Abstract
Purpose
Axillary lymph node dissection (ALND) and radiation therapy (RT) are commonly recommended for mastectomy patients with positive sentinel lymph node biopsy (SLNB). Effective alternatives to ALND that reduce lymphedema risk are needed. We evaluated rates of lymphedema in mastectomy patients who received SLNB with RT, compared to ALND with or without RT.
Methods
627 breast cancer patients who underwent 664 mastectomies between 2005–2013 were prospectively screened for lymphedema, median 22.8 months follow-up (range 3.0–86.9). Each mastectomy was categorized as: SLNB-no RT, SLNB+RT, ALND-no RT, or ALND+RT. RT included chest wall +/− nodal radiation. Perometer arm volume measurements were obtained pre- and post-operatively. Lymphedema was defined as ≥10% arm volume increase. Kaplan-Meier and Cox regression analyses were performed to determine lymphedema rates and risk factors.
Results
Of 664 mastectomies, 52% (343/664) were SLNB-no RT, 5% (34/664) SLNB+RT, 9% (58/664) ALND-no RT, and 34% (229/664) ALND+RT. The two-year cumulative lymphedema incidence was 10.0% (95% CI: 2.6–34.4%) for SLNB+RT compared with 19.3% (95% CI: 10.8–33.1%) for ALND-no RT, and 30.1% (95% CI: 23.7–37.8%) for ALND+RT. The lowest cumulative incidence was 2.19% (95% CI: 0.88%–5.40%) for SLNB-no RT. By multivariate analysis, factors significantly associated with increased lymphedema risk included RT (p=0.0017), ALND (p=0.0001), greater number of lymph nodes removed (p=0.0006), no reconstruction (p=0.0418), higher BMI (p<0.0001) and older age (p=0.0021).
Conclusion
Avoiding completion ALND and instead receiving SLNB with RT may decrease lymphedema risk in patients requiring mastectomy. Future trials should investigate the safety of applying the ACOSOG Z0011 protocol to mastectomy patients.
Keywords: Mastectomy, Lymphedema, Quality of Life, Radiation Therapy, Sentinel Lymph Node Biopsy
INTRODUCTION
Quality of life and long-term effects of treatment have become increasingly important for breast cancer patients due to improved survival outcomes [1–3]. A potential side effect of treatment is lymphedema, a chronic condition characterized by swelling of the arm, hand, breast, or trunk, which may develop from the accumulation of lymphatic fluid in the interstitial tissues. Lymphedema is known to have detrimental effects on quality of life due to body image changes, alterations in arm function, and increased complications such as infection and cellulitis [3–6].
Axillary lymph node dissection (ALND) and radiation therapy (RT) are commonly recommended for mastectomy patients with positive sentinel lymph node biopsy (SLNB). Previous studies have consistently identified ALND as the most significant risk factor for lymphedema, with a reported incidence of >20% compared with 3.5–11% for SLNB [7–12]. In addition, studies have shown that RT – particularly regional lymph node radiation (RLNR)— may contribute to even greater lymphedema risk [13,14]. Effective alternatives to ALND that reduce the risk of lymphedema are needed.
Findings from recent studies may impact treatment of the axilla in breast cancer patients. The ACOSOG Z0011 trial demonstrated no significant difference in the rates of regional recurrence, disease free survival (DFS), or overall survival (OS) in 891 women with limited nodal involvement randomized to completion ALND or SLNB followed by radiation to the breast without RLNR [15,16]. Participants did not receive a third field for RLNR, however, it is possible that they had high tangents which can cover a significant part of the axilla [17]. The trial excluded patients requiring mastectomy and therefore applicability of these findings are limited to women undergoing breast conserving therapy (BCT).
We sought to determine the rates of lymphedema in mastectomy patients who received SLNB with RT, compared to ALND with or without RT. Arm volume measurements, patient demographics and treatment characteristics were analyzed to identify risk factors for development of lymphedema.
MATERIAL & METHODS
Study Design
Beginning in 2005, with Institutional Review Board approval, we prospectively obtained bilateral arm volume measurements on women diagnosed with breast cancer using a Perometer. The Perometer is an optoelectronic device that utilizes infrared beams to measure circumferences of the limb and calculates volume based on these measurements. Measurements were obtained pre- and post-operatively, during treatment for breast cancer, and at follow-up oncology visits after completion of breast cancer treatment. The protocol for lymphedema screening has previously been published [18].
