of cannabinoids in relieving/reducing the following:
Spasticity in patients with multiple sclerosis (MS)
Central pain and painful spasms in MS (pain could be from any etiology, including spasticity, but excluding neuropathic pain)
Bladder dysfunction in MS
Involuntary movements, including tremor, in MS
Dyskinesias of Huntington disease (HD), levodopa-induced dyskinesias of Parkinson disease, cervical dystonia, and tics of Tourette syndrome
Seizure frequency in epilepsy
DESCRIPTION OF THE ANALYTIC PROCESS
The American Academy of Neurology (AAN) Guideline Development Subcommittee convened an expert panel to develop the systematic review (appendices e-1 and e-2 on the Neurology® Web site at Neurology.org). We searched Medline, EMBASE, PsycINFO, Web of Science, and Scopus. Appendix e-3 presents the original search strategy employed for Medline (1948–January 2013) and updated strategies for all databases searched. The search yielded 1,729 abstracts. We examined the listed conditions and excluded non-neurologic pain (e.g., cancer, surgery-related) as well as other non-neurologic conditions (e.g., nausea). Surveys, case reports/series, and non–placebo-controlled trials were excluded. Of the 1,729 abstracts reviewed, we reviewed the full text of 63 articles and found that 33 met inclusion criteria. An updated search through November 2013 yielded one article for inclusion. We classified these following the AAN's therapeutic scheme (appendix e-4). Some articles were used to answer more than one question. Unless otherwise stated, multiple outcomes were corrected by the author panel with a Bonferroni correction. Study data are presented in tables e-1 through e-5.
ANALYSIS OF EVIDENCE
Question.
Do cannabinoids relieve spasticity in patients with MS?
Analysis.
We found 4 Class I,5–8 4 Class II,9–12 and 9 Class III13–21 studies addressing the issue of spasticity. Because of the possible confusion of varying evidence for the different agents, we present our analysis by type of cannabinoid studied.
Nabiximols.
A Class I multicenter study of 160 patients compared self-titrated nabiximols with placebo for treating the most troublesome symptom measured by a 100-point visual analog scale (VAS).6 No significant change from baseline was seen at 6 weeks (difference −5.93; 95% confidence interval [CI] −13.52 to 1.65; p = 0.124). Among patients reporting spasticity as the worst symptom (n = 37), there was a significant reduction in VAS rating (mean reduction 31.2 in treated vs 8.4 in placebo [p < 0.001, post–Bonferroni correction, 95% CI −10 to −35]).
There were 3 Class II prospective studies. A multicenter investigation studied 189 subjects with definite MS and spasticity; 124 received nabiximols and 65 received placebo.9 The primary endpoint was a change in a patient-recorded numeric rating score (NRS) (0–11 points).9 The intention-to-treat (ITT) population (n = 184) analysis showed a 0.52-point treatment difference favoring nabiximols (p = 0.048; 95% CI −1.029 to 0.004 points).
One Class II multicenter study randomized 337 subjects to receive nabiximols or placebo.10 The primary outcome was the change from baseline in mean NRS (0–10) assessed at treatment weeks 2, 6, 10, and 14. Clinically meaningful improvement was defined as ≥30% improvement on the NRS. In the ITT analysis, the treatment difference of 0.23 was nonsignificant (p = 0.219). Secondary endpoints were not significantly improved.
Another Class II study randomized 36 patients receiving nabiximols for at least 12 weeks for spasticity relief.12 The hazard ratio for treatment failure was 0.335 (95% CI 0.162–0.691) favoring treatment.
A Class III trial did not demonstrate efficacy.13 Another Class III trial did not perform statistics on the data. After Bonferroni correction, there were no significant differences on post hoc statistics.21
Oral cannabis extract and THC.
