Abstract
Background: Alcoholic beverages are consumed by humans for a variety of dietary, recreational, and other reasons. It is uncertain whether the drinking effect on risk of all-cause mortality is different between women and men. We conducted a meta-analysis to evaluate the effect of drinking on the risk of all-cause mortality in women compared with men.
Methods: We selected cohort studies with measures of relative risk (RR) and 95% confidence interval (CI) for all-cause mortality for drinkers versus nondrinkers by sex. Sex-specific RR and 95% CI were used to estimate the female-to-male ratio of RR (RRR) and 95% CI. Pooled estimates of RRR across studies were obtained by the fixed-effects model or the random-effects model (if heterogeneity was detected). Second-order fractional polynomials and random effects meta-regression models were used for modeling the dose-risk relationship.
Results: Twenty-four studies were considered eligible. A total of 2,424,964 participants (male: 1,473,899; female: 951,065) were enrolled and 123,878 deaths (male: 76,362; female: 47,516) were observed. Compared with nondrinkers, the pooled female-to-male RRR for drinkers was 1.07 (95% CI: 1.02, 1.12). Subgroup analyses showed that the increased risk among female drinkers appeared to be consistent. J-shaped dose–response relationship was confirmed between alcohol and all-cause mortality in men and women, respectively. Moreover, the female-to-male RRR of all-cause mortality were 1.52 (95% CI: 1.01, 2.29), 1.95 (95% CI: 1.08, 3.49), and 2.36 (95% CI: 1.15, 4.88), respectively, for those who consumed 75, 90, and 100 g/day of alcohol.
Conclusions: Females had an increased risk for all-cause mortality conferred by drinking compared with males, especially in heavy drinkers. The present study suggested that female drinkers, particularly heavy drinkers, should moderate or completely reduce their level of consumption to have a health benefit.
Introduction
Alcoholic beverages are consumed by humans for a variety of dietary, recreational, and other reasons. World Health Organization's Global status report on alcohol and health (2011) revealed that 65% of men and 45% of women drank alcohol worldwide. It was estimated that about 4% of all deaths were attributed to alcohol in the world.1 Furthermore, the global burden of disease and injury attributable to alcohol was 7.4% for men and 1.4% for women.1 However, women may face greater risks to their health than their male counterparts.2,3 For instance, Nakamura et al. suggested that women were more susceptible than men to alcohol-related diseases such as alcoholic liver disease.4 Female heavy drinkers were also more susceptible to brain volume shrinkage compared to male drinkers.5 Moreover, an earlier meta-analysis of 34 prospective studies by Di Castelnuovo, including a total of 1,015,835 subjects and 94,533 deaths, investigated the relationship between alcohol dosing and all-cause mortality. The authors found that doses of alcohol intake of >4 drinks per day in men and >2 drinks per day in women were associated with an increase in all-cause mortality.6
Nonetheless, it is still uncertain whether the drinking effect on risk of all-cause mortality is different between women and men. Several inconsistent results might be due to various factors such as racial difference, classification of alcohol consumption and adjustment factors.7–9 In 2006, Di Castelnuovo conducted an excellent and insightful meta-analysis that showed a J-shaped relationship between all-cause mortality and alcohol intake in both men and women and reported the inverse association in women apparently disappears at doses lower than in men.6 However, this study did not directly report an effect of sex differences for drinking. In order to reduce the role of extraneous, between-study factors, we could conduct direct comparisons of the relation between drinking and all-cause mortality in men and women through internal, within-study comparisons, using the female-to-male ratio of relative risks (RRR).10 Therefore, a more thorough meta-analysis is needed. To explore whether alcohol drinking conferred different risk of all-cause mortality for women compared with men, we performed an updated meta-analysis of cohort studies that present the relationship between alcohol consumption and all-cause mortality after stratification by gender.
Materials and Methods
Search strategy and selection criteria
We carried out a systematic review of the published work without language restrictions (up to March 20, 2012) according to the Meta-Analysis of Observational Studies in Epidemiology guidelines. We selected relevant studies from Embase, PubMed, and Cochrane Library databases with the following combined terms and medical subject heading (MeSH) search strategy: “alcohol” or “ethanol” or “drinking” or “drink” or “beverages”; “mortality” or “all cause mortality” or “death” or “all cause death”; “cohort” or “prospective” or “follow-up.” We also manually screened the reference lists of the retrieved studies to identify other relevant publications. We included studies that provided relative risks (RR) of all-cause mortality for drinkers versus nondrinkers in males and females (age adjusted or multiple adjusted). Studies were excluded if they did not provide the standard error or 95% confidence interval (CI) of point estimate, if they did not report relative risk adjusted at least for age, or if they did not use nondrinkers as the reference category.
Data extraction
Three investigators (C. Wang, H. Xue, and Q. Wang) completed the search and data extraction independently. Any discrepancies in data abstraction were further examined and resolved by consensus. The following information data elements were extracted from each study: title, name of first author, year of publication, study design, sample size, duration of follow-up, and characteristics of the participants (such as country, average age or age range, prevalence of drinking, etc.).
Statistical analysis
To derive an overall RR within the study, we combined RR between all-cause mortality and drinking across levels of alcohol intake. Then sex-specific RR and 95% CI within each study were used to estimate the female-to-male ratio of RR and 95% CI. Pooled estimates of RRR across studies were obtained by means of the fixed-effect model. Nonetheless, the RRR was pooled using the random-effect model if heterogeneity was detected.10 We estimated percentage of variability between studies attributable to between-study heterogeneity with the Cochrane Q statistic and I2 statistic. The existence of heterogeneity between studies was evaluated using Cochran's Q statistic.11 Moreover, we quantified the effect of heterogeneity by using I2, which describes the percentage of the observed between-study variability attributable to heterogeneity rather than chance. I2 takes values between 0% and 100% and there should be important heterogeneity when I2 >50%.12 To detect whether the results were robust, sensitivity analyses were conducted to estimate RRR only among those studies using lifetime abstainers as reference group or among those studies excluding ex-drinkers from nondrinkers.
