Abstract
Magnetic resonance imaging (MRI) of disease biomarkers, especially cancer biomarkers, could potentially improve our understanding of the disease and drug activity during preclinical and clinical drug treatment and patient stratification. MRI contrast agents with high relaxivity and targeting capability to tumor biomarkers are highly required. Extensive work has been done to develop MRI contrast agents. However, only a few limited literatures report that protein residues can function as ligands to bind Gd3+ with high binding affinity, selectivity, and relaxivity. In this paper, we focus on reporting our current progress on designing a novel class of protein-based Gd3+ MRI contrast agents (ProCAs) equipped with several desirable capabilities for in vivo application of MRI of tumor biomarkers. We will first discuss our strategy for improving the relaxivity by a novel protein-based design. We then discuss the effect of increased relaxivity of ProCAs on improving the detection limits for MRI contrast agent, especially for in vivo application. We will further report our efforts to improve in vivo imaging capability and our achievement in molecular imaging of cancer biomarkers with potential preclinical and clinical applications.
INTRODUCTION
Molecular imaging of disease biomarkers, especially cancer biomarkers, could potentially improve our understanding of the disease and drug activity during preclinical and clinical drug treatment and patient stratification.1–4 For the preclinical setting, applications of molecular imaging are useful to conduct novel therapeutic analysis. A noninvasive way to monitor disease progression and effect of drug treatment could be very helpful for developing therapeutics for prevention and treatment of disease and understanding the molecular level biological basis of pathological processes. The success in translating in vitro discovery, to cellular response, to preclinical small animal research, and finally to the human body, requires a sensitive and reliable imaging technique.5–10 Additionally, the application of molecular imaging for human diagnosis will allow clinicians to tailor disease treatment specifically for individuals that express certain biomarkers. A noninvasive imaging technique with high sensitivity and specificity is important to perform both preclinical and clinical molecular imaging analysis.
Among clinical imaging modalities capable of imaging all parts of the human body: computed tomography (CT), magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET), MRI is potentially the ideal technique for molecular imaging with preferred capability and superb soft tissue contrast. MRI is able to obtain three-dimensional images and their dynamic changes with outstanding depth penetration (from 1 mm to 1 m) and high resolution. The resolution of the preclinical MRI scanner can reach 20 μm or even less.11 Without the use of ionizing radiation, MRI enables the noninvasive and repetitive assessment of biological or pathological processes of the same living subject at different time points for monitoring treatment response and disease progression with preferred safety and convenience.5–9 Today, MRI has been applied to acquire anatomic structures, compare volume changes, tumor metabolism, and probe the vascular properties of tumors by dynamic contrast enhanced-MRI (DCE-MRI) technique.12 MRI has also gained the potential to be the most powerful technique for allowing direct translation of preclinical findings to clinical applications by significantly reducing the number of animals required to evaluate a new compound and associated experimental errors. With improvement of the technology, MRI has become one of the leading imaging techniques for diagnostics, monitoring treatment, and progression of many types of diseases, such as central nervous system (CNS) disorders, cardiovascular disorders, and cancer.4–9,13 Applications of noninvasive MRI techniques with high resolution becomes even more important for imaging-guided targeted therapy and drug delivery against biomarkers, molecular targets, and personalized medicine.
The current application of MRI, however, is largely limited because it lacks the proper sensitivity. Most MRI images are generated on the basis of the different relaxation properties of protons in different organs and tissues based upon the water found in those tissues. Such differences are very small with a large background water concentration of 18 M, which results in significantly lower sensitivity than other imaging modalities, such as PET/SPECT. In order to increase the sensitivity of MRI scans, more than 30% of scans utilize the injection of MRI contrast agents intravenously.14 Based on paramagnetic, ferromagnetic, or super paramagnetic properties of metal ions, these MRI contrast agents change the longitudinal (T1) and transverse (T2) relaxation times of water in vivo and thus produce images with altered signal intensities. The lanthanide gadolinium (Gd3+) is the most frequently used metal ion for MRI contrast agent due to its very high magnetic moment and a symmetric electronic ground state. Its capability to create bright MR images by decreasing T1 without causing substantial line broadening makes it more desirable than other contrast agents such as superparamagnetic iron oxides with T2/T2* shortening which produce dark images. To date, while remarkable progress for developing contrast agents has been made in the last 20 years,15–20 MRI contrast agents capable of molecular imaging with high sensitivity and specificity remain elusive to the market.
To extend the application of MRI in clinical diagnostics and preclinical drug development, especially for molecular imaging of biomarker changes, contrast agents must be developed with several desired features. First, MRI contrast agents should have significantly improved sensitivity which is controlled by the degree an MRI contrast agent alters the relaxation of water. This parameter is called relaxivity. Current clinically approved MRI contrast agents have per Gd relaxivity (r1) values around 4–6 mM–1 s–1 which is significantly lower than theoretical maximum value around 100 mM–1 s–1 for one water molecule coordinated in the Gd3+ inner shell (Figure 1). On the basis of a recent simulation of the relationship between relaxivity and detection limits by Sherry et al.,21 the in vitro detection limits for contrast agents are about 10, 4, and 0.69 μM if the per Gd3+ r1 relaxivity of contrast agents are 5, 20, or 100 mM–1 s–1, respectively. With relaxivity values of approximately 4 mM–1 s–1, an injection dose of 0.1–0.2 mmol/kg of clinical MRI contrast agents is required to generate a detectable contrast with a local Gd3+ concentration of about 100 μM. Second, an ideal contrast agent should have the proper size for efficient tissue penetration and distribution to enable accurate detection of changes of the biomarkers during biological and pathological processes. Third, an ideal contrast agent should target to biomarkers with high specificity and high binding affinity for molecular imaging. Fourth, an ideal contrast agent should have favorable pharmacokinetics for targeting biomarkers and excretion.
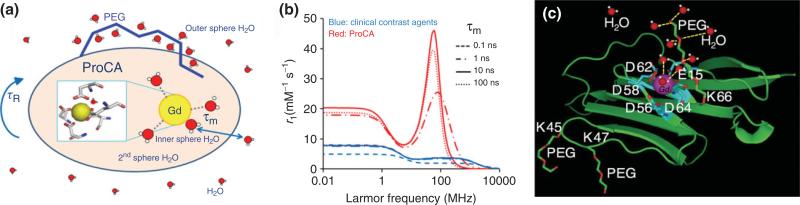
FIGURE 1.