We found 3 Class I studies.5,7,8 In one study,5 patients received either escalating doses of an oral cannabis extract (OCE) containing THC and CBD for 14 days followed by placebo, or placebo for 7 days and then treatment for 14 days. No significant difference was seen for the primary outcome measure, the Ashworth Spasticity Scale, in the 50 patients designated for ITT. Among 37 patients who received at least 90% of their prescribed dose, active treatment was associated with greater improvement in the secondary outcome of spasm frequency (p = 0.013), without improvement on the Ashworth scale. Because of the small number of patients included, this study lacked statistical precision to detect differences.
In a second Class I study, 277 patients with “stable MS” and “muscle stiffness for at least 3 months” received either cannabis extracts containing THC and CBD (titrated to maximum daily dose of 25 mg THC) or placebo.8 A 2-week titration was followed by 10 weeks of maintenance, with assessments at 2, 4, 8, and 12 weeks. The primary endpoint was an 11-point category rating scale (CRS) (0 = very much better, 5 = no difference, and 10 = very much worse). The CRS is similar to the NRS used in other studies. Relief of muscle stiffness was equated with categories 0–3, with an odds ratio (OR) of 2.26 for improvement at 12 weeks (95% CI 1.24–4.13; p = 0.004).
In a third Class I study, 630 patients with MS-related spasticity received THC or a combination of THC and CBD, or placebo for each, titrated over 5 weeks and maintained for 8 weeks.7 There was no significant treatment effect on the primary endpoint of Ashworth scale for THC (mean change 1.86 vs placebo 0.92, 95% CI 7 to 95; p = 0.94) or THC + CBD (mean change 1.24 vs 0.92, 95% CI 6 to 60; p = 0.32). However, a beneficial effect was seen for both active treatments on secondary outcomes of patient-reported spasticity and pain (p = 0.003). A 12-month continuation study, rated Class II because of loss at follow-up,11 found a mean improvement in Ashworth score from study beginning to study end of 1.82 (from baseline mean 22) in patients treated with THC (computed 95% CI 0.54 to 3.1), 0.10 in the patients treated with THC/CBD extract (computed 95% CI −0.98 to 1.18), and −0.2 in the placebo group (p = 0.01 adjusted for ambulatory status and center, adjustment for center because of center size).
Five Class III studies of oral cannabinoids showed inconsistent results.16–20
Smoked marijuana.
One Class III trial demonstrated a mean decrease in modified Ashworth score in 30 subjects who completed the trial (2.95 in treated subjects, 0.21 in placebo; 95% CI 2.20–3.14; p < 0.001).14 A Class III double-blind trial of 20 subjects, each of whom smoked a single marijuana cigarette, found worsened posture and balance after 10 minutes (p = 0.018 post–Bonferroni correction).15
Conclusions.
For patients with spasticity:
OCE is established as effective for reducing patient-reported scores (2 Class I studies7,8). OCE is probably ineffective for reducing objective measures at 12–15 weeks (1 Class I study7) but possibly effective at 1 year (1 Class II study11).
THC is probably effective for reducing patient-reported scores (1 Class I study7). THC is probably ineffective for reducing objective measures at 15 weeks (1 Class I study7) but possibly effective at 1 year (1 Class II study11).
Nabiximols is probably effective for reducing patient-reported symptoms at 6 weeks (1 Class I study6) and probably ineffective for reducing objective measures at 6 weeks (1 Class I study6).
Smoked marijuana is of uncertain efficacy (insufficient evidence).
Clinical context.
Standard medical therapy was continued in these studies, so no comment can be made as to comparative effectiveness.
Multiple methods of measuring spasticity exist. A recent study used correlations with changes on a standard Patient Global Impression of Change scale22 to determine that a ∼30% change in spasticity, as measured by the patient-reported NRS, best represented a clinically important difference.23 More improvements were seen in subjective measures than objective measures, possibly explained in part by the overall improvements in “feelings” or well-being provided by marijuana, or by pain relief allowing improved mobility.
Question.
What is the efficacy of using cannabinoids to treat central pain or painful spasms in MS?
Analysis.
There were 5 Class I,5–8,24 2 Class II,10,11 and 6 Class III13,15,18–20,25 studies of cannabinoids for treating central pain or spasms.
Nabiximols.