In order to test the robustness of the combined estimates, we did subgroup analyses by region (Asian vs. not Asian), average daily alcohol consumption among alcohol consumers regardless of gender (<13.0 g vs ≥13.0 g), year of publication (1992–2000 vs. after 2000), sample size (<20,000 participants vs ≥20,000 participants), adjustment for cardiovascular disease (CVD) (yes vs. no), and duration of follow-up (using 5 and 15 years as cut-points). Meta-regression models were employed to investigate potential sources of between-study heterogeneity.12,13
For the dose–response meta-analysis, we used a random-effects meta-regression model in a nonlinear dose–response relationship framework, providing the best-fitting second-order fractional-polynomial model. The best-fitting model, defined as the one with the lowest Akaike's information criterion (AIC) among the set of the 36 second-order fractional polynomial models tested, was selected as the final dose–response model.6,14 Because different studies used different units to measure alcohol intake (grams, milliliters, ounces, or drinks consumed every day, week, month, or year), alcohol consumption was converted into grams of ethanol per day using the following conversion factors: 0.8 g/mL, 28.0 g/ounce, and 12.5 g/drink.14,15 Occasional drinkers and ex-drinkers were not considered in the analysis.14,15 Since the levels of alcohol intake were often given by a range, we assigned the midpoint of the ranges in each category as the average consumption. If the highest category was open-ended, we assumed the width of the interval to be the same as in the preceding category. Nondrinkers were used as the reference category. This method required the number of cases and controls for case-control studies, or events and subjects at risk for cohort studies and the risk estimates with their corresponding variance estimates for at least three quantitative exposure categories.14 Therefore, we excluded studies that did not provide these data. Publication bias was evaluated using inverted funnel plot and Egger's test. All statistical tests were two-sided, using a level of significance of p values<0.05. Statistical analyses were carried out with Stata software (version 10.0; Stata Corporation) and SAS (version 9.2; SAS Institute Inc.).
Results
Search results and study characteristics
Initially, a total of 5,124 studies were yielded through the search strategy from electronic databases, of which 151 studies were potentially related to our issue for further scrutiny. Of the related studies, 129 were excluded for the following reasons: the data of 18 studies partially overlapped with others; 29 studies did not provide sex-stratified estimates of RR; alcohol was considered as a covariate in 29 studies which did not provide the RR of alcohol on all-cause mortality; 23 studies did not provide the RR adjusted for covariates or standard error of point estimates; 17 studies did not use “nondrinkers” as the control group; 13 studies did not provide information about the outcome variables. Therefore, 22 studies were finally eligible for inclusion. One of these studies included six large-scale population-based prospective cohorts.7 In addition, we used data from two unpublished studies: the data of mainland China (including 16 cohorts) from the Asia Pacific Cohort Studies Collaboration and a cohort of China National Hypertension Survey Epidemiology Follow-Up Study.16,17 Overall, 24 studies including 44 cohorts were included in our analysis (Fig. 1)7–9,16–36 Among these cohorts, 26 were conducted in Asia, 7 in Europe, 7 in America, and 3 in Australia, while Deev et al.'s8 cohort included both American and Russian populations.
FIG. 1.
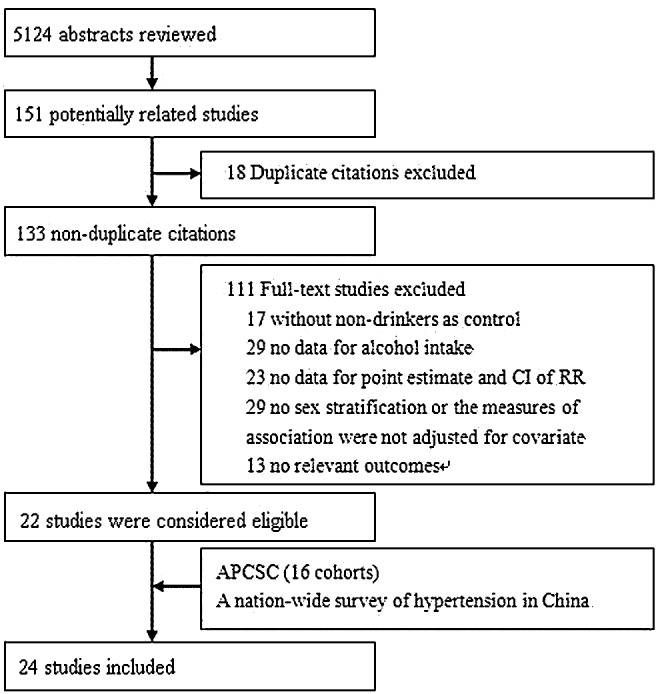
Study selection process.
In total, 2,424,964 participants (male: 1,473,899; female: 951,065) were available for the primary analysis, in which 123,878 deaths occurred (male: 76,362; female: 47,516). The mean follow-up period ranged from 4 to 23 years. All participants were older than 18 years. Twenty-three studies reported the sex-specific prevalence of drinking, which was 30.1∼95.3% in men and 2.8∼82.0% in women. The prevalence of drinking was higher in men than in women within each study. Asians tended to have a lower prevalence of drinking than Europeans and Americans. The detailed characteristics of the 24 studies included were shown in Table 1.
Table 1.