For this study, we utilized a weight-adjusted arm volume change equation which calculates change in arm volume compared to a pre-operative measurement and accounts for temporal changes in patient weight, which may cause arm size changes unrelated to lymphedema [19]. Weight-adjusted arm volume change (WAC) was calculated for the left and right arm independently at each post-operative assessment according to the formula, , where A1 is pre-operative arm volume, A2 is arm volume at a post-operative assessment, and W1 and W2 are the patient’s weights at these time points [19]. Use of the WAC equation allowed for inclusion of patients who underwent bilateral mastectomy, since arm volume change could be assessed in each arm individually and did not rely on comparison of size between arms. All patients included in this analysis had a pre-operative measurement and at least one follow-up measurement occurring >3 months after surgery.
Lymphedema was defined as a measurement with ≥ 10% WAC, based on consensus in the literature [20,21]. Measurements recorded within the first 3 months after surgery were not utilized for lymphedema assessment, because patients may experience transient increases in measured arm volume during this period related to post-surgical changes [22]. Measurements obtained after a patient was diagnosed with distant metastasis or local recurrence were excluded to avoid potential confounding.
Patient Population
627 patients who underwent 664 mastectomies for a diagnosis of primary breast cancer between 9/2005 – 2/2013 at our institution were included in this analysis, with a median post-operative follow-up of 22.8 months (range 3.0– 86.9). Prophylactic mastectomies were excluded. Each breast was considered individually, and mastectomies were categorized into four treatment groups: SLNB-no RT, SLNB+RT, ALND-no RT, ALND+RT. RT included chest wall with or without regional lymph node radiation (supraclavicular and/or axillary radiation). Mastectomies were categorized into these groups to replicate the treatment assignments for patients receiving breast conservation therapy (BCT) in the ASCOGO Z11 protocol [16]. Patient demographics, surgical, radiation and medical oncology treatments for the cohort were collected via medical record review to analyze as risk factors. The variable “reconstruction” included patients who underwent immediate breast reconstruction with implant-based or autologous reconstruction at the time of mastectomy. Patients who did not undergo immediate breast reconstruction or who underwent delayed breast reconstruction were classified as “no reconstruction”. At the time of this analysis, only 4 patients had undergone delayed breast reconstruction, and were classified as “no reconstruction” with measurements occurring after reconstruction excluded from the analysis.
Statistical Analysis
Univariate and multivariate Cox proportional hazard models were used to evaluate risk factors for lymphedema. Time-dependent covariates were included for use of systemic therapies and radiation fields such that cases were included in the unexposed group prior to initiation of a given treatment and in the exposed group thereafter. Regression parameters in the Cox models were estimated using a robust sandwich covariance matrix estimate to account for the correlation induced by including data from both sides for women who had bilateral mastectomies [23]. The Kaplan-Meier method was used to estimate and plot cumulative incidence of lymphedema by treatment group (SLNB-no RT, SLNB-RT, ALND-no RT, ALND-RT).
RESULTS
Patient Population
627 patients were included in this analysis, of whom 345 underwent unilateral mastectomy for unilateral breast cancer, and 282 underwent bilateral mastectomy for unilateral (245) or bilateral (37) breast cancer (Table 1). Median age at breast cancer diagnosis was 50 years (range 22–85), and median BMI at diagnosis was 25.0 kg/m2 (16.5–59.0). Of the total 664 mastectomies, 52% (343/664) were SLNB-no RT, 5% (34/664) SLNB+RT, 9% (58/664) ALND-no RT, and 34% (229/664) ALND+RT. In the SLNB-RT group, 38% (13/34) received RT to the chest wall only, and 62% (21/34) received radiation to the chest wall with RLNR. 15 of the 34 (44%) SLNB-RT mastectomies had a positive SLNB, and 7 of the 34 (21%) SLNB-RT mastectomy patients received neoadjuvant chemotherapy. In the ALND-RT group, 6% (14/229) received RT to the chest wall only, 92% received chest wall radiation with RLNR (211/229), and radiation fields were unknown for 2% (4/229).
Table 1.
