Abstract
Objective:
To understand the pathologic and clinical correlates of patients with chronic meralgia paresthetica (MP) undergoing lateral femoral cutaneous nerve (LFCN) neurectomy.
Methods:
A retrospective cohort approach was utilized to identify 7 patients undergoing LFCN neurectomy for intractable pain. Control autopsied LFCN was obtained. Clinical, radiologic, and electrophysiologic features were reviewed.
Results:
In identified cases, preoperative symptoms included severe lateral thigh pain and numbness. The duration of symptoms prior to surgery ranged from 2 to 15 years. Body mass index (BMI) varied from 20 kg/m2 to 44.8 kg/m2 (normal–morbidly obese), with 6 out of 7 patients being obese. No patients were diabetic. Focal nerve indentation at the inguinal ligament was seen intraoperatively and on gross pathology in 4 of 7 cases. Multifocal fiber loss, selective loss of large myelinated fibers, thinly myelinated profiles, regenerating nerve clusters, perineurial thickening, and subperineurial edema were seen. None of these features were observed in control nerve. Morphometric analysis confirmed loss of large myelinated fibers with small and intermediate size fiber predominance. Five patients had varying degrees of intraneural and epineurial inflammation. Six of 7 reported improved pain after neurectomy, sometimes dramatic.
Conclusions:
Patients with chronic MP and intractable pain have an LFCN mononeuropathy with loss of nerve fibers. Pathologic and clinical study supports a compressive pathogenesis as the primary mechanism. Abnormal nerve inflammation coexists and may play a role in pathogenesis. These selected patients typically benefited from neurectomy at a site of inguinal ligament compression.
Classification of Evidence:
This study provides Class IV evidence that patients with chronic MP LFCN neurectomy experience improvement in MP-related pain.
Meralgia paresthetica (MP) has been found to be relatively common, with an incidence of 32.6–43 per 100,000 patient-years.1,2 The incidence is greatest in older persons (55–64 years) with elevated body mass index (BMI), which supports the theory that mechanical compression plays a role.1 However, a strong association between diabetes and MP was also found, which was not explained by the increase in BMI alone, suggesting that diabetes may be an independent risk factor for MP. Specifically considered in the lateral femoral cutaneous nerve (LFCN), pathologic mechanisms of MP are (1) compression at the inguinal ligament from obesity; (2) injury susceptibility related to elevated blood sugars; or (3) an inflammatory or autoimmune mechanism as occurs in diabetic and nondiabetic lumbosacral plexitis, which commonly affects the femoral nerve.1,3
Pathologic descriptions and the outcomes of patients undergoing neurectomy would provide great insight into the disease mechanism and is the objective of the presented work.
METHODS
Standard protocol approvals, registrations, and patient consents.
Approval for this study was received from our Institutional Review Board and Biospecimens Committee.
Primary research questions were as follows: (1) to assess the pathologic features of the LFCN and the clinical features of patients with severe and chronic MP; and (2) to retrospectively evaluate the effectiveness of LFCN neurectomy in treatment of chronic MP-related pain (Class IV evidence).