Main Characteristics of Included Studies
| Author | County | Baseline study dates | Men | Women | Mean age (SD) or age range, years | Outcome | Mean duration, years | Current drinker (Men) | Current drinker (Women) | Number of outcome event (%, women) | Maximum adjustment available |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Klatsky et al., 199225 | United States (U.S.) | 1978–1985 | 56,926 | 72,008 | 40.6 | Mortality from all-cause | 8.0 | 88.6% | 81.7% | 1730 (40.0) | Age, race, BMI, education, marital status, smoking, coffee and tea consumption |
| Cullen et al ., 199320 | Western Australia | 1966 | 1,085 | 1,086 | ≥40 | Mortality from all-cause, CVD | 23.0 | 78.5% | 53.4% | 436 (42.7) | Age, sex, occupation, smoking, BP, probable or suspected CHD, forced expiratory volume, diabetes, cholesterol, uric acid, and treatment for hypertension. |
| Berberian et al., 199419 | Netherlands | 1975–1978 | 760 | 860 | >20 | Mortality from all-cause, CVD, cancer | 10.0 | 93.2% | 81.6% | 55 (44.7) | Age, BMI, serum cholesterol, SBP, DBP, pulse rate, cigarette smoking, and history of anti-hypertension drug use |
| Serdula et al., 199533 | U.S. | 1971–1975 | 3,573 | 4,614 | 59 | Mortality from all-cause, IHD, Non-IHD | 14.2 | 75.0% | 59.0% | 1059 (43.4) | Age, race, educations, smoking status, BMI, and physical activity at baseline |
| Simons et al., 199634 | Australia | 1988–1989 | 1,236 | 1,569 | >60 | Mortality from all-cause | 6.4 | 78.0% | 52.0% | 236 (43.6) | Age, marital status, smoking status, alcohol intake, BP, diabetes status, peak expiratory flow, prior CHD, atrial fibrillation, disability, and self-rated health |
| Deev et al., 19988 | U.S. and Russia | 1972–1976;1975–1977 | 3,808 | 4,356 | US: 40–69 Russia:40–59 |
Mortality from all-cause, CVD | 13.0 | 89.0%, 95.3 % | 79.0%, 82.0% | 686 (38.8) | Age, education, heart rate, total cholesterol, and BMI |
| Maskarinecet al., 199828 | U.S. | 1975–1980 | 13,870 | 13,808 | >30 | Mortality from all-cause, CHD | 20.0 | 37.0% | 13.0% | 2016 (40.2) | Age, ethnicity, years of school, BMI, and smoking status |
| Hoffmeister et al., 199923 | Germany | 1985, 1988, 1991 | 7,677 | 7,732 | 25–69 | Mortality from all-cause, CVD | 7.0 | 81.9% | 55.5% | 66 (41.5) | Age, smoking, and social status |
| Liao et al., 200026 | U.S. | 1988, 1990 | 17,821 | 25,874 | ≥40 | Mortality from all-cause,CHD | 6.0 | 69.0% | 57.5% | 2957 (53.4) | Age, race, smoking status, and history of hypertension, diabetes, and heart disease, marital status, number of years of education, and self-perceived health status |
| Trevisan et al., 200135 | Italy | 1978 | 8,980 | 6,669 | 30–59 | Mortality from all-cause, cancer | 7.0 | 88.9% | 66.3% | 165 (23.7) | Age and current smoking |
| Rehm et al., 200130 | U.S. | 1984 | 2,037 | 3,035 | ≥18 | Mortality from all-cause | 11.3 | 72.9% | 53.9% | 260 (48.9) | Age and ethnicity, income, marital status, smoking status |
| Diem et al., 200321 | Swiss | 1974–1977 | 162 | 125 | 46.2 | Mortality from all-cause, CHD | 12.6 | 66.7% | 20.8% | 24 (34.3) | Age, duration of diabetes, BMI, cholesterol, SBP, and nicotine consumption |
| Sempos et al., 200332 | U.S. | 1971–1975 | 768 | 1,286 | 25–75 | Mortality from all-cause | 19.0 | 72.4% | 60.0% | 437 (50.9) | Age, BMI, cigarette smoking, and physically very active |
| Wellmannet al., 200436 | Southern Germany | 1987–1988 | 1,345 | 1,365 | 38–67 | Mortality from all-cause | 10.0 | 84.0% | 56.0% | 84 (34.6) | Age, smoking, physical activity, partner status, education, BMI, total cholesterol, and hypertension |
| Makela et al., 200527 | Finland | 1969, 1976, 1984 | 3481 | 2,913 | 25–69 | Mortality from all-cause | 15.2 | 88.1% | 70.4% | 398 (34.8) | Age, period, marital status, education, and smoking status |
| Baglietto et al., 200618 | Australia | 1990–1994 | 14,557 | 22,427 | 40–69 | Mortality from all-cause | 10.5 | 83.0% | 59.0% | 654 (42.8) | Country of birth, smoking, fruit and vegetable intake, total energy intake, saturated fat, physical activity, education, and BMI |
| Paganini-Hill et al., 200729 | U.S. | 1981 | 4,980 | 8,644 | 74 | Mortality from all-cause | 23.0 | 78.0% | 72.0% | 6930 (60.9) | Age, smoking, exercise, BMI, caffeine consumption, and histories of hypertension, angina, heart attack, stroke, diabetes, rheumatoid arthritis, and cancer |
| Friesema et al., 200822 | Netherland | 1996 | 1,562 | 1,573 | 45–70 | Mortality from all-cause, CVD | 5.0 | NA | NA | 204 (38.2) | Age, region, smoking, BMI, fat intake, physical activity, education, income, and medical histories of CVD, hypertension, diabetes, and hypercholesterolemia |
| Sun et al., 20099 | Hong Kong | 1998–2000 | 18,750 | 37,417 | >65 | Mortality from all-cause | 4.1 | 30.1% | 8.9% | 1988 (52.1) | Age, education, housing type, BMI, and smoking status. |
| Sadakane et al., 200931 | Japan | 1992–1995 | 3,444 | 5,490 | 56.4 | Mortality from all-cause | 12.0 | 76.1% | 24.1% | 240 (37.7) | Age, tobacco smoking, education level, marital status, BMI, and physical activity index |
| Inoue et al., 20127,* | Japan | 1988–1994 | 144,012 | 165,070 | 35–79 | Mortality from all-cause, cancer, heart disease,cerebrovascular disease | 12.4 | 77.0% | 27.0% | 13541 (37.8) | Age and area, smoking, BMI, history of hypertension, history of diabetes, and leisure-time sports or physical exercise |
| Kim et al., 201024 | Korea | 2000 | 919,199 | 422,194 | Male:49.0 Female:48.3 |
Mortality from all-cause, cancer | 5.0 | 69.6% | 20.3% | 3267 (16.9) | Age, residential, smoking status, regular exercise, BMI, SBP, DBP, and fasting blood sugar |
| CHEFS** (Unpublished data) | China | 1991 | 66,078 | 71,048 | >40 | Mortality from all-cause, cancer | 8.3 | 38.5% | 2.8% | 7678 (43.9) | Age, BMI, SBP, cigarette smoking, high school education, physical inactivity, urban or rural residence, northern or southern China, and diabetes. |
| China data from APCSC*** (Unpublished data) | China | NA | 177,788 | 69,902 | >18 | Mortality from all-cause, cancer | 7.5 | 32.6% | 4.2% | 2405 (27.2) | Age, smoking, SBP, education |
The pooled analyses included the JPHC-I, JPHC-II, JACC, MIYAGI, OHSAKI, and TAKAYAMA studies.