(a) Schematic representation of a Gd3+ chelate surrounded by bulk water with one inner sphere and three second sphere water molecules and several outer sphere water molecules. Modification of contrast agents by PEG could change the water properties which could further influence relaxivity. (b) Simulated magnetic field-dependent relaxivity r1 of clinical MRI contrast agents and ProCA based on the given τR, τm, τv, and Δ2 and Solomon-Bloembergen-Morgan (SBM) theory using combinations of τR (100 ps for clinical MRI contrast agents and 10 ns for ProCA), τm (0.1, 1, 10, and 100 ns), τv (10 ps), and Δ2 (5 × 1019 s–2) under a magnetic field strength from 0.01 MHz to 10,000 MHz. (c) Model structure of ProCA1 with PEG modification. (Reprinted with permission from Ref 32. Copyright 2012 Elsevier)
There have been many efforts in improving relaxivity of MRI contrast agents by covalently linking Gd-chelates to nano-carriers, such as dendrimers,22 liposomes,23 nanoparticle emulsions24 viral capsids,25 and nanotubes.26 Non-covalent binding between Gdchelators and protein, such as MS-325, have shown dramatic increase of relaxivity.27 For recent progress please see these excellent reviews.14,19,28–31 However, only limited literature reports using protein residues function as ligands to bind Gd3+. In this review, we will focus on reporting our current progress on designing a novel class of protein-based Gd3+ MRI contrast agents (ProCAs) (Figure 1) equipped with several desirable capabilities for in vivo application of MRI and to meet a pressing need to develop MRI contrast agents with sufficient sensitivity and specificity to image disease biomarkers. ProCAs exhibits more than 10 times higher relaxivity and dose efficiency than that of the clinical MRI contrast agents. We will first discuss our strategy for improving the relaxivity by a novel protein-based design. We will then discuss the stability of ProCAs. We next discuss the effect of increased relaxivity of ProCAs on improving the detection limits for MRI contrast agent, especially for in vivo application. We will further report our efforts to improve in vivo imaging capability and our achievement in molecular imaging of cancer biomarkers with potential preclinical and clinical applications.
RELAXATION THEORY AND CHALLENGES FOR INCREASING RELAXIVITY
Proton relaxivity ri (in units of mM–1s–1) represents the efficiency of a paramagnetic contrast agent to enhance the relaxation rate of water protons. Water molecules directly interact with metal ion such as Gd3+ to change the relaxation properties. The ri is composed of the contributions from inner sphere , second coordination sphere , and outer sphere . As shown in Figure 1(a), the relaxivity (ri) of the contrast agents are influenced by many factors, such as rotational correlation time (τR), water number (q), and second and outer sphere water. The inner sphere and second sphere relaxivity can be characterized by Solomon-Bloembergen-Morgan (SBM) equation.33–37 The outer sphere effects of a Gd3+-based agent is usually characterized by a hard sphere model, where the relaxivity is mainly determined by the diffusion constant of the water and the closest distance of proton nuclear spin and the gadolinium electron spin. For details, please read this excellent review.38
τR is one of the major factors influencing high relaxivity. Contrast agents based on small chelators such as DTPA have a τR of approximately 54 ps at 37°C.39 This value is much smaller than the electron relaxation time (Tie)(T1e is about 2.8 ns at 60 MHz based on simulation) and the residence time of the bound water (τm)(143 ns).39,40 Therefore, τR determines the value of overall correlation time (τc), which subsequently determines T1m and thus restricts the relaxivity to values less than 10 mM–1s–1, regardless of the adjustment of the other parameters for typical Gd3+-based agents. As shown in Figure 1(b), when τR increases from 100 ps to 10 ns, r1 increases from 3.7 to 46.1 mmol–1 s–1 at 60 MHz (τm = 10 ns). The theoretical calculation by the SBM equation33–37 shows that the maximum per Gd3+ relaxivity, r1, that can be achieved is up to 100 mM–1s–1 for Gd3+ contrast agents with q = 1 and optimized τR (around 10 ns). Therefore, τR is the limiting factor for r1 at clinical magnetic field strengths. Optimizing τR can effectively increase per Gd3+ relaxivity.
Because optimizing τR can increase relaxivity and τR increases with molecular weight, one of the common strategies for increasing relaxivity is by covalent conjugation of Gd-chelators to macromolecules such as polymer,41 dendrimers,22 proteins25 or non-covalent conjugation to macromolecules such as proteins (e.g., serum albumin).28 Gd3+-chelators have been covalently or non-covalently bonded to proteins to alter their in vitro and in vivo properties of MRI contrast agents. Covalent conjugation of small chelating molecules to proteins such as albumin, immunoglobulin G (IgG), or polylysine to some degree increases relaxivity.38 For example, covalently linking of Gd-chelates to albumin increases the relaxivity and changes the distribution of Gd3+ with applications in DCE-MRI to probe tumor vasculature changes.42,43 Non-covalent and reversibly binding small chelator MS-325 to albumin increases the blood circulation time and improves the per Gd3+ relaxivity up to 42 mM–1 s–1 at 20 MHz 37°C.44
However, the improvement in proton relaxivity is much smaller than the theoretical prediction based on the molecular-weight increase. Bryant et al. have shown that r1 and r2 of a polyamidoamine (PAMAM) dendrimer conjugated with DOTA derivative and grown to high generation numbers (G = 5, 7, 9, and 10) do not continue to increase with macromolecular size but reach a plateau.45 Uncontrolled local motion of Gd-vector and slow water exchange rate τm are suggested to be the challenging factors to prevent a further increase of relaxivity.19,28,46 Recently, a substantial increase of molecular weight up to several million Daltons using polymerized liposomes and nanoparticle emulsions were shown to result in per Gd3+ T1 relaxivities of about 11–50 mM–1s–1. Naturally, several million Daltons of contrast agent are far too big in size for proper in vivo distribution, disease tissue penetration, and excretion. In developing new contrast agents, increasing relaxivity without compromising desired in vivo diffusion rate and good tissue/organ penetration associated with unfavorable size is challenging and of the upmost importance.
Another way one can optimize the relaxivity for a Gd3+-based contrast agent is to optimize the second and outer shell effects. Some contrast agents have an inner sphere q value of zero yet still produce appreciable relaxivity.28 Therefore, second shell effects significantly contribute to the relaxivity of an agent. However, the second sphere contributions to the overall relaxivity are usually not significant for small molecules due to negligible water interaction interface, fast water diffusion, fast molecular tumbling, and extended proton Gd3+-proton distance. For example, conjugation of hydrophilic chain of polyethylene glycol (PEG) to a small chelator contrast agent DTPA results in a modest increase in relaxivity.47 In some cases, the relaxivities of small chelator-based contrast agents were decreased because of the addition of long PEG chain that limits the water exchange rate.48
Contributions from the second and outer sphere to the relaxivity become significant when calculating a macromolecule complex with an optimized τc and large interface for water molecule interactions. Based on simulations at 20 and 60 MHz with , τR = 10 ns, τm = 10 ns, and q = 4, the second sphere per Gd3+ relaxivity for a protein metal complex could potentially reach 3.3 and 8.8 mM–1 s–1, respectively.