Clinical and pathologic characteristics of patient cohort
| Median (Range) | n = 664 (100%) | |
|---|---|---|
|
| ||
| Patient Characteristics | ||
|
| ||
| Age at diagnosis, years | 50 (22–85) | - |
| BMI at diagnosis, kg/m2 | 25.0 (16.5–59.0) | - |
|
| ||
| Breast Surgery | ||
|
| ||
| Unilateral Mastectomy | - | 345 (52%) |
| Bilateral Mastectomy | - | 319 (48%) |
|
| ||
| Breast Reconstruction | ||
|
| ||
| Yes | - | 467 (70%) |
| No | - | 197 (30%) |
|
| ||
| Axillary Surgery | ||
|
| ||
| SLNB | - | 337 (57%) |
| ALND | - | 287 (43%) |
| # LN’s removed | 4 (0–43) | - |
|
| ||
| Pathologic Characteristics | ||
|
| ||
| Invasive Carcinoma | - | 594 (89%) |
| Ductal Carcinoma in Situ (DCIS) | - | 70 (11%) |
| Invasive tumor size, cm | 1.8 (0.01–12.5) | - |
| # Positive LN’s | 0 (0–39) | - |
|
| ||
| Radiation Therapy* | ||
|
| ||
| Yes | - | 401 (60%) |
| No | - | 263 (40%) |
|
| ||
| Neoadjuvant Chemotherapy | ||
|
| ||
| Yes | - | 102 (15%) |
| No | - | 562 (85%) |
|
| ||
| Adjuvant Chemotherapy | ||
|
| ||
| Yes | - | 350 (53%) |
| No | - | 314 (47%) |
|
| ||
| Hormonal Therapy | ||
|
| ||
| Yes | - | 481 (72%) |
| No | - | 183 (28%) |
Abbreviations: BMI = Body Mass Index, ALND = Axillary lymph node dissection, SLNB = Sentinel lymph node biopsy, LN = lymph node
Radiation therapy included patients treated with chest wall alone and chest wall with regional lymph node radiation
Cumulative Incidence of Lymphedema
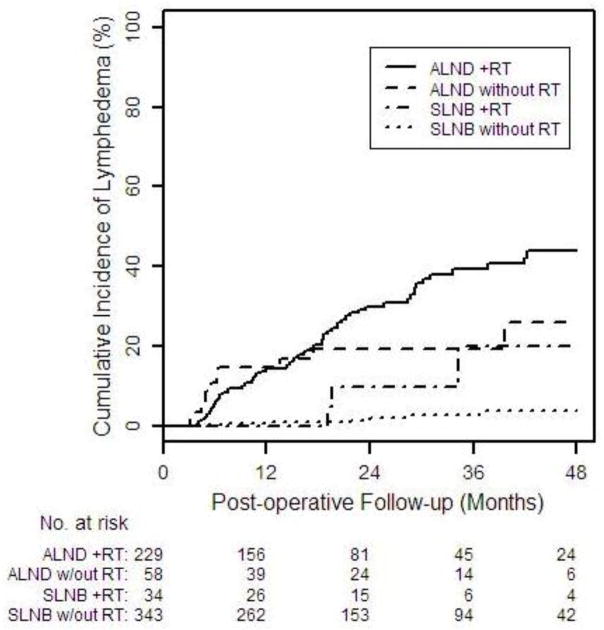
The overall two-year cumulative incidence of lymphedema was 13.8% (95% CI: 11.0–17.3%), 10.0% (95% CI: 2.6–34.4%) for SLNB+RT compared with 19.3% (95% CI: 10.8–33.1%) for ALND-no RT and 30.1% (95% CI: 23.7–37.8%) for ALND+RT (Table 2, Figure 1). The lowest cumulative incidence of lymphedema was 2.19% (95% CI: 0.88%–5.40%) for SLNB-no RT.
Table 2.
2-year cumulative incidence of lymphedema after mastectomy (≥10% WAC) overall and by treatment group
| N | 2-year cumulative incidence | 95% Confidence Interval | ||
|---|---|---|---|---|
| Entire cohort | 664 | 13.82% | 11.01% | 17.27% |
| SLNB-no RT | 343 | 2.19% | 0.88% | 5.40 % |
| SLNB-RT | 34 | 10.00% | 2.60% | 34.40% |
| ALND-no RT | 58 | 19.27% | 10.79% | 33.05% |
| ALND-RT | 229 | 30.08% | 23.66% | 37.77% |
Abbreviations: SLNB = sentinel lymph node biopsy, ALND = axillary lymph node dissection, RT = radiation therapy
Figure 1.