The design and rationale for the cohort of China National Hypertension Survey Epidemiology Follow-Up Study (CHEFS) referred to reference 17.
The design and rationale for the cohort of mainland China from the Asia Pacific Cohort Studies Collaboration (APCSC) referred to reference 16.
APCSC, Asia Pacific Cohort Studies Collaboration; BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; IHD, ischemic heart disease; NA, not applicable; SBP, systolic blood pressure; JPHC-I, Japan Public Health Center-based Prospective Study, Cohort I; JPHC-II, Japan Public Health Center-based Prospective Study, Cohort II; JACC, Japan Collaborative Cohort Study; MIYAGI, Miyagi Cohort Study; OHSAKI, Ohsaki National Health Insurance Cohort Study; TAKAYAMA, Takayama Study.
Age-adjusted RRR
Nine studies with 547,764 individuals and 44,432 events reported age-adjusted and gender-stratified RR. We calculated RRR (female-to-male) and 95% CI based on a fixed-effect model. The RRR was 1.18 (95% CI: 1.09, 1.28), which suggested that alcohol affected women more harmfully than men. There was no significant heterogeneity between studies (I2=3.3%, p=0.41). Visual inspection of the funnel plot and Egger's test suggests that there was no publication bias (p=0.09).
Multiple-adjusted RRR
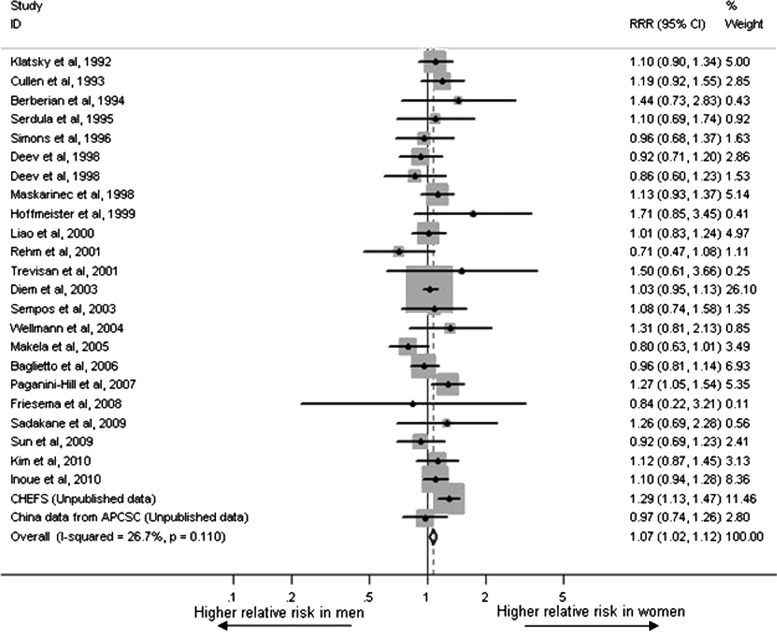
Twenty-four studies with 123,878 events among 2,424,964 individuals reported multiple-adjusted and gender-stratified RR. Data from the study conducted by Deev et al. involved Americans and Russians, so it was included in our analysis as two studies. The risk of alcohol on all-cause mortality in women and men was 0.92 (95% CI: 0.86, 0.99) and 0.88 (95% CI: 0.83, 0.93), respectively. RRR (female-to-male) was 1.07 (95% CI: 1.02, 1.12) with no evidence of between-study heterogeneity (I2=26.7%, p=0.11), which was combined using a fixed-effect model (Fig. 2). Visual inspection of the funnel plot and Egger's test suggested that there was no publication bias (p=0.93). In the sensitivity analysis with data for 548,035 participants and a total of 38,722 deaths, we calculated RRR and 95% CI comparing drinkers with lifetime abstainers (RRR=1.06, 95% CI: 0.98, 1.14). There was no significant heterogeneity between studies (I2=13.9%, p=0.31).
FIG. 2.
Multiple-adjusted female-to-male relative risk ratios for all-cause mortality for drinkers compared with non-drinkers.