A Class I study involved 66 patients with neuropathic and central pain. Patients with only spasticity or painless spasms were excluded.24 Patients were randomized to nabiximols or placebo, and they rated their pain on an 11-point NRS. During the study's fourth week, nabiximols was superior to placebo in reducing pain intensity (treated: 41% decrease, placebo: 22% decrease) and in decreasing both the mean pain intensity of −2.7 (95% CI 3.4–2.0, placebo −1.4, 95% CI 2.0–0.8; p = 0.005) and pain-related sleep disturbances.
In a Class II study of 337 patients, 167 receiving nabiximols and 170 placebo, no significant difference was seen in the secondary outcomes of change in spasm frequency (treated: −0.86, placebo: −0.85; p = 0.955) and pain NRS (treated: −1.22, placebo: −1.14; p = 0.763).10 A Class III study did not show a significant effect of nabiximols on mean pain VAS score over 6 weeks.13
OCE and THC.
In a previously described Class I study,7 pain reduction after 14 weeks of treatment was described by 50% of those receiving THC, 46% of those receiving THC/CBD, and 30% of those receiving placebo. These differences were significant after Bonferroni correction (p = 0.022 for THC, p = 0.033 for THC/CBD). The Class II continuation study included 356 patients reporting pain and 438 reporting muscle spasms.11 Pain improvement was reported in 28% receiving THC, 31% receiving THC/CBD, and 23% receiving placebo (p = 0.002). Muscle spasm reduction was reported in 29% of those receiving THC, 36% receiving THC/CBD, and 23% receiving placebo (p = 0.002).
Another Class I study,8 described previously, which included pain and spasms as a secondary outcome, found that the proportion of patients with self-reported relief (0–3 on CRS) was greater in the cannabis group than in the placebo group at all visits (p < 0.025).
In a Class I study described previously,6 at 6 weeks no significant difference in ratings was seen for pain or spasms between the 2 groups (pain: n = 36, +8.73, 95% CI −10.39 to 27.84; spasms: n = 38, difference −5.3, 95% CI −19.81 to 9.22). Because of the large placebo response to pain, this part of the study could not be interpreted.
In a Class I randomized, placebo-controlled, crossover study, 50 patients with MS were treated for 14 days with THC/CBD capsules, which decreased spasm frequency from 1.0 to 0.7/day (treated) relative to 0.9–0.8/day (placebo) (95% CI of the difference between periods of placebo and cannabis: 0.99–3.19; p = 0.058).5 The study had limited power to detect differences.
Four Class III studies found varying results after Bonferroni correction.18–20,25
Smoked marijuana.
There were 2 Class III studies.14,15 In a crossover study of smoked marijuana, 30 patients with MS and spasticity showed smoking marijuana significantly reduced pain: decrease to 8.27 from 16.61 in patients receiving treatment vs 11.52 from 14.51 in patients receiving placebo (95% CI 2.48–10.01; p = 0.008).14
A study of dynamic posturography among 10 adult patients with spasticity and 10 matched normal volunteers found smoking marijuana impairs posture and balance in patients with spasticity.15
Conclusion.
For patients with MS with central pain or painful spasms, OCE is effective for reduction of central pain (2 Class I studies7,8). THC or nabiximols (1 Class I study each7,24) are probably effective for treating MS-related pain or painful spasms. Smoked marijuana is of unclear efficacy for reducing pain (2 Class III studies that examined different issues14,15).
Question.
Do cannabinoids help treat bladder dysfunction in MS?
Analysis.
Nabiximols.
A Class I study of 135 patients with MS and detrusor overactivity showed no difference in mean daily episodes of incontinence (−1.08 using nabiximols spray relative to −0.98 placebo; p = 0.056).26 After Bonferroni correction, the significant secondary outcomes were the Overall Bladder Condition rating scale score20 (p = 0.008), the patients' Global Impression of Change score27 (p = 0.04), and number of voids per 24 hours (p = 0.008). Another Class I study, which looked at bladder complaints as a secondary outcome, did not show improvement.6
Oral cannabinoids and THC.