Cumulative incidence of lymphedema after mastectomy (≥10% WAC)according to treatment group
Univariate and Multivariate Analysis
By univariate analysis, factors significantly associated with increased risk of lymphedema included RT (p<0.0001), ALND (p<0.0001), greater number of lymph nodes removed (p<0.0001), no breast reconstruction (p<0.0001), neoadjuvant (p<0.0001) and adjuvant chemotherapy (p=0.0083), higher BMI (p<0.0001), and older age at breast cancer diagnosis (p=0.0021) (Table 3). Unilateral compared with bilateral mastectomy (p= 0.0521) and hormonal therapy (p= 0.2781) were not significant predictors of lymphedema.
Table 3.
Univariate analysis of risk factors for lymphedema after mastectomy (≥10% WAC)
| Hazard Ratio | 95% Confidence Interval | P Value | ||
|---|---|---|---|---|
|
| ||||
| Patient Characteristics | ||||
|
| ||||
| Age at diagnosis, years | 1.030 | 1.011 | 1.050 | 0.0021 |
| BMI at diagnosis, kg/m2 | 1.086 | 1.055 | 1.117 | <0.0001 |
|
| ||||
| Surgical Characteristics | ||||
|
| ||||
| Bilateral vs. unilateral mastectomy | 0.654 | 0.427 | 1.004 | 0.0521 |
| Breast reconstruction | 0.283 | 0.186 | 0.430 | <0.0001 |
| ALND vs. SLNB | 11.819 | 6.178 | 22.612 | <0.0001 |
| # LN’s removed | 1.094 | 1.079 | 1.110 | <0.0001 |
|
| ||||
| Pathologic Characteristics | ||||
|
| ||||
| Invasive Tumor Size, cm | 1.225 | 1.115 | 1.346 | <0.0001 |
| # Positive LN’s | 1.091 | 1.066 | 1.116 | <0.0001 |
|
| ||||
| Systemic/Radiation Therapy | ||||
|
| ||||
| Neoadjuvant Chemotherapy | 2.669 | 1.697 | 4.197 | <0.0001 |
| Adjuvant Chemotherapy | 1.810 | 1.165 | 2.812 | 0.0083 |
| Hormonal Therapy | 1.317 | 0.801 | 2.165 | 0.2781 |
| Radiation Therapy | 4.440 | 2.976 | 6.625 | <0.0001 |
Abbreviations: BMI = Body Mass Index, SLNB = Sentinel lymph node biopsy, ALND = Axillary lymph node dissection, LN = lymph node
Age, BMI, invasive tumor size, # LN’s removed and # positive LN’s analyzed as continuous variables such that the hazard ratios reflect the change in lymphedema risk associated with a 1-unit increase in the variable
Radiation Therapy includes radiation to the breast/chest wall +/− regional lymph node radiation (RLNR)
By multivariate analysis, factors significantly associated with increased risk of lymphedema included RT (p=0.0017), ALND (p=0.0001), greater number of lymph nodes removed (p= 0.0006), no breast reconstruction (p=0.0418), higher BMI (p<0.0001), and older age at breast cancer diagnosis (p=0.0021) (Table 4). Multivariate analysis revealed a significant interaction between ALND and RT (p=0.005). Risk of lymphedema was significantly greater for SLNB-RT compared with SLNB-no RT (p=0.0017), but there was no significant difference in risk for SLNB-RT compared with ALND-RT (p=0.9327) or ALND-no RT (p=0.9709).
Table 4.