Outer sphere effects of a Gd3+-based agent are usually characterized by a hard sphere model, where the relaxivity is mainly determined by the diffusion constant of the water, the closest distance of proton nuclear spin, and the gadolinium electron spin. Gd(C11-DOTP)5– is known Gd3+ complex with q = 0. Owing to the contribution of outer sphere mechanism, the relaxivity of Gd(C11-DOTP)5– is about 4.6 at 20 MHz at 25°C. After binding to human serum albumin (HSA), the per Gd3+ relaxivity of Gd(C11-DOTP)5– -albumin complex can increase to about 40 mM–1 s–1 at 5°C. Since Gd(C11-DOTP)5– does not have any inner sphere water, such remarkable relaxivity is mainly due to second and outer sphere water. Relaxivity increases when the temperature decreases from 37 to 5°C, which indicates that the τm of second and outer sphere water is approaching an optimized condition when Gd(C11-DOTP)5– binds to albumin.49 Recently, the relaxivities of the monocrystalline superparamagnetic iron oxide nanoparticle (MION) were shown to be influenced by the coating thickness of the hydrophilic PEG, due to their influence of the water retention to the core of the MION. As the thickness of PEG increases, R2 decreases and R1 increases.50,51 In this case, both physical abundance of protons and water residency time were suggested to alter the relaxation rates. The coating molecules of contrast agents could change the water abundance on the surface, hinder water diffusion, and immobilize water molecules through hydrogen bonds.
An additional tactic to increase the relaxivity for Gd3+-based contrast agents is to increase q, or the number of water ions that fill the coordinate shell around Gd3+. Pioneered by Meade et al. a class of smart contrast agents has been developed by modulating the number of q upon responses to chemical events such as calcium, zinc, and enzymatic actions.52–55 Theoretically, when q is doubled from 1 to 2, the relaxivity of the contrast agents should also be doubled. However, this is a very tricky parameter to optimize because one must strike a balance between allowing as many water molecules to coordinate with gadolinium as possible while keeping the gadolinium ion tightly chelated within the contrast agent structure. It is important that q is increased carefully in order to ensure Gd3+ is not made liable to dissociation. Raymond et al.,19,56 developed hydroxypyridinone-based MRI contrast agents with q = 2 or 3. Competition experiments between Gd3+ and other metal ions suggest that these contrast agents have comparable stability to clinical MRI contrast agents. These contrast agents are also resistant to the competition from other anions, such as phosphate.
RATIONAL DESIGN OF PROTEIN-BASED MRI CONTRAST AGENTS
In the past three decades, Gd3+-chelators were covalently or non-covalently bonded to proteins to alter in vivo properties of MRI contrast agents. For example, MS-325 can reversibly bind to albumin with increased blood circulation time and improve the relaxivity. Because of these improvements, MS-325 is applied to imaging blood vessel abnormalities.44 Gd-chelates were also covalently linked to proteins such as albumin. Covalent linkage of Gd3+ to albumin increases the relaxivity and changes the distribution of Gd3+. Meade et al.41 developed protein-polymer MRI contrast agents. In their design, DO3A-based Gd3+ chelators were linked to lysine-containing random-coil polymers. The per Gd3+ relaxivity is up to 14 mM–1 s–1 and per particle relaxivity is about 461 mM–1s–1.
ProCA is different from any of these Gd3+ labeled proteins in which Gd3+-chelates covalently or non-covalently linked to proteins. ProCA uses side chains from the scaffold protein to directly generate a Gd3+ binding protein and our designed protein itself serves as a chelator to tightly bind to Gd3+ (Figure 1(c)). Similar strategies have been applied by Caravan et al.27 and Liepold et al.57 where the Gd3+ binding sites were formed by the amino acid side chains of helix-loop-helix peptide or virus particles. Caravan et al. de novo designed a metallo-peptide with helix-loop-helix structure, which bind to DNA with a 100% increase of per Gd3+ relaxiviy (r1 = 42.4 mM–1 s–1 at 60 MHz and 37°C). Liepold and his colleagues developed a Gd3+-loaded Cowpea chloroticmottle virus (CCMV), and 180 Gd3+ were able to load in CCMV. A T1 relaxivity of Gd3+-loaded particle is 202 mM–1 s–1 (at 61 MHz).57
We hypothesize that MRI contrast agents with high relaxivity can be achieved by directly designing Gd3+ binding sites into stable proteins to improve the three key factors of τc, q, and outer coordinate shell contributions without sacrificing desired in vivo diffusion of water and stability of Gd3+. Figure 1(c) shows the modeled structure of designed ProCA1 in domain one of CD2 using our developed computational methods. A Gd3+ binding pocket is formed by several oxygen atoms from carboxyl side chains such as Asp and Glu from different stretches of the host protein and one side of Gd3+ binding pocket is designed to open to allow Gd3+ to have fast water exchange. Different from previous studies using existing Gd3+ chelators to attach or bind to larger macromolecules, we have designed protein-based contrast agents by creating binding sites directly in proteins for Gd3+ with desired metal-binding affinity and relaxation properties. The development of our protein-based MRI contrast agents is based on our cumulated efforts in understanding metal coordination and key determinants for metal-binding affinity, selectivity, conformational change, and dynamic properties of metalloproteins using protein design.58–64 To understand metal selectivity, we have performed extensive analysis of structural parameters such as the ligand types, coordination numbers, water numbers, and bond angles and lengths of different classes of metal-binding sites in proteins.65,66
In designing ProCA three main parameters were tuned in order to obtain high relaxivity. These three parameters are τc: a time constant that refers to local magnetic fluctuations; outer and second shell optimization: interactions between the contrast agent and water molecules outside the inner sphere; and q: the number of bound water molecules.
First, directly coordinating Gd3+ ions to protein ligand residues eliminates the high internal mobility of the paramagnetic moiety associated with polymers and protein conjugates. The proton T1 relaxivity can be dramatically increased due to the increase in correlation time. We selected a rigid host structure as the scaffold protein for our contrast agent because of its high resistance to pH and salt denaturation and tolerance to mutations as well as its correlation time τR of 9.2 ns, which is very close to the most optimized τR based on the SBM equation.33–37 Second, contribution of the second and outer layer spheres can be explored by protein engineering and modification. Third, the different coordination shells provide us with the possibility of increasing water q without sacrificing metal-binding affinity and selectivity. We have previously shown that metal selectivity is also largely dependent on the ligand types and chemical properties such as electrostatic interactions of protein environment, as well as long range interactions.58,60,63,67 We successfully designed metal binding sites in a scaffold protein with strong target metal selectivity in the presence of excess physiological metal ions.58–64 Structure determination by solution nuclear magnetic resonance (NMR) revealed that the actual coordination geometry in a designed variant is the same as our design, verifying the computational methods and the design strategy of incorporating metal-binding sites in proteins.58–64 Fourth, targeted contrast agent with improved specificity to certain biomarkers can be developed by expression as a fusion protein or by grafting of the protein. Additionally, proteins are biocompatible and have been modified to protein drugs to overcome adverse effects such as immune responses due to rapid current advances in biotechnology and pharmacology.68
The per Gd3+ relaxivity of ProCA1 is 117 mM–1 s–1 at 1.5 T, 48 mM–1 s–1 at 3T, and 6 mM–1 s–1 at 9.4 T, and is much higher than most of clinical MRI contrast agents. Such substantial increase of relaxivity is likely due to optimized q, τc, and outer sphere relaxivity. Tb3+ luminescent lifetime experiments indicate that ProCA1 has a q = 2.1. Strikingly, we overcome the instability of contrast agents when q is increased. Both r1 and r2 increase when temperature decrease, indicating that ProCA1 has fast water exchange. ProCA1 has much higher metal selectivity (pGd/pMg>10.0, pGd/pZn = 5.3) than clinical MRI contrast agents (pGd/pMg = 4.3, pGd/pZn = 4.2 for Gd-DTPA). No precipitation was found when ProCA1 was supplemented with Ca2+ and phosphate, indicating that Ca2+ and phosphate cannot compete for Gd3+.64 We have also designed a novel generation of ProCA with multiple Gd3+ binding sites and improved stabilities (named ProCA2 and ProCA3) (Xue et al., unpublished results).