One Class I study examined bladder complaints as a secondary outcome, and no improvement was noted with either THC or OCE.7 Another Class I study, which examined bladder complaints over time, also noted no improvement of self-reported bladder complaints.5
A Class II substudy (255 of the original 630 patients) of the Cannabinoids in MS study measured incontinence episodes.28 Fewer than half the patients were analyzed, and thus the results cannot inform reliable conclusions.
Conclusion.
Nabiximols is probably effective for reducing the number of bladder voids per day at 10 weeks (1 Class I study26). THC and OCE are probably ineffective for reducing bladder complaints (1 Class I study7). Nabiximols is of unknown efficacy in reducing overall bladder symptoms (contradictory Class I studies).
Question.
Do cannabinoids help treat tremor in MS?
Analysis.
Tremor was included as a secondary outcome in 3 Class I studies,5–7 1 Class II study,10 and 2 Class III studies.29,30
Nabiximols.
In a Class I study, the oral spray nabiximols produced no change in VAS report of tremor in 13 patients (−21.42 treated vs −25.17 placebo, p = 0.810).6 Because of the small number of patients included, the study was underpowered to detect differences.
In a Class II study of 337 patients, an unspecified number of whom rated tremor on NRS, no response was reported with nabiximols as compared with placebo (−0.56 vs −0.31, p = 0.255).10
Oral cannabinoids and THC.
In a Class I study of 630 patients, tremor was listed as a symptom in diaries kept by 391 patients receiving capsules of THC, THC/CBD, or placebo.7 Neither self-report (p = 0.398) nor physician assessment (p = 0.052) noted a response.
In the subset of 26 patients reporting tremor in a third Class I study of 50 patients, no response to CBD was observed by patient report (p = 0.9) or physician report (p = 0.82).5 This study had limited power to detect differences.
Two Class III studies examined tremor; no improvement was seen with oral nabilone29 or THC/CBD.30
Conclusions.
THC and OCE are probably ineffective for treating MS-related tremor (1 Class I study7). Nabiximols is possibly ineffective (1 Class II study10).
Question.
Do cannabinoids reduce symptoms in involuntary movement disorders?
Analysis.
Huntington disease.
A Class I crossover study evaluated nabilone for symptomatic HD treatment (n = 37, two 5-week treatment periods separated by a 5-week washout period).31 There was no significant difference on the primary outcome of Unified Huntington's Disease Rating Scale (UHDRS)32 total motor score (treatment difference 0.86, 95% CI −1.8 to 3.52; p = 0.5), with a 1-point change in UHDRS likely to be clinically significant.33 This study was underpowered to detect anything but a large difference.
A 2012 AAN guideline specifically examined the efficacy of marijuana for treating chorea in HD.34
In another Class III crossover study (15 patients), the efficacy of CBD capsules (10 mg/kg/d in 2 divided doses) was evaluated for symptomatic HD treatment.35 This study was underpowered to detect differences.
Conclusion.
Whereas these 2 studies31,35 suggest lack of benefit, both were underpowered to detect differences, and thus no reliable conclusions can be drawn.
Levodopa-induced dyskinesias in Parkinson disease.
A Class I double-blind crossover study examined the effectiveness of CBD extract in 1.25- or 2.5-mg capsules with an average daily dose of 0.146 mg/kg/d in the treatment of levodopa-induced dyskinesias in 19 patients.36 The primary outcome was score on Part IV (dyskinesia section, items 32–34) of the Unified Parkinson's Disease Rating Scale (UPDRS).37 The overall treatment effect was +0.52 on items 32–34 of the UPDRS, which indicated a worsening but was nonsignificant (p = 0.09). No secondary outcomes were affected by treatment. A Class III study examined dopamine-induced dyskinesias and showed improvement in 7 patients.38
Conclusion.
OCE is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson disease (1 Class I study36).
Tourette syndrome.