The medical charts of individuals undergoing open LFCN neurectomy proximal to the inguinal ligament for intractable pain were reviewed. An electronic retrieval system was utilized to assure all cases within the nerve pathology database were identified. Nine LFCNs were identified; 7 of these 9 cases were performed within our hospital system and had associated clinical and follow-up information. The clinical diagnosis of MP was confirmed in exclusion of alternative disorders such as neoplastic infiltration, acute nerve injury by external trauma, and upper lumbar radiculopathy. Age, sex, BMI, and diabetes status were determined. Available imaging and electrophysiology was reviewed. Because of the lack of normal values for the LFCN in terms of nerve morphology, fiber number, and distribution, a control LFCN from autopsy was obtained. Teased fiber, paraffin and epoxy sections, as well as morphometric procedures were performed by standard techniques.4 LFCN conduction studies were reviewed and performed by published technique.5
RESULTS
Seven LFCN biopsies were identified from individuals undergoing neurectomy for intractable pain: 4 men and 3 women, age 27–78 years at the time of biopsy (table 1). Six patients had previously undergone ultrasound-guided steroid injections in proximity to the LFCN at the inguinal ligament with initial but unsustained benefit. BMIs ranged from 20.14 kg/m2 to 44.81 kg/m2 (normal to morbid obesity [>40 kg/m2]). Six out of the 7 patients had BMIs at or above 30 kg/m2 (obese Class I). None of the patients was diabetic. Symptom duration at neurectomy ranged from 2 to 15 years. Six out of 7 patients had continuous painful lateral thigh numbness and paresthesias; 1 patient had intermittent symptoms, severe on standing (case 1). Position-related pain occurred with standing (n = 5), lying (n = 2), or both (n = 1). Symptoms were unilateral in 5 and bilateral in 2 (cases 4 and 5). LFCN conductions were normal in 1 patient (case 1), unilaterally absent in 2 patients (cases 2 and 3), and bilaterally absent in 2 patients (cases 4 and 6). All patients underwent EMG without evidence for a diffuse femoral neuropathy or upper lumbar radiculopathy. In case 4, MRI of the nerve was available and revealed LFCN fusiform enlargement with T2 hyperintensity; compression and distortion of the nerve were noted during surgery. At neurectomy, 2 additional patients had focal compression at the inguinal ligament with visible distal enlargement of the LCFN (cases 2 and 3). A fourth also had visible compression at time of surgery of the LFCN at the inguinal ligament with associated erythema (case 5).
Table 1.
Patients with meralgia paresthetica undergoing neurectomy
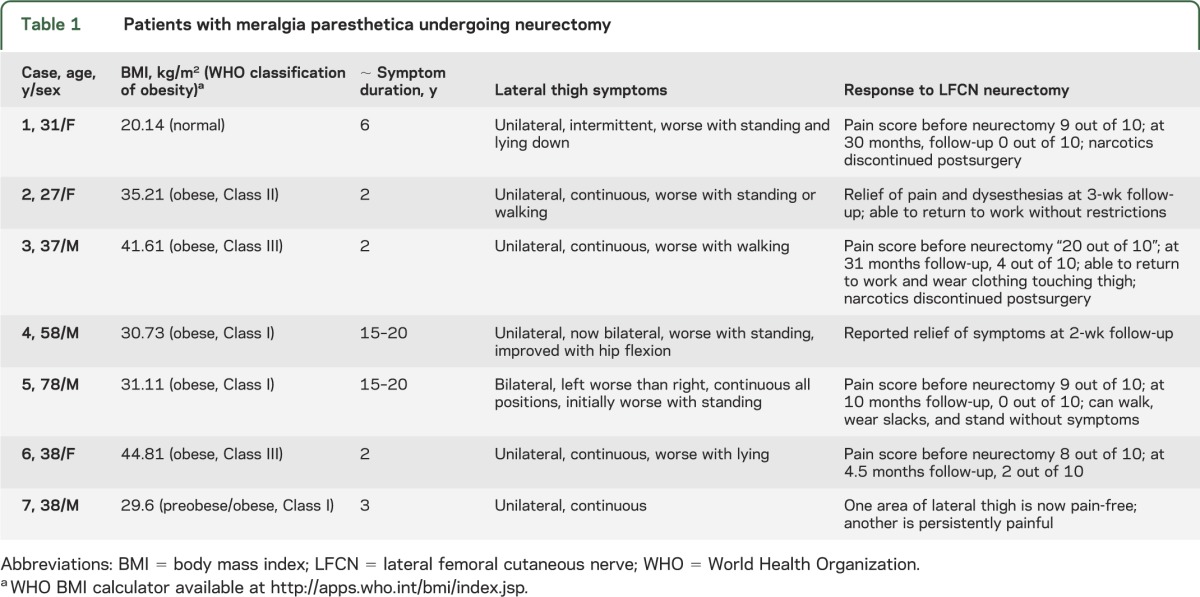
All patients had transient worsening of neuropathic pain immediately postneurectomy that subsequently improved. Sustained clinical improvement of pain postoperatively was reported in 6 out of 7 patients (follow-up period between 2 weeks and 31 months with a median follow up of 4.5 months) (table 1). Many had dramatic improvements in pain scores going from a level of 9 out of 10 preoperatively to 0 out of 10 at follow-up. Two patients were on narcotics preoperatively and were able to discontinue them (cases 1 and 3).