In addition to taking advantage of the larger hydration surface of the protein as opposed to a small molecule, we further increased relaxivity of ProCA1 by addition of a secondary shell with PEGylation. The PEGylation modifications dramatically increased longitudinal and transverse relaxivities of the ProCA1 at different field strengths tested (0.47, 1.4, 3.0, and 9.4 T). The r1 and r2 values of ProCA1-PEG0.6k show an increase of almost 66 and 110% compared with ProCA1. The r1 and r2 values of ProCA1-PEG2.4k increased by 100 and 125% and the r1 and r2 values of ProCA1-PEG12k increased by 252 and 130% from ProCA1. By comparison with Gd-DTPA, whose r1 and r2 values are less than 10 mM– s–1 at any magnetic field strength, the ProCA1 and PEGylated ProCA1 showed dramatically higher r1 and r2 values. ProCA1-PEG12k exhibits 19-fold higher r1 and r2 values compared with Gd-DTPA. PEGylated ProCA1 displayed relaxivities that are even higher than nanoparticle-based contrast agents. Additionally intriguing, at high field strength of 9.4 T, ProCA1-PEG2.4k still exhibited great increase of relaxivity values r1 and r2.32
Improving the relaxivity of the contrast agents has two advantages. First, increasing the relaxivity of the contrast agents can decrease the required injection dosage, which could potentially decrease the Gd3+ accumulation in vivo. Second, improving the relaxivity can potentially improve the detection limits for an MRI contrast agent, which greatly benefits molecular imaging using MRI. As shown in Table 1, the injection dosage of only 0.0024–0.0048 mmol Gd/kg for ProCA was used to generate excellent in vivo imaging (Figure 2). It is estimated that 0.02 mmol Gd/kg is needed for the molecular imaging of human epidermal growth factor receptor 2 (HER-2) biomarkers.64,32,69 On the other hand, clinical contrast agents usually require injection of 0.1–0.2 mmol Gd/kg to obtain magnetic resonance (MR) images with good contrast. The molecular imaging of HER-2 by three-step targeting requires as high as 0.145 mmol/kg of avidin-Gd-DTPA to clearly image tumor.70 Nanoparticles, such as G-5 dendrimers, have an injection dosage of 0.03 mmol Gd/kg. Indeed, by improving the relaxivity, the dosage efficiency of ProCA is significantly improved compared with other contrast agents. Our HER-2 MRI results69 indicate that by incorporating targeting moieties, ProCAs could be applied to imaging other cancer biomarker because of efficient targeting and high relaxivity.
TABLE 1.
The Size, Organ/Tissue Enhancement, Biomarker Recognition, Relaxivity, and Dosages of Typical MRI Contrast Agents
| Targeting or Not | Contrast Agents | Size | Organ/Tissue Enhancement | Biomarker Recognition | Per Gd3+, r1, mM–1 s–1 | Field Strength, T | Temperature, °C | Dosage, μmol Gd/kg | References |
|---|---|---|---|---|---|---|---|---|---|
| Nontargeted contrast agents | Gd-DTPA | 547 Da | Whole body, brain, CNS, kidney | None | 3.5 | 1.5 | 20 | 100–200 | 71 |
| MS-325 | Blood vessel | None | 42 | 0.47 | 37 | 30 | 44, 72 | ||
| Gadomer-17 | 35 kDa | Blood vessel | None | 17.3 | 0.47 | 40 | 25–100 | 73 | |
| PAMAM-G4 | 59 kDa | Liver, kidney, blood vessel | None | 29 | 1.5 | 20 | 30 | 15, 45 | |
| NanoglobularMRI CAs (G3) | 3.2 nm | Blood vessel, tumor, vasculature | None | 10 | 3 | RT | 10–30 | 74 | |
| 27 kDa | |||||||||
| Gold nanoparticles | 1.9 nm | Kidney, bladder | None | 4.1 | 7 | RT | [Gd3+] 5 mM, 300 μl | 75 | |
| ProCA | ProCA1, ProCA1.affi, ProCA1.GRP | 12 kDa | Kidney, liver, and tumor biomarkers | Nontarget, HER-2, GRPR | 117 | 1.4 | RT | 2.4 | 64 |
| 2–3 nm | |||||||||
| Targeted contrast agents | EP-3533 | Collagen-rich scar, collagen-rich liver | Collagen | 18.7 | 0.47 | 37 | 25 μmol particle/kg, 20 μmol particle /kg | 76, 77 | |
| EP-3600 | Myocardium | Collagen | 21.3 | 1.4 | 37 | 12.3 μmol particle/kg | 78 | ||
| Gd-DOTA-R832 | Inflammation lesions in liver | VCAM-1 | 8.5 | 0.47 | 37 | 100 | 79 | ||
| Peptoid-(Gd)8-dendron | ~8.6 kDa | Tumor | VEFGR2 | 13.8 | 0.54 | 37 | 8 μmol particle /kg, 32 μmol Gd/kg | 80 | |
| αvβ3-Integrin-targeted nanoparticles | 270 nm | Tumor | αvβ3-integrin | 20 (1,800,000) | 0.47 | 40 | 81 | ||
| 167 and 236 nm | Tumor | αvβ3-integrin | 2 mL/kg, 0.2 nM | 82 | |||||
| 273 nm | Tumor | integrin | 19.1 | 0.47 | 37 | 3 | 83 | ||
| 0.47 T | |||||||||
| 273 nm | Angiogenesis in atherosclerosis | αvβ3-integrin | 17.7 | 1.5 | 0.5 mL/kg | 84 | |||
| bG4D-Gd-SA | 4182 Da | Her-2 positive tumor | HER2 by multiple step | 145 | 70 | ||||
| Gd-perfluorocarbon nanoparticles | <250 nm | Vulnerable plaques | Fibrin | 18 | 0.25–0.5 mL/kg | 24 | |||
| Dendrimer nanoclusters | 75–150 nm | Subcutaneous tumor xenografts | Folate receptor | 12.3 | 1.4 | 40 | 300 | 22 | |
| P947 | Atherosclerotic plaques | MMPs, ACE, and APN | 85 |
CNS, central nervous system; MRI CA, magnetic resonance imaging contrast agents; GRP, gastrin-releasing peptide; GRPR, gastrin-releasing peptide receptor; MMP, matrix metalloproteinases; ACE, angiotensin-converting enzyme; APN, aminopeptidases N
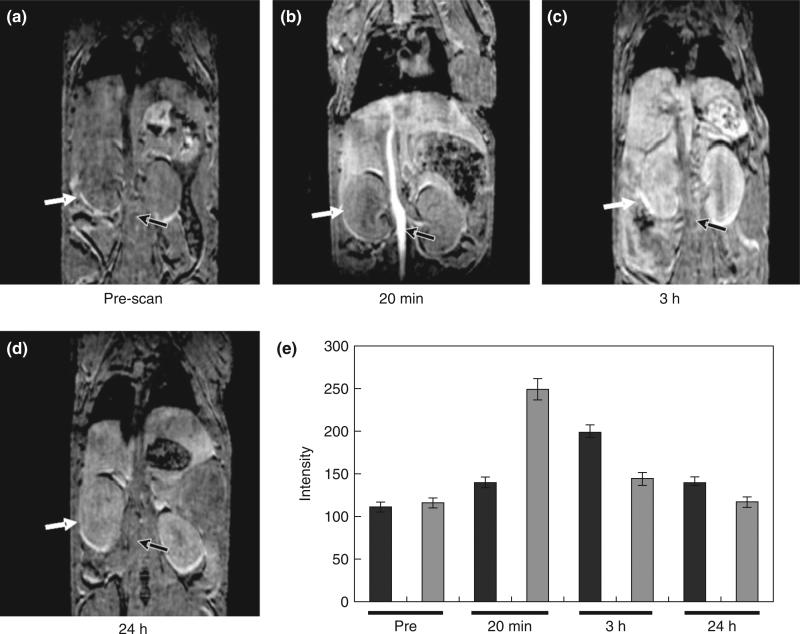
FIGURE 2.