Subgroup analyses
We performed subgroup analyses across a number of key study characteristics (Table 2). Stratified by sample size, the pooled RRR (female to male) of drinkers compared with nondrinkers was 1.04 (95% CI: 0.97, 1.11) in smaller studies (<20, 000), and 1.10 (95% CI: 1.03, 1.17) in larger studies (≥20, 000). Compared with nondrinkers, the pooled RRR of drinkers was 1.06 (95% CI: 0.97, 1.16) in studies that published between 1992 and 2000, and 1.07 (95% CI: 1.02, 1.13) in studies that published after 2000. Stratified by region, the pooled female-to-male RRR of drinkers compared with non-drinkers was 1.15 (95% CI: 1.06, 1.25) for studies conducted in Asia, and 1.04 (95% CI: 0.99, 1.10) for studies in other regions. Compared with non-drinkers, the pooled RRR of drinkers was 1.13 (95% CI: 1.01, 1.27) in studies adjusting for CVD diseases, and 1.06 (95% CI: 1.01, 1.11) in studies that did not adjust for CVD disease. Moreover, 13 studies with data for dose–response analysis were stratified by average daily alcohol consumption. We found that the pooled RRR of drinkers compared with nondrinkers was 1.12 (95% CI: 1.02, 1.22) in the studies with higher doses (≥13.0 g/day), and 1.05 (95% CI: 0.95, 1.16) in the studies with lower doses (<13.0 g/day). When using 5 and 15 years as cut-points for follow-up duration, we found that drinkers tended to have the strongest pooled RRR in the studies with the longest follow-up (15 years or more). The increased risk of all-cause mortality among female drinkers appeared to be consistent in subgroup analyses stratified by sample size, region, year of publication, adjustments for CVD disease, average daily alcohol consumption, and duration of follow-up. In meta-regression analysis, we explored the influence of the six key characteristics mentioned above in the heterogeneity (Table 2). There were no significant impacts on the main results.
Table 2.
Subgroup Analyses of Multiple-Adjusted Female-to-Male Relative Risk Ratios for Drinkers Compared with Non-Drinkers
| Heterogeneity test | Meta-regression | ||||
|---|---|---|---|---|---|
| Subgroup analyses | No. of studies | RRR (95% CI) | p value | I2 (%)a | (p value) |
| Sample size | |||||
| <20,000 | 14 | 1.04 (0.97, 1.11) | 0.12 | 30.8 | 0.48 |
| ≥20,000 | 10 | 1.10 (1.03, 1.17) | 0.25 | 20.8 | |
| Region | |||||
| Asian | 6 | 1.15 (1.06, 1.25) | 0.20 | 31.0 | 0.24 |
| Not Asian | 18 | 1.04 (0.99, 1.10) | 0.23 | 18.5 | |
| Year of publication | |||||
| 1992–2000 | 9 | 1.06 (0.97, 1.16) | 0.66 | 0.0 | 0.96 |
| After 2000 | 15 | 1.07 (1.02, 1.13) | 0.20 | 46.9 | |
| Adjustment for cardiovascular disease | |||||
| Yes | 6 | 1.13 (1.01, 1.27) | 0.54 | 0.0 | 0.37 |
| No | 18 | 1.06 (1.01, 1.11) | 0.07 | 34.6 | |
| Average daily alcohol consumption (regardless of gender)b | |||||
| <13.0g | 7 | 1.05 (0.95, 1.16) | 0.62 | 0.0 | 0.34 |
| ≥13.0g | 6 | 1.12 (1.02, 1.22) | 0.23 | 27.2 | |
| Duration of follow-up | |||||
| ≤5.0 years | 3 | 1.03 (0.85, 1.24) | 0.56 | 0.0 | 0.59 |
| 5.1–14.9 years | 16 | 1.06 (1.01, 1.12) | 0.16 | 25.8 | |
| ≥15.0 years | 5 | 1.10 (0.99, 1.22) | 0.05 | 58.1 | |
Excluded studies which did not provide the required data in dose-response analysis.
I2 is interpreted as the proportion of total variation across studies that are due to heterogeneity rather than chance.
CI, confidence interval; RRR, relative risk ratios.
Dose–response meta-analysis
The dose–response meta-analysis included 13 studies with daily alcohol intake.7,18,20,23,25,26,28–30,32–34,36 Figure 3 showed the dose–response relationship, giving the RR function and the corresponding 95% CI for the best-fitting relationship between alcohol consumption and risk of all-cause mortality in men and women, respectively. The relationship was modeled by the random-effects second-order fractional polynomials with p1=1 and p2=2, which indicated that the AIC value was the lowest among the set of the 36 second-order fractional polynomial models tested [i.e., ln (RR)=dose+dose2] in both men and women.14,15
FIG. 3.
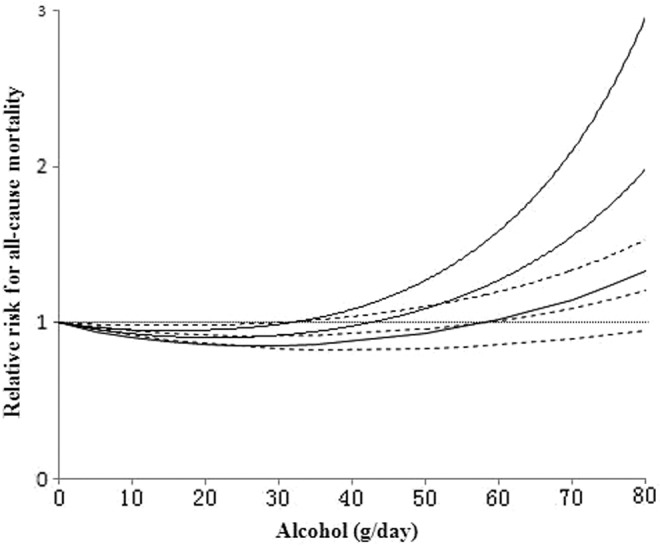
Relative risk function and the corresponding 95% confidence interval, describing the best-fitting dose–response relationship between alcohol dose (g/day) and relative risk of all-cause mortality in men (dashed lines) and women (solid line), respectively.