Multivariable analysis of risk factors for lymphedema after mastectomy (≥10% WAC)
| Hazard Ratio | 95% Confidence Interval | P Value | ||
|---|---|---|---|---|
|
| ||||
| BMI at diagnosis, kg/m2 | 1.071 | 1.037 | 1.106 | <0.0001 |
|
| ||||
| Age at diagnosis, years | 1.032 | 1.012 | 1.053 | 0.0021 |
|
| ||||
| Immediate breast reconstruction | 0.623 | 0.395 | 0.983 | 0.0418 |
|
| ||||
| # LN’s Removed | 1.046 | 1.020 | 1.073 | 0.0006 |
|
| ||||
| ALND vs. SLNB | - | - | - | 0.0001 |
|
| ||||
| Radiation Therapy | - | - | - | 0.0017 |
|
| ||||
| Interaction, ALND*RT | - | - | - | 0.0050 |
| SLNB-RT vs. SLNB-no RT | 6.4902 | 2.0183 | 20.8698 | 0.0017 |
| ALND-no RT vs. SLNB-RT | 0.9803 | 0.3367 | 2.8541 | 0.9709 |
| ALND-RT vs. SLNB-RT | 1.0468 | 0.3623 | 3.0245 | 0.9327 |
| ALND-RT vs. ALND-no RT | 1.0678 | 0.6662 | 1.7114 | 0.7853 |
Abbreviations: BMI = Body Mass Index, ALND = Axillary lymph node dissection, LN = lymph node
Note: HR’s for ALND vs. SLNB and Radiation Therapy are blank due to the inclusion of the interaction term (ALND*RT)
DISCUSSION
In this series of 664 therapeutic mastectomies from breast cancer patients prospectively screened for lymphedema at our institution, the two-year cumulative incidence of lymphedema was 10% for SLNB with RT, compared with 19% for ALND without RT and 30% for ALND with RT. By multivariate analysis, RT (p=0.0017) and ALND (p=0.0001) were significant risk factors for lymphedema, however, there was no significant difference in risk for SLNB-RT compared with ALND-no RT (p=0.9709) or ALND-RT (p=0.9327). Patients who require post-mastectomy RT after ALND remain at the highest risk for lymphedema; therefore, avoiding completion ALND and instead receiving SLNB with RT may decrease the risk of lymphedema.
Axillary lymph node dissection is the most frequently cited risk factor for lymphedema, with an incidence of >20% following ALND [7,9,11,12]. Post-mastectomy RT is routinely recommended for patients with advanced primary tumors and 4 or more positive lymph nodes, and may also be recommended for patients with 1–3 positive lymph nodes [24–26]. In addition to ALND, regional lymph node radiation (RLNR) is also frequently cited as a risk factor for lymphedema [13,14]. Studies limited to mastectomy patients have shown an increased risk of lymphedema associated with post-mastectomy RT [27]. Thus patients who undergo mastectomy with ALND and RT are at a significant risk for lymphedema, and alternative treatment strategies that minimize this risk are necessary.
Results from recent studies may impact surgical and radiation approaches for treatment of the axilla in breast cancer patients. The ACOSOG Z0011 trial demonstrated no significant difference in the rates of regional recurrence, disease free survival (DFS), or overall survival (OS) in 891 women with limited nodal involvement randomized to completion ALND or SLNB followed by radiation to the breast without RLNR [15,16]. The trial was limited to women undergoing BCT, and lymphedema rates have not yet been reported. Based on these findings, BCT patients with limited nodal disease may receive SLNB with RT and avoid completion ALND. This will likely decrease the risk of lymphedema in these patients given the significantly lower incidence of lymphedema associated with SLNB compared with ALND (<11% versus >20%, respectively) [9,11,12].
We hypothesized that application of the ACOSOG Z0011 treatment protocol for patients requiring mastectomy could similarly reduce the risk of lymphedema. We evaluated the risk of lymphedema in mastectomy patients following SLNB with RT, combining patients who received radiation to the chest wall only with those who also received RLNR to increase sample size. We found that patients who underwent SLNB with RT had a lower incidence of lymphedema (10%) compared to patients who received completion ALND with (30%) or without RT (19%). However, due to small sample size and correspondingly large confidence intervals, we were unable to show a statistically significant reduction in lymphedema risk for the SLNB-RT group.