Multiple organ enhancement of magnetic resonance imaging (MRI) of mice after injection of ProCA1 for 20 min (b), 3 h (c), 24 h (d) compared with pre-scan (a). (e) MRI intensity changes of kidney (black) and liver (gray) before and after injection of ProCA1. (Reprinted with permission from Ref 32. Copyright 2012 Elsevier)
STABILITY OF PROTEIN-BASED MRI CONTRAST AGENTS
Free Gd3+ is toxic with LD50 about 0.2 mmol/kg in mice; thus, it is essential that designed MRI contrast agents have strong metal-binding affinities and selectivity. We applied several spectroscopic methods to evaluate the metal-binding affinity and selectivity of ProCAs. The first generation of ProCA, ProCA1, has a very good metal selectivity for Gd3+ over physiological metal ions, such as Zn2+, Mg2+, and Ca2+ (log (KGd/KZn) = 5.34 log (KGd/KMg) > 10.06 and log (KGd/KCa) > 9.84), although the binding affinity is weaker than clinical MRI contrast agents. The high Gd3+ stability and selectivity of ProCA is further supported by the observation that the r1 is not changed in the presence of 10 mM Ca2+.64 The new generation of ProCA, ProCA3, has a thermodynamic stability constant (pGd) of 21.6, (Xue, unpublished data) which is comparable to most of the clinical MRI contrast agents and much high than Gd-DTPA-BMA (pGd = 16.6).
The stability of protein in the circulation system is essential for the in vivo application of ProCA. To test the serum stability, ProCAs were incubated with 75% human serum for 3 and 6 h. The stability of the ProCAs was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and matrix-assisted laser desorption/ionization (MALDI) mass spectrometry, and the ProCAs remains intact after 6 h of incubation. Taken together, ProCAs are stable for in vivo application.
MOLECULAR IMAGING OF BIOMARKERS BY MRI
As discussed above, to achieve molecular imaging of biomarkers, targeted contrast agents with high specificity are required in addition to its sensitivity. To date, many studies have been devoted to developing targeted MR contrast agents to achieve molecular imaging by MRI (Table 1). Commonly, antibody, peptide or small ligand, and small protein domains such as affibodies have been used to achieve targeting.4,22,24,70,76–82,84,83,85 To enhance the sensitivity of the contrast agents, these targeting moieties are usually linked to high payload MRI contrast agents, such as nanoparticles. Conjugation of fibrin antibody to emulsion nanoparticles has been successfully applied to image vulnerable plaques.24 Also, conjugation of arginine-glycineaspartic acid (RGD) peptide to emulsion nanoparticles is successfully applied to image integrin, which is up-regulated in many diseases such as cancer and atherosclerosis.81–84 However, owing to the large size and low tissue penetration, nanoparticles may be limited to imaging biomarkers on the blood vessel. Many strategies have been applied to overcome the limitation of extravasation and diffusion barriers for the macromolecule. Bhujwalla and coworkers applied a multistep targeting and prelabeling strategy using antibody-tagged magnetic particles.70,86 EP-3533, a small collagen targeting peptide conjugated with Gd3+-chelates, is able to target to collagen in the collagen-rich scar in liver.76,77 In addition, synthesized small molecules such as peptoid-(Gd)8-dendron were also applied for molecular imaging to vascular endothelial growth factor receptor 2 (VEGFR2), the angiogenesis biomarker highly expressed in tumors.80 However, although great achievement has been made over the last decade, the development of MRI contrast agents for molecular imaging by MRI is largely hampered by relatively low sensitivity compared with PET/SPECT, inadequate perfusion to diseased tissue, instability of the peptide targeting moieties, and targeting specificity and selectivity.
Figures 3(a) and 4(b) show our developed targeted contrast agents against two biomarkers of HER2 and GRPR that are over-expressed in several different types of cancer cell surfaces. We will show our effects on the molecular imaging of cancer biomarkers by targeted ProCAs in the next few sections.
Molecular Imaging of HER-2 Expression Level by Targeted Contrast Agent ProCA1.affi for Breast Cancer Diagnostics
Biomarkers such as the epidermal growth factor receptors EGFR and HER2/Neu are highly expressed in various diseases including breast and ovarian cancers and play important roles in disease progression and survival. HER-2 is a major prognosis biomarker expressed in 30% breast cancer and 60–70% of ductal carcinoma in situ (DCIS) tissue. Monitoring the spatial and temporal changes of several molecular biomarkers such as HER-2/EGFR sharing the same signaling pathway during cancer progression and treatment is the key for understanding the molecular basis of cancers, early and accurate diagnosis, and for developing effective drugs with synergistic effects to treat this deadly disease. They are also major drug targets for targeted therapy. The clinical application of targeted therapy is largely limited because current methods for assessment of these cancer biomarkers involve invasive methods, such as biopsy, and because the effectiveness of the target therapy largely depends on the preselection of patients over-expressing these biomarkers. To date, one of five HER2/Neu clinical tests, including biopsy and immunostaining (IHC, immunihistochemistry) provides incorrect results, leading to improper selection of appropriate patients for personalized treatment using biomarker targeted therapies.87,88 To achieve MRI of HER-2, Bhujwalla and coworkers has applied a three-step strategy for the targeting: biotinylated trastuzumab binds to HER-2, avidin binds to biotin, and bG4D-Gd-SA binds to avidin.70,86 There is an urgent need to develop noninvasive and accurate methods to facilitate diagnosis and selection of patients and to monitor biomarker levels/distribution and their changes upon treatment by targeted drugs.