LFCN histopathologic findings.
Gross abnormalities of the resected nerve included focal indentation (4 of 7) and enlargements distal to the indentations (2 of 7) (table 2, figure 1). All nerve biopsies had reduced fiber density with multifocal fiber loss (7 of 7), selective loss of large myelinated fibers (7 of 7), perineurial thickening (7 of 7), and Renaut bodies (7 of 7). Six cases had subperineurial edema; 4 had regenerating clusters and thinly myelinated profiles.
Table 2.
Pathologic findings of lateral femoral cutaneous nerve in meralgia paresthetica
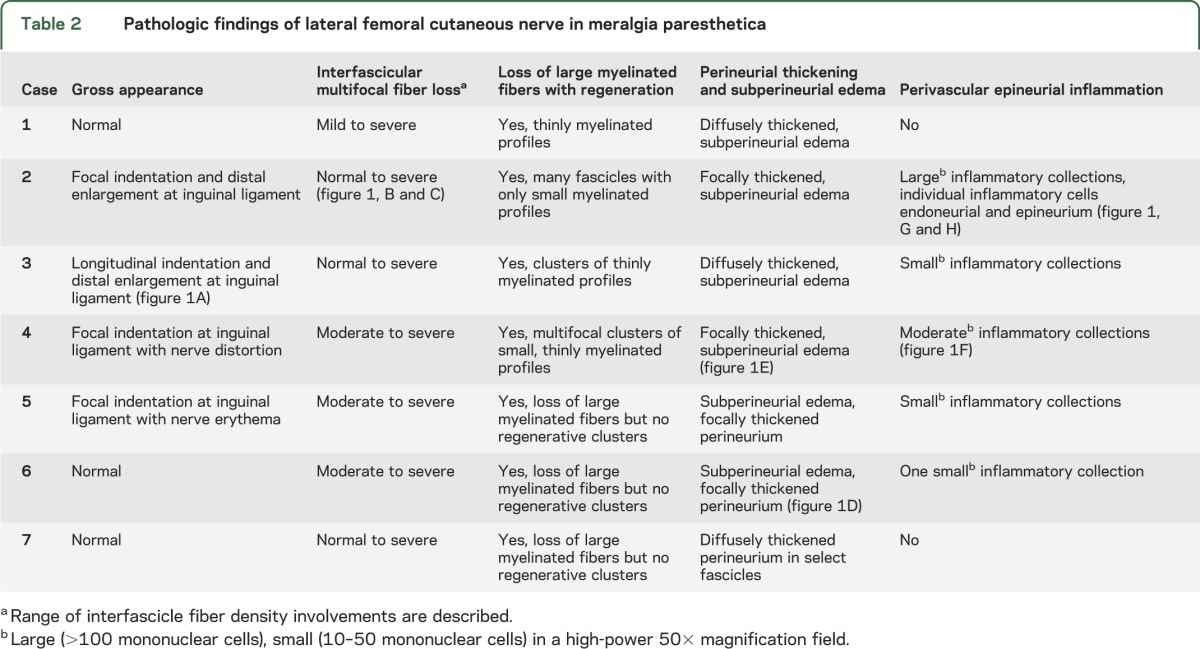
Figure 1. Characteristic findings in lateral femoral cutaneous nerve biopsies from patients with chronic meralgia paresthetica who underwent neurectomy.