A Class II study examined 9 measures in 12 patients in a placebo-controlled, crossover study, using a single-dose THC capsule (5.0, 7.5, or 10.0 mg).39 Because of the small number of patients and the large number of items tested, this trial lacks statistical power to enable reliable conclusions to be drawn.
In a Class III placebo-controlled study of tics in 24 patients, patients received up to 10 mg/d of THC orally over 6 weeks.40 After Bonferroni correction, there were no significant differences.
Conclusion.
For patients with Tourette syndrome, data are insufficient to support or refute efficacy of THC for reducing tic severity (1 Class II study, 1 Class III study).39,40
Cervical dystonia.
One Class III study examined the effect of dronabinol on cervical dystonia.e1 No differences were detected in any outcome measure, but the study was underpowered to detect differences.
Conclusion.
For patients with cervical dystonia, data are insufficient to support or refute the efficacy of dronabinol.
Question.
Do cannabinoids decrease seizure frequency in epilepsy?
Analysis.
There were no Class I–III studies. There were 2 Class IV studies that did not demonstrate a significant benefit and did not show adverse effects (AEs) over 3–18 weeks of treatment.e2,e3
Conclusion.
For patients with epilepsy, data are insufficient to support or refute the efficacy of cannabinoids for reducing seizure frequency (no Class I–III studies).
Clinical context.
Neither the present review, nor a Cochrane review, which includes abstracts, non–peer-reviewed literature, and anecdotal reports of smoked cannabis use by patients with seizure disorders,e4 concluded there is sufficient evidence to prescribe CBDs or recommend self-treatment with smoked marijuana.
ADVERSE EFFECTS
In looking at marijuana-related AEs, we excluded studies that reanalyzed earlier studies, used a single dose of medication, or had Class IV evidence or unclear information about AEs.15,17,28,38,e2,e3 See table e-6 for details.
Overall, 1,619 patients were treated with cannabinoids for less than 6 months. Meta-analysis of simple proportions yielded 6.9% (95% CI 5.7%–8.2%) who stopped the medication because of AEs. Of the 1,118 who received placebo, 2.2% (95% CI 1.6%–3.5%) stopped because of AEs. Data on the symptoms that caused medication withdrawal were often incomplete. Among patients treated with cannabinoids, the following symptoms appeared in at least 2 studies: nausea, increased weakness, behavioral or mood changes (or both), suicidal ideation or hallucinations (or both), dizziness or vasovagal symptoms (or both), fatigue, feelings of intoxication. Psychosis, dysphoria, and anxiety are associated with higher concentrations of THC, which are not typical of the studies we analyzed. There was one death “possibly related” to treatment (a seizure, followed by fatal aspiration pneumonia).18
A single Class II study looked at the effects of cannabinoids at 1 year. Thirty-one of 207 patients treated with cannabis extract (15%) stopped medication, as did 28 of 197 treated with THC (14%) and 10 of 207 given placebo (5%).11 However, AEs were not necessarily the reason medication was stopped. For example, cannabinoids inhibit many enzymes of the cytochrome P-450 system, which will cause interactions with other medications being taken, especially opiates for pain. No direct fatalities (overdoses) have been attributed to marijuana, even in recreational users of increasingly potent marijuana, possibly because of the lack of endocannabinoid receptors in the brainstem. Clearly, deleterious effects on judgment can indirectly endanger patients who perform dangerous tasks such as driving. In addition, smoking and possibly even use of vaporized preparations expose users to carbon monoxide and other respiratory toxins.
Clinical context.