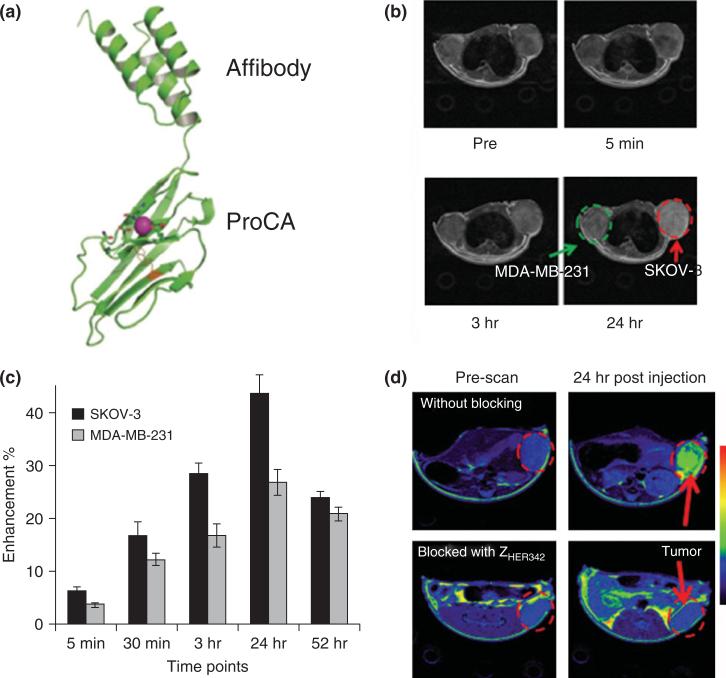
HER-2 targeted contrast agent ProCA1.affi was created by fusing the C-terminal of ProCA1 with a HER-2 affibody. We use HER-2 affibody instead of an antibody for several important reasons. It has a comparable binding affinity to an antibody and strongly and selectively binds to HER-2 with a Kd of 22 pM.89 On the other hand, it has a size of 5.8 kDa which is significantly smaller than antibodies (~150 kDa). The fusion protein, named ProCA1.affi, has a molecular weight of 17 kDa which means ProCA1.affi is more ideal for tumor penetration. Furthermore, the binding site by affibody is different from Herceptin, a therapeutic antibody, allowing a clinician to monitor the change of HER-2 expression during drug treatment. Because ProCA1.affi has high relaxivity, high tumor penetration, and high binding affinity to HER-2, ProCA1.affi can potentially be applied to image HER-2 expression level in tumor.69
Figure 3 shows that ProCA1.affi is able to differentially enhance several cancer cells with different expression levels. We implant tumors with different expression levels in each mouse. SKOV-3 has high HER-2 expression level (106 receptors/cell), whereas MDA-MB-231 tumor has low HER-2 expression level (104receptors/cell). As shown in Figure 3, after tail vein injection of contrast agents with 10-fold lower injection dose than Gd-DTPA, ProCA1.affi can specifically enhance the SKOV-3 tumor which has high expression level of HER-2. To evaluate the specificity of HER-2 enhancement in tumor, HER-2 in xenograft mice were first blocked with HER-2 affibody without contrast agents, then ProCA1.affi was injected for the MRI, no enhancement were found in tumor after blocking (Figure 3(d)). These results support that tumor enhancement is due to ProCA1.affi and HER-2 interaction, and tumor enhancement is not due to blood perfusion, blood vessel permeability, or necrosis. These results indicate that ProCA1.affi can be used to evaluate the expression level of HER-2 biomarkers by the molecular imaging of MRI. ProCA1.affi could be further applied to quantitatively evaluate tumor biomarker expression and receptor occupancy using MRI.69
FIGURE 3.
Molecular imaging of HER-2 by ProCA1.affi. (a) Model structure of ProCA1.Affi. (b) Magnetic resonance imaging (MRI) of HER-2 xenograft tumor (SKOV-3 and MDA-MB-231) before and after injection of ProCA1.affi. (c) Tumor intensity changes over time postinjection of ProCA1.affi. SKOV-3 has much higher HER-2 expression and SOKV-3 has more MRI signal enhancement than that of MDA-MB-231. The MRI signal intensities of SKOV-3 or MDA-MB-231 tumor regions from six adjacent slides were quantified by Image J software. The average signal intensities and the standard derivation were then calculated from these six adjacent slides. (d) MRI of the mouse SKOV3 tumor pre-blocked by affibody ZHER2:342 (bottom) and without blocking (top). (Reprinted with permission from Ref 69. Copyright 2011 PloS ONE)
Optimizing Peptide Targeting Capability to Tumor Biomarkers by Grafting Approach
Fusing or conjugating short peptide fragments with affinity to biomarkers (peptide targeting) has been commonly used in molecular imaging because of its small size and advances in peptide synthesis.28 However, the application of this approach faces two limitations: (1) peptides are more easily degraded by enzymatic cleavage with a short half-life in vivo and (2) the undefined structure from a peptide could decrease binding specificity and binding affinity to the target biomarker. In the effort to improve peptide targeting, we applied a grafting approach to overcome these two limitations (Figure 4(b)).
FIGURE 4.
Design (a, b) and binding test (c) of ProCA1.GRP for the molecular imaging of gastrin-releasing peptide receptor (GRPR). GRP peptide was linked either at C-terminal (a, named ProCA1.GRPC) and in the middle of ProCA1 with grafting approach (b, named ProCA1.GRP(52)). (c) Radioactive binding test of ProCA1, ProCA1.GRPC, and ProCA1.GRP(52) to GRPR high-expression cells (PC3 and DU154) and GRPR low-expression cells (H441). ProCA1.GRP(52) shows the best binding among three contrast agents. (Reprinted with permission from Ref 90. Copyright 2010 Springer)
Gastrin-releasing peptide receptor (GRPR) is a biomarker for many cancers such as prostate, breast, and small cell lung cancer.90,91–93 Its natural peptide ligand, gastrin-releasing peptide (GRP), binds to GRPR with high affinity.94 We use grafting approach to link GRP peptide in the middle of ProCA1 to achieve molecular imaging of GRPR in a prostate tumor model (Figure 4(a) and (b)). As we put peptide in the middle of the ProCA by grafting approach, the targeting peptide is effectively protected from protease degradation. We added flexible linkers to the end of the peptide to give some flexibility to the peptide for targeting. Interestingly, when the peptide is grafted in the middle of ProCA1, much higher targeting capability is observed than when GRP is linked to the C-terminal (Figure 4(c)). Using these strategies, we successfully imaged GRPR in prostate tumor cells through intratumoral injection.90 Moreover, our unpublished data shows that GRP is stable for at least 6 h when it grafted in ProCA1, while literature shows the proGRP (a precursor of GRP containing GRP peptide) decreased by 6–28% after 2 h at room temperature.95 Thus, grafting approach protect peptide from degradation. The grafting approach could be applied to other peptide-based targeting for the molecular imaging of tumor biomarkers.