Compared with nondrinkers, the RR of all-cause mortality were 0.95 (95% CI: 0.92, 0.98), 0.92 (95% CI: 0.85, 0.99), 0.96 (95% CI: 0.83, 1.10), 1.15 (95% CI: 0.92, 1.43), 1.36 (95% CI: 1.02, 1.80), and 1.56 (95% CI: 1.12, 2.19), respectively, for men who consumed 10, 25, 50, 75, 90, and 100 g/day of alcohol. Compared with nondrinkers, the RR of all-cause mortality were 0.93 (95% CI: 0.90, 0.96), 0.91 (95% CI: 0.85, 0.96), 1.09 (95% CI: 0.93, 1.27), 1.74 (95% CI: 1.23, 2.47), 2.65 (95% CI: 1.59, 4.42), and 3.70 (95% CI: 1.95, 7.04), for women who consumed 10, 25, 50, 75, 90, and 100 g/day of alcohol, respectively. Notably, the female-to-male RRR of all-cause mortality were 0.98 (95% CI: 0.94, 1.02), 0.99 (95% CI: 0.90, 1.09), 1.14 (95% CI: 0.92, 1.40), 1.52 (95% CI: 1.01, 2.29), 1.95 (95% CI: 1.08, 3.49), and 2.36 (95% CI: 1.15, 4.88), respectively, for those who consumed 10, 25, 50, 75, 90, and 100g/day of alcohol (Fig. 4).
FIG. 4.
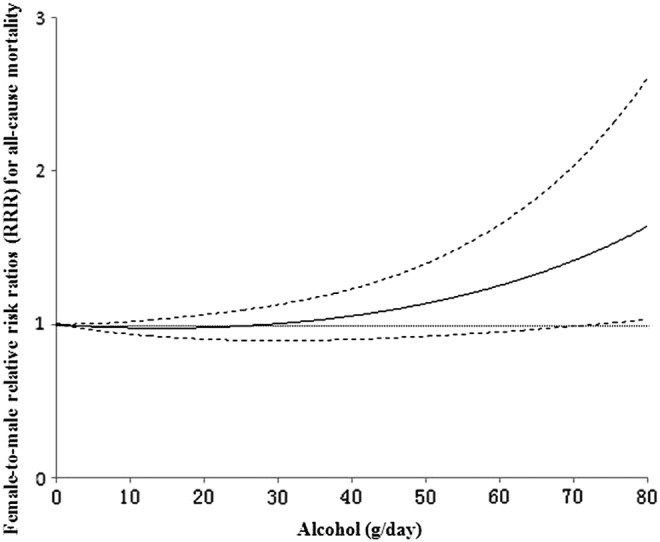
Relative risk ratios function (solid line) and the corresponding 95% confidence interval (dashed lines), describing the dose–response relationship between alcohol dose (g/day) and female-to-male relative risk ratios for all-cause mortality.
Discussion
Nowadays the harmful use of alcohol drinking is a major global contributing factor for death, leading to approximately 2.5 million deaths each year. Globally, 6.2% of all male deaths were attributable to alcohol, compared with 1.1% of female deaths.1 Compared with women, men were more likely to drink and consume more alcohol.1 Some studies have used J-shaped or U-shaped curves to describe the relationship between alcohol consumption and all-cause mortality. However, the impact of alcohol consumption across the globe might differ between women and men, or in other words, the effect of drinking on risk of all-cause mortality between men and women is still uncertain. Quantifying sex difference in the relative effect of drinking on all-cause mortality risk is of great importance both clinically and from a public health perspective.
The current study focused on investigating the effects of alcohol intake on the risk of all-cause mortality in women compared with men. We found women had an increased risk of alcohol drinking with all-cause mortality compared with men, especially among heavy drinkers. Female drinkers, particularly heavy drinkers, should reduce their level of alcohol intake to obtain a health benefit. In our study, compared with non-drinkers, female drinkers showed a 7% excess risk of all-cause mortality than male drinkers after adjustment for multiple covariates. The results of sensitivity analyses did not substantially differ from the main result. The increased all-cause mortality risk in female drinkers was consistently found in all of the subgroup analyses. In addition, we assessed the dose–response relationship of alcohol and all-cause mortality in men and women respectively. Dose response curves were similar for men and women when alcohol intake was light, but differed in heavier drinkers. We found the inverse association disappeared at a lower dose in women than in men, which was similar to the finding by Di Castelnuovo et al.6 Furthermore, the female-to-male RRRs of all-cause mortality were 1.52 (95% CI: 1.01, 2.29), 1.95 (95% CI: 1.08, 3.49), and 2.36 (95% CI: 1.15, 4.88), respectively, for those who consumed 75, 90, and 100 g/day of alcohol, which indicated that women were exposed to the higher risk of all-cause mortality at higher level of alcohol consumption compared with men.
Actually, Mattisson et al.37 found that although the subset of female heavy drinkers was small, women with alcohol use disorders had a higher mortality than men, which was consistent with previous findings.37,38 The sex difference in risk of all-cause mortality was likely due to inherent biological differences between men and women. Frezza et al.39 presented that alcohol dehydrogenase activity in women was lower than that in men and that the first stage of alcohol metabolism was slowed down in women, resulting in more alcohol being absorbed into the blood circulation. Women also typically had lower total body water content; thus, alcohol was diluted less and blood alcohol level might be about 30% higher in women than that in men.40 Additionally, women differed from men in several parameters of alcohol metabolism (mainly because of a smaller gastric metabolism in women) which resulted in a greater generation of hepatotoxic products (such as acetaldehyde and probably oxygen radicals). Thereby, it may increase the vulnerability of women to the risk of alcohol-related diseases such as liver disease.41 Some investigators suspected that women were more exposed than men for all-cause mortality at moderate to high levels of alcohol consumption, which might be explained by an increased risk of cancer in women.6,42 However, data concerning an increase of (breast) cancer according to alcohol intake are inconclusive, especially regarding drinking in moderation.
In addition, the present study showed higher risk of all-cause mortality in women with heavy alcohol consumption. In order to reduce excessive alcohol consumption for women, some effective interventions are needed. Supportive counseling and educational sessions have contributed to help women reduce their alcohol consumption.43,44 It should be recommended and emphasized in public policy to provide psychological and educational intervention programs to female heavy drinkers.