AEs are a significant concern with marijuana use. Outside the setting of treatment trials, cognitive impairment is more likely to be of concern. One study of patients with MS who smoked cannabis at least once a month showed an increase in cognitive impairment.e5 Another article showed that patients with MS who used cannabis were twice as likely to be classified as globally cognitively impaired as those who did not use cannabis.e6 Some patients who have neurologic conditions may have preexisting cognitive dysfunction, which may increase their susceptibility to cannabinoids' toxicities.e5,e6 Moreover, it is especially concerning that a medication that may have an AE of suicide may be prescribed in a population such as patients with MS who already are at increased suicide risk.e7
RECOMMENDATIONS FOR FUTURE RESEARCH
Placebo effect, which has been reported to be as high as 70%,35 interferes with proof of efficacy, although the ability to recognize treatment was mitigated by preparations with less THC and thus less psychoactive effects. Although masking may be lost due to the non-naive subject's recognition of his or her assigned group (treatment vs control),2,3,e8,e9 interviews of subjects also found many who guessed incorrectly which group they were in, especially the first time cannabis was used.e10
Recruitment into studies of a drug currently classified as Schedule I in the United States may be difficult due to the stigma attached or the additional burden placed on researchers, although in British studies the prohibition from driving for the duration of the study was more likely to dissuade patients from enrolling.
The many formulations and doses we studied make comparative analysis of cannabinoid efficacy difficult. Cannabis smoked in cigarettes or pipes, the most familiar form of cannabis, was the least studied outside of user surveys, which often are generated by anonymous questionnaires, sources from which detailed information is difficult to obtain. Even in surveys of identifiable subjects, such as those derived from support groups, reliability suffers.e11
In addition, the need to use many subjective measures such as patient-driven symptom rating scales, especially of pain, is a fundamental problem in this field.7,8,e10,e12–e14 Even “objective” measures such as walking times and the Ashworth Spasticity Scale have poor reliability,e15 as they will be influenced by patient's improved pain control or general improvement in well-being. Despite this, from a patient's perspective insights gained from subjective outcomes are probably even more important than objective outcomes.
Future research with randomized controlled studies is necessary in order to determine the efficacy of this medication class. The present review downgraded some studies for inadequate outcome concealment and comparison of baseline characteristics. Some studies were underpowered to detect differences; others had too many dropouts for reliable conclusions to be drawn. Other questions concerning the anti-inflammatory and immunologic effects of cannabinols evolved from the presence of CB-2 receptors in the lymphatic system and observation of neuroprotective effects in animal models of diseases such as amyotrophic lateral sclerosis.4 Disease-modifying effects in MS were not confirmed in a recent clinical study.e16
Cannabinoids should be studied as other drugs are, to determine their efficacy, and when evidence is available, should be prescribed as other drugs are. Twenty states and the District of Columbia have legalized the medical use of marijuana, and 2 have decriminalized all use. This should encourage researchers to continue seeking answers to the benefits of marijuana use in patients who have neurologic illness.
Supplementary Material
GLOSSARY
- AAN
American Academy of Neurology
- AE
adverse effect
- CBD
cannabidiol
- CI
confidence interval
- CRS
category rating scale
- HD
Huntington disease
- ITT
intention-to-treat
- MS
multiple sclerosis
- NRS
numeric rating score
- OCE
oral cannabis extract
- THC
Δ-9-tetrahydrocannabinol
- UHDRS
Unified Huntington's Disease Rating Scale
- UPDRS
Unified Parkinson's Disease Rating Scale
- VAS
visual analog scale
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Barbara Koppel: study concept and design, acquisition of data, analysis or interpretation of data, critical revision of the manuscript for important intellectual content. John Brust: analysis or interpretation of data, critical revision of the manuscript for important intellectual content. Terry Fife: study concept and design, acquisition of data, analysis or interpretation of data, critical revision of the manuscript for important intellectual content, study supervision. Jeff Bronstein: analysis or interpretation of data, critical revision of the manuscript for important intellectual content. Sarah Youssof: analysis or interpretation of data, critical revision of the manuscript for important intellectual content. Gary Gronseth: study concept and design, acquisition of data, analysis or interpretation of data, critical revision of the manuscript for important intellectual content, study supervision. David Gloss: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
This systematic review was developed with financial support from the American Academy of Neurology. None of the authors received reimbursement, honoraria, or stipends for their participation in the development of this systematic review.
DISCLOSURE
B. Koppel, J. Brust, T. Fife, J. Bronstein, S. Youssof, and G. Gronseth report no disclosures relevant to the manuscript. D. Gloss serves as an evidence-based medicine consultant to the American Academy of Neurology. Go to Neurology.org for full disclosures.