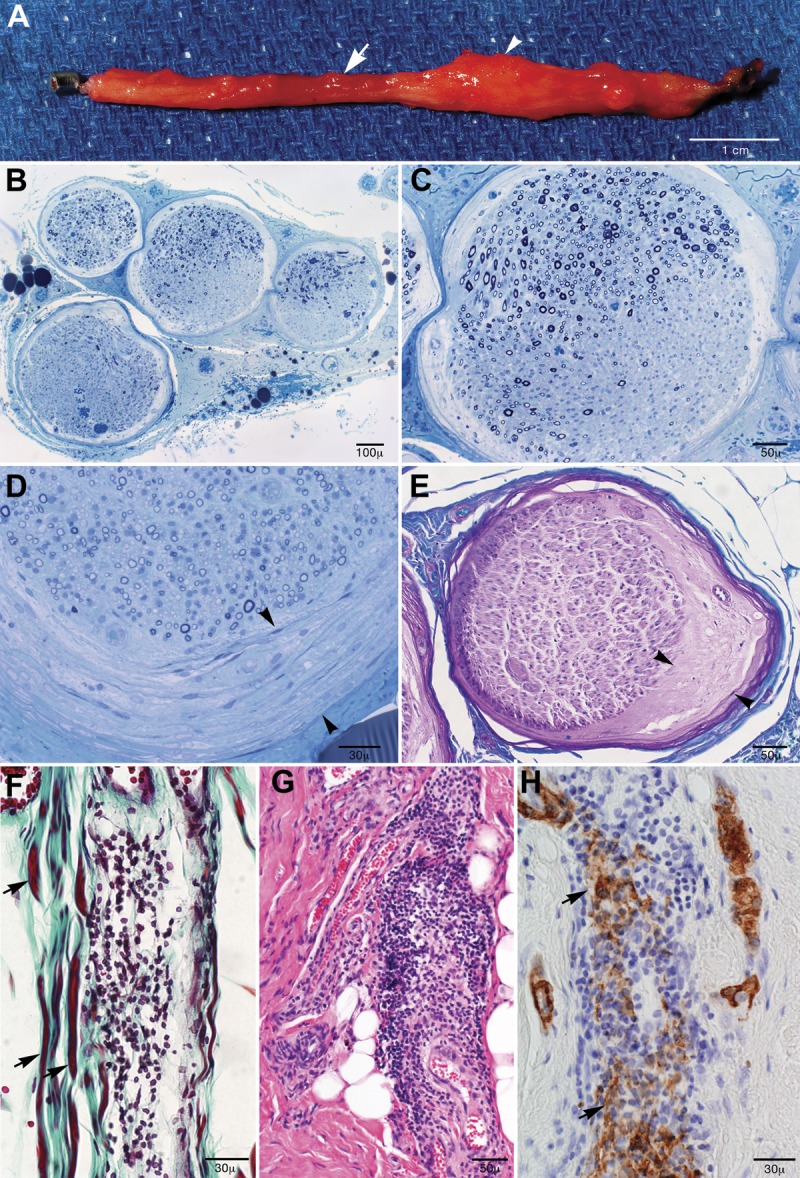
(A) Nerve indentation was frequently seen (4 of 7 biopsies, arrow) at the site of inguinal ligament compression with associated swellings distal (arrowhead) to the compression (2 of 7 cases). Epoxy-embedded semithin sections stained with methylene blue showed (B) interfascicular and (C) intrafascicular multifocal fiber loss without selective subperineurial fiber loss and (D) perineurial thickening. (E) On Luxol fast blue/periodic acid–Schiff stain, subperineurial edema was seen. (F) Trichrome paraffin preparation of case 4 where a moderate collection of inflammatory mononuclear cells was seen within the epineurium juxtaposed to myelinated fibers (arrows). (G) Hematoxylin & eosin stain shows marked epineurial inflammation in case 2 that was 3 months post unsuccessful neurolysis of the same nerve, and (H) smooth muscle actin (SMACTIN) preparation shows evidence for vessel wall fragmentation (arrows).