Our meta-analysis might have underestimated the true RR difference between women and men. First, previous studies have suggested that females were physically more damaged by their heavy use of alcohol.37,45 However, it was more common among women to underreport their amounts of alcohol consumption and alcohol problems because of shame and guilt. Thus, female drinkers might be misclassified as nondrinkers, and the risk of alcohol on all-cause mortality in women will be underestimated. Second, men drink more than women. If there were no differences between men and women in terms of alcohol consumption, the relative risk of alcohol-related diseases, mortality or all-cause mortality in women might be much higher than men.46,47 Finally, the long-term effect of alcohol consumption on all-cause mortality may not be immediately apparent due to its lag effects. Thus, the stronger lag effects may result in underestimating the risk of alcohol on all-cause mortality in women.
Our study had the following advantages. First, we included the largest sample from studies reporting gender-specific association between drinking and risk of all-cause mortality. The consistency in our findings with no publication bias supported the robustness of the current results. Second, the RR was computed with multiple-adjusted estimates, which could reduce confounding effects of other factors as much as possible. Finally, the results from our sensitivity analysis suggested that the findings were stable.
Conclusions
In summary, the present meta-analysis of 24 prospective cohort studies showed that females had an increased risk for all-cause mortality conferred by drinking compared with male after adjustment for multiple covariates, especially in heavy drinkers. Our findings strongly suggest that female drinkers, particularly heavy drinkers, should moderate or completely reduce alcohol consumption to have a health benefit. National governments, the World Health Organization, and other organizations with an interest in population-level health should emphasize psychological supportive counseling and health education to help female heavy drinkers reduce their alcohol consumption to moderate levels.
Acknowledgments
This work was supported by grants of the National Science and Technology Pillar Program (2011BAI09B03 and 2011BAI11B03) from the Ministry of Science and Technology, China. The authors thank Professor Vincenzo Bagnardi and Matteo Rota, University of Milan-Bicocca, Milan, Italy, for providing the SAS macro.
Disclosure Statement
No competing financial interests exist.
References
- 1.World Health Organization. Global status report on alcohol and health 2011. Available at: www.who.int/substance_abuse/publications/global_alcohol_report/en/index.html Accessed February11, 2011
- 2.Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: Are women more vulnerable? Alcohol Clin Exp Res 2005;29:896–901 [DOI] [PubMed] [Google Scholar]
- 3.Urbano-Marquez A, Estruch R, Fernandez-Sola J, Nicolas JM, Pare JC, Rubin E. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. JAMA 1995;274:149–154 [DOI] [PubMed] [Google Scholar]
- 4.Nakamura S, Takezawa Y, Sato T, Kera K, Maeda T. Alcoholic liver disease in women. Tohoku J Exp Med 1979;129:351–355 [DOI] [PubMed] [Google Scholar]
- 5.Jacobson R. The contributions of sex and drinking history to the CT brain scan changes in alcoholics. Psychol Med 1986;16:547–559 [DOI] [PubMed] [Google Scholar]
- 6.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: An updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437–2445 [DOI] [PubMed] [Google Scholar]
- 7.Inoue M, Nagata C, Tsuji I, et al. Impact of alcohol intake on total mortality and mortality from major causes in Japan: A pooled analysis of six large-scale cohort studies. J Epidemiol Community Health 2012;66:448–456 [DOI] [PubMed] [Google Scholar]
- 8.Deev A, Shestov D, Abernathy J, Kapustina A, Muhina N, Irving S. Association of alcohol consumption to morality in middle-aged U.S. and Russian men and women. Ann Epidemiol 1998;8:147–153 [DOI] [PubMed] [Google Scholar]
- 9.Sun W, Schooling CM, Chan WM, Ho KS, Lam TH, Leung GM. Moderate alcohol use, health status, and mortality in a prospective Chinese elderly cohort. Ann Epidemiol 2009;19:396–403 [DOI] [PubMed] [Google Scholar]
- 10.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet 2011;378:1297–1305 [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 14.Rota M, Bellocco R, Scotti L, et al. Random-effects meta-regression models for studying nonlinear dose-response relationship, with an application to alcohol and esophageal squamous cell carcinoma. Stat Med 2010;29:2679–2687 [DOI] [PubMed] [Google Scholar]
- 15.Tramacere I, Pelucchi C, Bagnardi V, et al. A meta-analysis on alcohol drinking and esophageal and gastric cardia adenocarcinoma risk. Ann Oncol 2012;23:287–297 [DOI] [PubMed] [Google Scholar]
- 16.Woodward M, Barzi F, Martiniuk A, et al. Cohort profile: The Asia Pacific Cohort Studies Collaboration. Int J Epidemiol 2006;35:1412–1416 [DOI] [PubMed] [Google Scholar]
- 17.He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med 2005;353:1124–1134 [DOI] [PubMed] [Google Scholar]
- 18.Baglietto L, English DR, Hopper JL, Powles J, Giles GG. Average volume of alcohol consumed, type of beverage, drinking pattern and the risk of death from all causes. Alcohol Alcohol 2006;41:664–671 [DOI] [PubMed] [Google Scholar]
- 19.Berberian KM, van Duijn CM, Hoes AW, Valkenburg HA, Hofman A. Alcohol and mortality. Results from the EPOZ (Epidemiologic Study of Cardiovascular Risk Indicators) follow-up study. Eur J Epidemiol 1994;10:587–593 [DOI] [PubMed] [Google Scholar]