DISCLAIMER
This statement is provided as an educational service of the American Academy of Neurology. It is based on an assessment of current scientific and clinical information. It is not intended to include all possible proper methods of care for a particular neurologic problem or all legitimate criteria for choosing to use a specific procedure. Neither is it intended to exclude any reasonable alternative methodologies. The AAN recognizes that specific patient care decisions are the prerogative of the patient and the physician caring for the patient, based on all of the circumstances involved. The clinical context section is made available in order to place the evidence-based systematic review(s) into perspective with current practice habits and challenges.
CONFLICT OF INTEREST
The American Academy of Neurology is committed to producing independent, critical, and truthful systematic reviews (SRs). Significant efforts are made to minimize the potential for conflicts of interest to influence the conclusions of this SR. To the extent possible, the AAN keeps separate those who have a financial stake in the success or failure of the products appraised in the SRs and the developers of the SRs. Conflict of interest forms were obtained from all authors and reviewed by an oversight committee prior to project initiation. AAN limits the participation of authors with substantial conflicts of interest. The AAN forbids commercial participation in, or funding of, SR projects. Drafts of the SR have been reviewed by at least 3 AAN committees, a network of neurologists, Neurology peer reviewers, and representatives from related fields. The AAN Guideline Author Conflict of Interest Policy can be viewed at www.aan.com. For complete information on this process, access the 2004 AAN process manual.e17
REFERENCES
- 1.Joy JE, Watson SJ, Bensen JA, Jr, eds. Institute of Medicine. Marijuana and Medicine: Assessing the Science Base. Washington, DC: National Academies Press; 1999. Available at: http://www.nap.edu. Accessed March 2013 [PubMed] [Google Scholar]
- 2.Zajicek JP, Apostu VI. Role of cannabinoids in multiple sclerosis. CNS Drugs 2011;25:187–201 [DOI] [PubMed] [Google Scholar]
- 3.Raichlen DA, Foster AD, Gerdman GL, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner's high.” J Exp Biol 2012;215:1331–1336 [DOI] [PubMed] [Google Scholar]
- 4.Grant I, Atkinson JH, Gouaux B, Wilsey B. Medical marijuana: clearing away the smoke. Open Neurol J 2012;6:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaney C, Heinzel-Gutenbrunner M, Jobin P, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler 2004;10:417–424 [DOI] [PubMed] [Google Scholar]
- 6.Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler 2004;10:434–441 [DOI] [PubMed] [Google Scholar]
- 7.Zajicek JP, Fox P, Sanders H, et al. ; UK MS Research Group. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet 2003;362:1517–1526 [DOI] [PubMed] [Google Scholar]
- 8.Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG; MUSEC Research Group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry 2012;83:1125–1132 [DOI] [PubMed] [Google Scholar]
- 9.Collin C, Davies P, Mutiboko IK, Ratcliffe S; Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol 2007;14:290–296 [DOI] [PubMed] [Google Scholar]
- 10.Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res 2010;32:451–459 [DOI] [PubMed] [Google Scholar]
- 11.Zajicek JP, Sanders HP, Wright DE, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 2005;76:1664–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notcutt W, Langford R, Davies P, Ratcliffe S, Potts R. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex® (nabiximols). Mult Scler 2012;18:219–228 [DOI] [PubMed] [Google Scholar]
- 13.Centonze D, Mori F, Kock G, et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci 2009;30:531–534 [DOI] [PubMed] [Google Scholar]
- 14.Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ 2012;184:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg HS, Werness SA, Pugh JE, Andrus RO, Anderson DJ, Domino EF. Short-term effects of smoking marijuana on balance in patients with multiple sclerosis and normal volunteers. Clin Pharmacol Ther 1994;55:324–328 [DOI] [PubMed] [Google Scholar]