Size is Essential for Contrast Agents Distribution and Excretion
The locations of cancer biomarkers are diversified. For example, VEGFR is a biomarker expressed on blood vessel surface of a tumor, while HER-2 is a biomarker deeply expressed in a tumor outside of the blood vessel. Thus, the ideal half-life for targeting varies in different biomarkers. In principle, longer circulation gives more time for Gd3+ dissociation and toxicity. Ideally, the contrast agents should have a short blood circulation time if the biomarker is expressed on the blood vessel surface. On the other hand, for molecular imaging of a tumor biomarker deeply expressed outside of the blood vessel, the contrast agents should have sufficient blood half-life to allow the contrast agents to penetrate deeply into the tumor and then quickly be excreted out of the body to reduce the toxicity. ProCA1 in mice has a half time varying from 30 min to hours depending on the surface modification32 and exhibits enhancement in various organs (Figure 2). At time of less than 1 h, an enhancement of the bladder was observed suggesting its renal excretion. Such in vivo behavior and half-life are very different from the minutes timescale observed in small chelators or days from nanopartciples that were uptaken by macrophages and accumulated in the liver and spleen. De Jong et al.96 studied the distribution of gold nanoparticles with a diameter of 10, 50, 100, and 250 nm. After 24 h, 10-nm gold nanoparticles distributed in various organs such as the blood, liver, kidney, spleen, heart, lung, and brain, whereas 50-nm gold nanoparticles mainly distributed in the blood, lung, liver, and spleen. The larger particles with a diameter larger than 50 nm mainly located in the blood, liver, and spleen96. Besides influencing tumor penetration and organ distribution, the size of the contrast agents could also influence renal excretion. A molecule with a molecular size greater than 7 nm in diameter (about 60 kDa) is not readily able to pass through the glomeruli, and the blood half-lives of these molecules in mice are usually much longer than 80 min.97 For example, lysozyme has a molecular weight of 15 kDa and hydrodynamic diameter of 3.4 nm, and the blood half-life is 12 min. When the molecular weight is increased to 152 kDa as an example of IgG, the blood half-life is 330 min. There is an increasing concern about the toxicity of nanoparticles with a size greater than 15 nm since the renal excretion is prevented for these large macromolecules because of the risk of accumulation in the human body.97
Size and Tissue Penetration is Essential for Molecular Imaging of Cancer Biomarkers
Besides high relaxivity and high dose efficiency, the size of the contrast agents is also essential for the molecular imaging of tumor biomarkers. To quantitatively evaluate tumor biomarker expression levels, contrast agents should be first homogeneously distributed in the tumor. Then, the nontargeted contrast agents should be easily washed out of the tumor to avoid unnecessary false positive enhancement under MRI.
Tumors have abnormal blood vasculature compared to normal tissue. The growth of a tumor is much faster than blood vessels; therefore the rapid proliferation of tumor cells forces the blood vessel apart. Tumor and stroma cells also secrete enzymes and growth factors to facilitate the formation of new blood vessels and extracellular matrix. The extracellular matrix could slow down the penetration of the molecule to the inside of the tumor. Furthermore, high interstitial fluid pressure in the tumor forms a barrier for drug and imaging reagent penetration to the tumor from the blood vessel.98 Enhanced permeability and retention (EPR) effects show that a molecule with a molecular size from 40 kDa to more than 778 kDa can accumulate in the tumor with high concentration.99 However, the optimized size for imaging tumor biomarkers could be much smaller. To evaluate tumor biomarkers outside of the blood vessel, the ideal contrast agents should be able to first efficiently penetrate to the tumor and then the unbounded form should be quickly washed away. Proteins with molecular weight less than 40 kDa could be quickly washed out from tumor. What is the possible good size for imaging reagents have efficient tumor penetration? Dreher et al.100 used fluorescent labeled dextrans with a molecular weight difference between 3.3 kDa to 2 MDa to evaluate the role of the drug size on the tumor accumulation and penetration. Consistent with EPR effects, 40–70 kDa dextrans has the highest accumulation in tumors within 30 min. However, the dextrans with a molecular weight larger than 40 kDa are mainly accumulated along the blood vessel of the tumor. Dextrans with a molecular weight of 4.7 and 10 kDa can become deeply (larger than 35 μm) distributed in the tumor within 30 min, whereas 2-MDa dextrans can only penetrate 5 μm.100 Thus, imaging reagents with a large size are not ideal for MRI imaging of tumors. Currently, MRI molecular imaging using Gd3+-based nanopaticles mainly target to cardiovascular and tumor vasculature biomarkers such as fibrin and integrin22,24,70,76–82,84,83,85 with limited penetration to tumor tissue. The molecular imaging of MRI to evaluate tumor biomarkers is limited by the lack of MRI contrast agents with high dose efficiency, high tissue penetration, proper blood retention time, and good renal excretion profile.
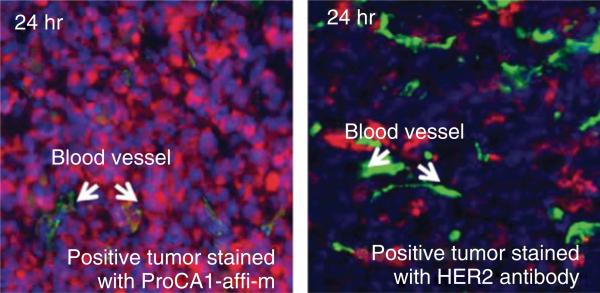
To test the penetration of contrast agents in tumors, we linked the HER-2 targeting moiety, affibody, to ProCA (named ProCA1.affi) and then compared the tumor penetration of antibody (MW about 150 kDa) and ProCA1.affi (MW about 17 kDa) at different time points. We iv-injected either HER-2 antibody or ProCA1.affi into the SKOV-3 tumor-bearing mice. ProCA1.affi can be stained in the tumor after 4-h postinjection. On the contrary, no antibody in tumors can be stained by immunofluorescence at the same time point. ProCA.affi are evenly distributed in tumors with high concentration 24-h postinjection, whereas the antibody is only localized in the region near the blood vessel with much lower accumulation (Figure 5). These results indicate that ProCA1.affi exhibits unprecedented tumor penetration, and such unique features have great potential for quantitative/semiquantitative evaluation of biomarker changes during disease progression and drug treatment.
FIGURE 5.
Immunofluoresence imaging of ProCA1.affi (left) or HER-2 antibody (right) in SKOV-3 xenograft tumors in mice after IV injection. ProCA1.affi and HER-2 antibody are stained with red color. Blood vessel is stained with green color. ProCA1.affi is evenly distributed in tumor 24-h postinjection, whereas HER-2 antibody only accumulated in near the blood vessel. (Reprinted with permission from Ref 69. Copyright 2011 PloS ONE)
CONCLUSION AND PERSPECTIVES
The application of MRI for molecular imaging is limited by the lack of MRI contrast agents with high relaxivity for sensitivity, biomarker selectivity, good tissue penetration, and in vivo properties. We have created a novel class of contrast agents by designing a Gd3+ binding pocket into a protein scaffold. High relaxivities of ProCA are achieved by tuning τR, q, and second and outer sphere relaxivity.