- 20.Cullen KJ, Knuiman MW, Ward NJ. Alcohol and mortality in Busselton, Western Australia. Am J Epidemiol 1993;137:242–248 [DOI] [PubMed] [Google Scholar]
- 21.Diem P, Deplazes M, Fajfr R, et al. Effects of alcohol consumption on mortality in patients with Type 2 diabetes mellitus. Diabetologia 2003;46:1581–1585 [DOI] [PubMed] [Google Scholar]
- 22.Friesema IH, Zwietering PJ, Veenstra MY, et al. The effect of alcohol intake on cardiovascular disease and mortality disappeared after taking lifetime drinking and covariates into account. Alcohol Clin Exp Res 2008;32:645–651 [DOI] [PubMed] [Google Scholar]
- 23.Hoffmeister H, Schelp FP, Mensink GB, Dietz E, Bohning D. The relationship between alcohol consumption, health indicators and mortality in the German population. Int J Epidemiol 1999;28:1066–1072 [DOI] [PubMed] [Google Scholar]
- 24.Kim MK, Ko MJ, Han JT. Alcohol consumption and mortality from all-cause and cancers among 1.34 million Koreans: The results from the Korea national health insurance corporation's health examinee cohort in 2000. Cancer Causes Control 2010;21:2295–2302 [DOI] [PubMed] [Google Scholar]
- 25.Klatsky AL, Armstrong MA, Friedman GD. Alcohol and mortality. Ann Intern Med 1992;117:646–654 [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, McGee DL, Cao G, Cooper RS. Alcohol intake and mortality: Findings from the National Health Interview Surveys (1988 and 1990). Am J Epidemiol 2000;151:651–659 [DOI] [PubMed] [Google Scholar]
- 27.Makela P, Paljarvi T, Poikolainen K. Heavy and nonheavy drinking occasions, all-cause and cardiovascular mortality and hospitalizations: A follow-up study in a population with a low consumption level. J Stud Alcohol 2005;66:722–728 [DOI] [PubMed] [Google Scholar]
- 28.Maskarinec G, Meng L, Kolonel LN. Alcohol intake, body weight, and mortality in a multiethnic prospective cohort. Epidemiology 1998;9:654–661 [PubMed] [Google Scholar]
- 29.Paganini-Hill A, Kawas CH, Corrada MM. Type of alcohol consumed, changes in intake over time and mortality: The Leisure World Cohort Study. Age Ageing 2007;36:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehm J, Greenfield TK, Rogers JD. Average volume of alcohol consumption, patterns of drinking, and all-cause mortality: Results from the US National Alcohol Survey. Am J Epidemiol 2001;153:64–71 [DOI] [PubMed] [Google Scholar]
- 31.Sadakane A, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K; Jichi Medical School Cohort Study G. Amount and frequency of alcohol consumption and all-cause mortality in a Japanese population: The JMS Cohort Study. J Epidemiol 2009;19:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sempos CT, Rehm J, Wu T, Crespo CJ, Trevisan M. Average volume of alcohol consumption and all-cause mortality in African Americans: The NHEFS cohort. Alcohol Clin Exp Res 2003;27:88–92 [DOI] [PubMed] [Google Scholar]
- 33.Serdula MK, Koong SL, Williamson DF, et al. Alcohol intake and subsequent mortality: Findings from the NHANES I Follow-up Study. J Stud Alcohol 1995;56:233–239 [DOI] [PubMed] [Google Scholar]
- 34.Simons LA, McCallum J, Friedlander Y, Simons J. Alcohol intake and survival in the elderly: A 77-month follow-up in the Dubbo study. Aust N Z J Med 1996;26:662–670 [DOI] [PubMed] [Google Scholar]
- 35.Trevisan M, Schisterman E, Mennotti A, et al. Drinking pattern and mortality: The Italian Risk Factor and Life Expectancy pooling project. Ann Epidemiol 2001;11:312–319 [DOI] [PubMed] [Google Scholar]
- 36.Wellmann J, Heidrich J, Berger K, Doring A, Heuschmann PU, Keil U. Changes in alcohol intake and risk of coronary heart disease and all-cause mortality in the MONICA/KORA-Augsburg cohort 1987–97. Eur J Cardiovasc Prev Rehabil 2004;11:48–55 [DOI] [PubMed] [Google Scholar]
- 37.Mattisson C, Bogren M, Ojehagen A, Nordstrom G, Horstmann V. Mortality in alcohol use disorder in the Lundby Community Cohort–a 50 year follow-up. Drug Alcohol Depend 2011;118:141–147 [DOI] [PubMed] [Google Scholar]
- 38.Batty GD, Hunt K, Emslie C, Lewars H, Gale CR. Alcohol problems and all-cause mortality in men and women: Predictive capacity of a clinical screening tool in a 21-year follow-up of a large, UK-wide, general population-based survey. J Psychosom Res 2009;66:317–321 [DOI] [PubMed] [Google Scholar]
- 39.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med 1990;322:95–99 [DOI] [PubMed] [Google Scholar]
- 40.Ely M, Hardy R, Longford NT, Wadsworth ME. Gender differences in the relationship between alcohol consumption and drink problems are largely accounted for by body water. Alcohol Alcohol 1999;34:894–902 [DOI] [PubMed] [Google Scholar]
- 41.Baraona E, Abittan CS, Dohmen K, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res 2001;25:502–507 [PubMed] [Google Scholar]
- 42.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med 2004;38:613–619 [DOI] [PubMed] [Google Scholar]
- 43.Stade BC, Bailey C, Dzendoletas D, Sgro M, Dowswell T, Bennett D. Psychological and/or educational interventions for reducing alcohol consumption in pregnant women and women planning pregnancy. Cochrane Database Syst Rev 2009:CD004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Connor MJ, Whaley SE. Brief intervention for alcohol use by pregnant women. Am J Public Health 2007;97:252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabine Schwarz MD. Important risk factors of common diseases in women at midlife and beyond. Journal of Public Health 2007;15:81–85 [Google Scholar]
- 46.Gearhart JG, Beebe DK, Milhorn HT, Meeks GR. Alcoholism in women. Am Fam Physician 1991;44:907–913 [PubMed] [Google Scholar]
- 47.Rehm J, Baliunas D, Borges GL, et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction 2010;105:817–843 [DOI] [PMC free article] [PubMed] [Google Scholar]