- 16.Killestein J, Hoogervorst EL, Reif M, et al. Safety, tolerability and efficacy of orally administered cannabinoids in MS. Neurology 2002;58:1404–1407 [DOI] [PubMed] [Google Scholar]
- 17.Ungerleider JT, Andyrsiak T, Fairbanks L, Ellison GW, Myers LW. Δ-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv Alcohol Subst Abuse 1987;7:39–50 [DOI] [PubMed] [Google Scholar]
- 18.Wade DT, Makela P, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler 2006;12:639–645 [DOI] [PubMed] [Google Scholar]
- 19.Novotna A, Mares J, Ratcliffe S, et al. ; Sativex Spasticity Study Group. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols (Sativex), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 2011;18:1122–1131 [DOI] [PubMed] [Google Scholar]
- 20.Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil 2003;17:21–29 [DOI] [PubMed] [Google Scholar]
- 21.Serpell MG, Notcutt W, Collin C. Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol 2013;260:285–295 [DOI] [PubMed] [Google Scholar]
- 22.Dworkin RH, Turk DC, Farrarr JT, et al. ; IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19 [DOI] [PubMed] [Google Scholar]
- 23.Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther 2008;30:974–985 [DOI] [PubMed] [Google Scholar]
- 24.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812–819 [DOI] [PubMed] [Google Scholar]
- 25.Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomized double blind placebo controlled crossover trial. BMJ 2004;329:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavia RB, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler 2010;16:1349–1359 [DOI] [PubMed] [Google Scholar]
- 27.Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther 2004;27:26–35 [DOI] [PubMed] [Google Scholar]
- 28.Freeman RM, Adekanmi O, Waterfield MR, Wright D, Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicenter, randomised placebo-controlled trial (CAMS-LUTS). Int Urogynecol J Pelvic Floor Dysfunct 2006;17:636–641 [DOI] [PubMed] [Google Scholar]
- 29.Fox SH, Kellett M, Moore AP, Crossman AR, Brotchie JM. Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov Disord 2002;17:145–149 [DOI] [PubMed] [Google Scholar]
- 30.Fox P, Bain PG, Glickman S, Carroll C, Zajicek J. The effect of cannabis on tremor in patients with multiple sclerosis. Neurology 2004;62:1105–1109 [DOI] [PubMed] [Google Scholar]
- 31.Curtis A, Mitchell I, Patel S, Ives N, Rickards H. A pilot study using nabilone for symptomatic treatment of Huntington's disease. Mov Disord 2009;24:2254–2259 [DOI] [PubMed] [Google Scholar]
- 32.Huntington Study Group. Unified Huntington's Disease Rating Scale: reliability and consistency. Mov Disord 1996;2:136–142 [DOI] [PubMed] [Google Scholar]
- 33.Beglinger LJ, O'Rourke JJ, Wang C, Langbehn DR, Duff K, Paulsen JS; Huntington Study Group Investigators. Earliest functional declines in Huntington disease. Psychiatry Res 2010;178:414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong MJ, Miyasaki JM. Evidence-based guideline: pharmacologic treatment of chorea in Huntington disease: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2012;79:597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Consroe P, Laguna J, Allender J, et al. Controlled clinical trial of cannabidiol in Huntington's Disease. Pharmacol Biochem Behav 1991;40:701–708 [DOI] [PubMed] [Google Scholar]
- 36.Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology 2004;63:1245–1250 [DOI] [PubMed] [Google Scholar]
- 37.Gancher ST. The Unified Parkinson’s Disease Rating Scale. Portland, OR: Demos Medical Publishing; 2002 [Google Scholar]
- 38.Sierzdan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson's disease: a pilot study. Neurology 2001;57:2108–2111 [DOI] [PubMed] [Google Scholar]
- 39.Müller-Vahl KR, Schneider U, Koblenz A, et al. Treatment of Tourette's syndrome with Δ-9-tetrahydrocannabinol (THC): a randomized crossover trial. Pharmacopsychiatry 2002;35:57–61 [DOI] [PubMed] [Google Scholar]
- 40.Müller-Vahl KR, Schneider U, Prevedel H, et al. Δ9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry 2003;64:459–465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