Teased fibers preparations revealed severe loss of large fibers with primarily empty strands in 3 cases; 4 cases had grouped empty strands typical of multifocal fiber loss. Increased rates of demyelination were noted in 2 of 7 cases, as can be seen in models of chronic compression (cases 1 and 2).6,7 Axonal degeneration was noted in 3 cases (2, 4, and 5); each of these cases had edema and inflammatory collections.
A loss of large myelinated fibers was seen in all cases. Morphometry supported this observation, where loss of large myelinated fibers was accompanied by a shift to smaller fibers, consistent with a chronic regenerative process (figure e-1 on the Neurology® Web site at Neurology.org). Five patients had varying but abnormal degrees of intraneural and epineurial inflammation; 1 patient had a large collection of inflammatory cells (greater than 100 cells) but had undergone neurolysis of the same nerve 3 months earlier (figure 1). Four biopsies had small (10–50 cells) and one biopsy had moderate (50–100 cells) perivascular collections. Perineurial thickening, loss of large myelinated fibers, and inflammation were not seen in the control LFC autopsied nerve.
DISCUSSION
Patients with chronic MP and intractable pain have an LFCN mononeuropathy with reduced myelinated nerve fiber density. Our pathologic and operative experience supports nerve compression with indentation at the inguinal ligament as the primary active pathogenesis. The sustained benefits seen by neurectomy would also support that mechanism. Inflammation and multifocal fiber loss associates with these findings and may suggest an additional pathogenic component.
The patients presented here have the predominant pathologic features described in animal models and autopsy series of chronic nerve compression.7–9 In animal models, experimental compression caused selective damage to large myelinated fibers with preservation of the small myelinated and unmyelinated fibers, similar to our patients. Also reported is demyelination and axonal degeneration under the areas of compression with axonal degeneration in the segment distal to compression as seen in several of our patients.6
Focal demyelination, thickened perineurium, endoneurial edema, Renaut bodies, loss of large myelinated fibers with numerous thinly myelinated fibers, and regenerating clusters have been described in autopsied specimens of human ulnar nerves at sites of compression.8 Similar to these cases of compression, our MP cases had perineurial thickening, loss of large myelinated fibers, Renaut bodies, subperineurial edema, and regenerating clusters.
We and others hypothesized that in some patients with MP inflammation may play a prominent pathologic role in MP.1,3 The multifocal fiber loss and inflammation seen in these cases is of note and might suggest a component of inflammation beyond a compressive etiology. Specifically, multifocal fiber loss has most commonly been reviewed to occur in inflammatory immune processes like vasculitis.10 Among our presented patients. limited or moderate inflammation was seen in 4 of 7 cases; 1 patient had a large inflammatory collection, but in that case, the inflammation may simply relate to prior neurolysis of the same nerve (3 months prior). Although earlier experimental work in compressive neuropathies suggested that focal compression may lead to ischemia caused by endoneurial edema, that fiber loss occurred in the subperineurial areas via a theorized “miniature compartment syndrome” mechanism.11 In our cases, selective subperineurial fiber loss was not seen. We therefore theorize either chronic sustained compression or a prior significant inflammatory insult not captured at the time of biopsy as the mechanism for the pattern of nerve fiber loss. In support of an inflammatory component is the initial but unsustained response to local steroid injection in our patients. But against a significant ongoing inflammatory process are absence of microfasciculation, neovascularization, and hemosiderin-laden macrophages typical of other nonsystemic nerve vasculitis as can specifically affect the femoral nerve in lumbosacral plexitis.12
Because our patients did not experience sustained benefit from steroid injection but had prolonged relief with neurectomy, a primary compressive mechanism is most strongly supported at the time of surgery. Our study has selection bias against cases where inflammatory mechanisms might predominate because such persons would be predicted to have a monophasic and self-limited course and would not come to biopsy. The majority of patients with MP do have a self-limited course.1
The pathologic findings in patients with chronic MP combined with the clinical responsiveness to neurectomy at the sites of LFCN compression supports chronic compression as the primary cause of their pain and axonal loss. Although inflammation may play a role in some cases, it does not appear to be the primary pathogenesis in unremitting chronic cases. From this anecdotal case series, it is suggested that patients having chronic intractable pain may benefit from removal of the LFCN at the site of compression.