Preliminary findings of the AMAROS phase III trial comparing axillary radiation to ALND for a positive SLNB in lumpectomy or mastectomy patients demonstrated no significant differences between treatment arms with regard to OS or DFS [28], with a 5-year axillary recurrence rate of 0.54% (4/744) after ALND versus 1.03% (7/681) after axillary radiation. Lymphedema was assessed in this study post-operatively and at 1 and 5 years post-treatment, and was based on subjective clinical assessment by the study investigator. Objective lymphedema measurements were not utilized. At 1 year post-treatment, rates of lymphedema were 15% for patients receiving SLNB with axillary radiation, compared with 25% for ALND without axillary radiation (p<0.001 vs SLNB +axillary radiation) and 59% for ALND with axillary radiation (p<0.001 vs SLNB +axillary radiation) [29]. Lymphedema rates were similar at 5 years post-treatment. We noted a similar trend in our series, in which ALND- RT was associated with a 30% rate of lymphedema, compared with 19% for ALND-no RT and 10% for SLNB-RT. It should be noted that in contrast with the AMAROS trial, our study was restricted to mastectomy patients and utilized prospectively collected objective measurements for lymphedema occurring at approximately 6-month intervals throughout oncology follow-up. Preliminary findings from the AMAROS trial and results from our hypothesis-generating study support possible alternative strategies for treatment of the axilla in mastectomy patients, which may reduce lymphedema risk without compromising local control.
In our series, multivariate analysis indicated a number of independent risk factors for lymphedema in addition to ALND and RT. After controlling for type of axillary surgery, greater number of lymph nodes removed was associated with an increased risk of lymphedema, which has been demonstrated in prior studies [7,14,30]. Increased BMI and age at breast cancer diagnosis were also associated with increased risk of lymphedema in our series, and have been previously identified as independent risk factors for lymphedema [7,9,10,14,31–33]. Interestingly, we found that patients who did not undergo immediate breast reconstruction were at a significantly increased risk for lymphedema by multivariate analysis. While two previous studies have indicated similar findings, further research is warranted regarding a potential mechanism by which immediate breast reconstruction could reduce lymphedema risk [34,35].
Our study has a number of limitations, including the non- randomized selection of patients for SLNB-RT versus ALND with or without RT. The number of patients who received SLNB-RT in this series was small (n=34), and as a result we were unable to demonstrate a statistically significant reduction in risk of lymphedema for this group. In addition, we could not precisely replicate the treatment groups utilized in the ACOSOG Z0011 protocol for the mastectomy patients included in this series. In our cohort, fewer than half (44%) of mastectomy patients who received SLNB-RT had a positive SLNB. Another potential limitation is our combining of patients who received radiation to the chest wall only with those who also received RLNR to increase sample size. Due to the median 2-year follow-up for patients in our cohort and non-random assignment of axillary treatment, we did not evaluate local recurrence rates. Owing to these limitations, further research is warranted utilizing a cohort of mastectomy patients randomized to SLNB-RT compared with ALND +/− RT to evaluate differences in lymphedema rates and axillary recurrence among these groups.
The current study also has many strengths, including use of a large cohort of patients prospectively screened for lymphedema with the Perometer, a device with demonstrated validity and high accuracy for lymphedema assessment [36,37]. To our knowledge, this cohort of 627 patients (corresponding to 664 mastectomies) represents the largest cohort of mastectomy patients prospectively screened for lymphedema in the literature. Patients in our series underwent pre-operative and regular post-operative measurements, and lymphedema was quantified using a validated weight-adjusted volume change (WAC) equation, which incorporates fluctuations in patient weight to account for arm size changes unrelated to lymphedema. The importance of obtaining pre-operative assessments to account for asymmetry between arms and adjustment for factors unrelated to lymphedema has been previously demonstrated [18,19,38,39].
In conclusion, findings from our hypothesis-generating study suggest that application of the ACOSOG Z0011 treatment protocol may reduce the risk of lymphedema for mastectomy patients. Preliminary findings from the AMAROS Phase III trial suggest that ALND or axillary radiation after a positive SLNB provide comparable regional control for lumpectomy and mastectomy patients. Future studies assessing the safety of applying the ACOSOG Z0011 protocol in mastectomy patients is warranted.
Acknowledgments
This project was supported by Award R01CA139118 (AGT), and Award P50CA089393 (AGT) from the National Cancer Institute.
Footnotes
Financial Disclosures: The authors have no financial disclosures to report.