Supplementary Material
GLOSSARY
- BMI
body mass index
- LFCN
lateral femoral cutaneous nerve
- MP
meralgia paresthetica
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Berini: design, acquisition and analysis of data, drafting and editing the manuscript. Dr. Spinner: concept, acquisition of the data, analysis, editing the manuscript. Dr. Jentoft: acquisition of the data, analysis, editing the manuscript. J.K. Englestad: creation of the figures, editing the figures, critical edits. Dr. Staff: acquisition of data, analysis and review of the manuscript. Dr. Suanprasert: biopsy interpretation, acquisition of data, review of manuscript. Dr. Dyck: acquisition of data, critical editing of the manuscript and figures. Dr. Klein: concept and design of the study, acquisition of the data, drafting and critically editing the manuscript.
STUDY FUNDING
Supported by the National Institute of Neurologic Disorders and Stroke K08 (NS065007).
DISCLOSURE
S. Berini, R. Spinner, M. Jentoft, J. Engelstad, N. Staff, N. Suanprasert, and P. Dyck report no disclosures relevant to the manuscript. C. Klein is coeditor of Journal of the Peripheral Nervous System. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Parisi TJ, Mandrekar J, Dyck PJ, Klein CJ. Meralgia paresthetica: relation to obesity, advanced age, and diabetes mellitus. Neurology 2011;77:1538–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Slobbe AM, Bohnen AM, Bernsen RM, Koes BW, Bierma-Zeinstra SM. Incidence rates and determinants in meralgia paresthetica in general practice. J Neurol 2004;251:294–297 [DOI] [PubMed] [Google Scholar]
- 3.Wartenberg R. Meralgia paresthetica. Neurology 1956;6:560–562 [DOI] [PubMed] [Google Scholar]
- 4.Dyck PJ, Dyck PJ. Pathologic alterations of nerves. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy, 4th ed 2005:737–780 [Google Scholar]
- 5.Shin YB, Park JH, Kwon DR, Park BK. Variability in conduction of the lateral femoral cutaneous nerve. Muscle Nerve 2006;33:645–649 [DOI] [PubMed] [Google Scholar]
- 6.Dyck PJ, Lais AC, Giannini C, Engelstad JK. Structural alterations of nerve during cuff compression. Proc Natl Acad Sci USA 1990;87:9828–9832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochoa J, Marotte L. The nature of the nerve lesion caused by chronic entrapment in the guinea-pig. J Neurol Sci 1973;19:491–495 [DOI] [PubMed] [Google Scholar]
- 8.Neary D, Eames RA. The pathology of ulnar compression in man. Neuropathol Appl Neurobiol 1975;1:69 [Google Scholar]
- 9.Ochoa J, Danta G, Fowler TJ, Gilliatt RW. Nature of the nerve lesion caused by a pneumatic tourniquet. Nature 1971;233:265–266 [DOI] [PubMed] [Google Scholar]
- 10.Gwathmey KG, Burns TM, Collins MP, Dyck PJ. Vasculitic neuropathies. Lancet Neurol 2014;13:67–82 [DOI] [PubMed] [Google Scholar]
- 11.Lundborg G, Myers R, Powell H. Nerve compression injury and increased endoneurial fluid pressure: a “miniature compartment syndrome.” J Neurol Neurosurg Psychiatry 1983;46:1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve 2002;25:477–491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