DISCLOSURES
The authors have no conflicts of interest to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Khan F, Amatya B, Pallant JF, Rajapaksa I. Factors associated with long-term functional outcomes and psychological sequelae in women after breast cancer. Breast. 2012;21 (3):314–320. doi: 10.1016/j.breast.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Rief W, Bardwell WA, Dimsdale JE, Natarajan L, Flatt SW, Pierce JP. Long-term course of pain in breast cancer survivors: a 4-year longitudinal study. Breast cancer research and treatment. 2011;130 (2):579–586. doi: 10.1007/s10549-011-1614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26 (35):5689–5696. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakorafas GH, Peros G, Cataliotti L, Vlastos G. Lymphedema following axillary lymph node dissection for breast cancer. Surgical oncology. 2006;15 (3):153–165. doi: 10.1016/j.suronc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26 (21):3536–3542. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 6.Jager G, Doller W, Roth R. Quality-of-life and body image impairments in patients with lymphedema. Lymphology. 2006;39 (4):193–200. [PubMed] [Google Scholar]
- 7.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. The lancet oncology. 2013;14 (6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 8.Wernicke AG, Goodman RL, Turner BC, Komarnicky LT, Curran WJ, Christos PJ, Khan I, Vandris K, Parashar B, Nori D, Chao KS. A 10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection or axillary clearance. Breast cancer research and treatment. 2011;125 (3):893–902. doi: 10.1007/s10549-010-1167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26 (32):5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilke LG, McCall LM, Posther KE, Whitworth PW, Reintgen DS, Leitch AM, Gabram SG, Lucci A, Cox CE, Hunt KK, Herndon JE, 2nd, Giuliano AE. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Annals of surgical oncology. 2006;13 (4):491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I, England D, Sibbering M, Abdullah TI, Barr L, Chetty U, Sinnett DH, Fleissig A, Clarke D, Ell PJ. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. Journal of the National Cancer Institute. 2006;98 (9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 12.Langer I, Guller U, Berclaz G, Koechli OR, Schaer G, Fehr MK, Hess T, Oertli D, Bronz L, Schnarwyler B, Wight E, Uehlinger U, Infanger E, Burger D, Zuber M. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Annals of surgery. 2007;245 (3):452–461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coen JJ, Taghian AG, Kachnic LA, Assaad SI, Powell SN. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. International journal of radiation oncology, biology, physics. 2003;55 (5):1209–1215. doi: 10.1016/s0360-3016(02)04273-6. [DOI] [PubMed] [Google Scholar]
- 14.Hayes SB, Freedman GM, Li T, Anderson PR, Ross E. Does axillary boost increase lymphedema compared with supraclavicular radiation alone after breast conservation? International journal of radiation oncology, biology, physics. 2008;72 (5):1449–1455. doi: 10.1016/j.ijrobp.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 15.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2011;305 (6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Annals of surgery. 2010;252 (3):426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haffty BG, Hunt KK, Harris JR, Buchholz TA. Positive sentinel nodes without axillary dissection: implications for the radiation oncologist. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29 (34):4479–4481. doi: 10.1200/JCO.2011.36.1667. [DOI] [PubMed] [Google Scholar]
- 18.Ancukiewicz M, Russell TA, Otoole J, Specht M, Singer M, Kelada A, Murphy CD, Pogachar J, Gioioso V, Patel M, Skolny M, Smith BL, Taghian AG. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. International journal of radiation oncology, biology, physics. 2011;79 (5):1436–1443. doi: 10.1016/j.ijrobp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller CL, Specht MC, Horick N, et al. A novel, validated method to quantify breast-cancer related lymphedema (BCRL) following bilateral breast surgery. Lymphology. 2013;46:64–72. [PubMed] [Google Scholar]
- 20.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphatic research and biology. 2005;3 (4):208–217. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- 21.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Annals of surgical oncology. 2009;16 (7):1959–1972. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 22.Armer JM, Stewart BR, Shook RP. 30-Month Post-Breast Cancer Treatment Lymphoedema. Journal of lymphoedema. 2009;4 (1):14–18. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee EW, Wei LJ, Amato D. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. Kluwer Academic; Netherlands: 1992. pp. 237–247. [Google Scholar]
- 24.Recht A, Edge SB. Evidence-based indications for postmastectomy irradiation. The Surgical clinics of North America. 2003;83 (4):995–1013. doi: 10.1016/S0039-6109(03)00033-1. [DOI] [PubMed] [Google Scholar]
- 25.Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ, Jr, Deshler A, Fulton S, Hendricks CB, Kemeny M, Kornblith AB, Louis TA, Markman M, Mayer R, Roter D. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. Journal of the National Cancer Institute. 2001;93 (13):979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 26.Harris JR, Halpin-Murphy P, McNeese M, Mendenhall NP, Morrow M, Robert NJ. Consensus Statement on postmastectomy radiation therapy. International journal of radiation oncology, biology, physics. 1999;44 (5):989–990. doi: 10.1016/s0360-3016(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 27.Hinrichs CS, Watroba NL, Rezaishiraz H, Giese W, Hurd T, Fassl KA, Edge SB. Lymphedema secondary to postmastectomy radiation: incidence and risk factors. Annals of surgical oncology. 2004;11 (6):573–580. doi: 10.1245/ASO.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Rutgers EJ, MD, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer patients: Final analysis of the EORTC AMAROS trial (10981/22023). Journal of clinical oncology : official journal of the American Society of Clinical Oncology; Proceedings of the ASCO Annual Meeting; 2013 May 31–June 4; Chicago, IL. 2013. (suppl; abstr LBA1001) [Google Scholar]
- 29.Donker M, Rutgers EJ, van de Velde CJH, et al. Axillary lymph node dissection versus axillay radiotherapy: A detailed analysis of morbidity. Results from the EORTC 10981-22023 AMAROS trial; European Cancer Congress; 2013 September 27–October 1; Amsterdam, Netherlands. 2013. Abstract #30. [Google Scholar]
- 30.Shah C, Wilkinson JB, Baschnagel A, Ghilezan M, Riutta J, Dekhne N, Balaraman S, Mitchell C, Wallace M, Vicini F. Factors associated with the development of breast cancer-related lymphedema after whole-breast irradiation. International journal of radiation oncology, biology, physics. 2012;83 (4):1095–1100. doi: 10.1016/j.ijrobp.2011.09.058. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast cancer research and treatment. 2011;130 (3):981–991. doi: 10.1007/s10549-011-1667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norman SA, Localio AR, Kallan MJ, Weber AL, Torpey HA, Potashnik SL, Miller LT, Fox KR, DeMichele A, Solin LJ. Risk factors for lymphedema after breast cancer treatment. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2010;19 (11):2734–2746. doi: 10.1158/1055-9965.EPI-09-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson K, Ohlsson K, Ingvar C, Albertsson M, Ekdahl C. Factors associated with the development of arm lymphedema following breast cancer treatment: a match pair case-control study. Lymphology. 2002;35 (2):59–71. [PubMed] [Google Scholar]
- 34.Card A, Crosby MA, Liu J, Lindstrom WA, Lucci A, Chang DW. Reduced incidence of breast cancer-related lymphedema following mastectomy and breast reconstruction versus mastectomy alone. Plastic and reconstructive surgery. 2012;130 (6):1169–1178. doi: 10.1097/PRS.0b013e31826d0faa. [DOI] [PubMed] [Google Scholar]
- 35.Avraham T, Daluvoy SV, Riedel ER, Cordeiro PG, Van Zee KJ, Mehrara BJ. Tissue expander breast reconstruction is not associated with an increased risk of lymphedema. Annals of surgical oncology. 2010;17 (11):2926–2932. doi: 10.1245/s10434-010-1112-2. [DOI] [PubMed] [Google Scholar]
- 36.Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR. Validation of an optoelectronic limb volumeter (Perometer) Lymphology. 1997;30 (2):77–97. [PubMed] [Google Scholar]
- 37.Tierney S, Aslam M, Rennie K, Grace P. Infrared optoelectronic volumetry, the ideal way to measure limb volume. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 1996;12 (4):412–417. doi: 10.1016/s1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 38.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112 (12):2809–2819. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 39.Ancukiewicz M, Miller CL, Skolny MN, O’Toole J, Warren LE, Jammallo LS, Specht MC, Taghian AG. Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: the flaws in current studies and need for universal methodology. Breast cancer research and treatment. 2012;135 (1):145–152. doi: 10.1007/s10549-012-2111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]