To avoid large molecular size, we graft affibody or peptide instead of an antibody to our contrast agents to target cancer biomarkers. We also carefully designed the proper protein size to optimize blood retention time and tumor penetration. The molecular imaging of several tumor biomarkers is achieved by our careful design of targeted ProCA. Currently, ProCAs have been developed to target HER-2 and GRPR which are over-expressed in many types of cancers including breast and prostate cancers. However, ProCA certainly is not limited to these two biomarkers. Theoretically, ProCAs could be targeted to various over-expressed biomarkers due to the ease of grafting targeting moieties onto the protein structure.
With the advantage of spatial resolution and depth penetration, MRI has been applied in both preclinical drug development and clinical diagnostics. The high relaxivity and organ distribution feature of nontargeted ProCA make it a promising tool for imaging tumor metastasis with high sensitivity, specificity, and dose efficiently. Owing to the longer blood retention time and slower tumor penetration than clinical contrast agents, nontargeted ProCA can also be applied to DCE-MRI to evaluate tumor vasculature with increased accuracy. Targeted imaging by MRI has great potential to evaluate the drug effects on cancer biomarkers changes with high accuracy. Our results show that targeted ProCA is able to semiquantitatively differentiate biomarker expression levels, which is a great advantage for semiquantitative evaluation of biomarker expression level changes in preclinical disease models after drug treatment. The repetitive administration of targeted ProCA in the same animal in preclinical studies could potentially save the cost of animals and enable tracking of biomarker changes over time in small animal studies. Besides, targeted ProCA may have a potential application to clinical diagnostics for patient selection and monitoring drug treatment.
ACKNOWLEDGMENTS
We thank Dr. Xiaoping Hu, Katheryn Lee Meenach, and Natalie White for their critical reviews of the manuscript. This work is supported in part by research grants from NIH EB007268, GM62999, and CA118113 to Jenny J. Yang and Zhi-Ren Liu, and Molecular Basis of Disease (MBD) fellowship for Shenghui Xue.
REFERENCES
- 1.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caruthers SD, Winter PM, Wickline SA, Lanza GM. Targeted magnetic resonance imaging contrast agents. Methods Mol Med. 2006;124:387–400. doi: 10.1385/1-59745-010-3:387. [DOI] [PubMed] [Google Scholar]
- 3.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 4.Gore JC, Manning HC, Quarles CC, Waddell KW, Yankeelov TE. Magnetic resonance in the era of molecular imaging of cancer. Magn Reson Imaging. 2011;29:587–600. doi: 10.1016/j.mri.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002;179:1193–1199. doi: 10.2214/ajr.179.5.1791193. [DOI] [PubMed] [Google Scholar]
- 6.Kim YR, Savellano MD, Weissleder R, Bogdanov A., Jr Steady-state and dynamic contrast MR imaging of human prostate cancer xenograft tumors: a comparative study. Technol Cancer Res Treat. 2002;1:489–495. doi: 10.1177/153303460200100609. [DOI] [PubMed] [Google Scholar]
- 7.Raghunand N, Howison C, Sherry AD, Zhang S, Gillies RJ. Renal and systemic pH imaging by contrast-enhanced MRI. Magn Reson Med. 2003;49:249–257. doi: 10.1002/mrm.10347. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Su MY, Nalcioglu O. Applications of dynamic contrast enhanced MRI in oncology: measurement of tumor oxygen tension. Technol Cancer Res Treat. 2002;1:29–38. doi: 10.1177/153303460200100104. [DOI] [PubMed] [Google Scholar]
- 9.Mueller GC, Hussain HK, Carlos RC, Nghiem HV, Francis IR. Effectiveness of MR imaging in characterizing small hepatic lesions: routine versus expert interpretation. AJR Am J Roentgenol. 2003;180:673–680. doi: 10.2214/ajr.180.3.1800673. [DOI] [PubMed] [Google Scholar]
- 10.Hu X, Norris DG. Advances in high-field magnetic resonance imaging. Annu Rev Biomed Eng. 2004;6:157–184. doi: 10.1146/annurev.bioeng.6.040803.140017. [DOI] [PubMed] [Google Scholar]
- 11.Petiet AE, Kaufman MH, Goddeeris MM, Brandenburg J, Elmore SA, Johnson GA. High-resolution magnetic resonance histology of the embryonic and neonatal mouse: a 4D atlas and morphologic database. Proc Natl Acad Sci U S A. 2008;105:12331–12336. doi: 10.1073/pnas.0805747105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yankeelov TE, Gore JC. Dynamic contrast enhanced magnetic resonance imaging in oncology: theory, data acquisition, analysis, and examples. Curr Med Imaging Rev. 2009;3:91–107. doi: 10.2174/157340507780619179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RA, Votaw JR, Salman K, Sharma P, Lurie C, Kalb B, Martin DR. Magnetic resonance imaging evaluation of renal structure and function related to disease: technical review of image acquisition, postprocessing, and mathematical modeling steps. J Magn Reson Imaging. 2011;33:1270–1283. doi: 10.1002/jmri.22335. [DOI] [PubMed] [Google Scholar]
- 14.Major JL, Meade TJ. Bioresponsive, cell-penetrating, and multimeric MR contrast agents. Acc Chem Res. 2009;42:893–903. doi: 10.1021/ar800245h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57:2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Atanasijevic T, Shusteff M, Fam P, Jasanoff A. Calcium-sensitive MRI contrast agents based on superparamagnetic iron oxide nanoparticles and calmodulin. Proc Natl Acad Sci U S A. 2006;103:14707–14712. doi: 10.1073/pnas.0606749103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo B, Pagel MD. A PARACEST MRI contrast agent to detect enzyme activity. J Am Chem Soc. 2006;128:14032–14033. doi: 10.1021/ja063874f. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro MG, Westmeyer GG, Romero PA, Szablowski JO, Kuster B, Shah A, Otey CR, Langer R, Arnold FH, Jasanoff A. Directed evolution of a magnetic resonance imaging contrast agent for noninvasive imaging of dopamine. Nat Biotechnol. 2010;28:264–270. doi: 10.1038/nbt.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta A, Raymond KN. Gd-hydroxypyridinone (HOPO)-based high-relaxivity magnetic resonance imaging (MRI) contrast agents. Acc Chem Res. 2009;42:938–947. doi: 10.1021/ar800250h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geraldes CF, Laurent S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol Imaging. 2009;4:1–23. doi: 10.1002/cmmi.265. [DOI] [PubMed] [Google Scholar]
- 21.Hanaoka K, Lubag AJ, Castillo-Muzquiz A, Kodadek T, Sherry AD. The detection limit of a Gd3+-based T1 agent is substantially reduced when targeted to a protein microdomain. Magn Reson Imaging. 2008;26:608–617. doi: 10.1016/j.mri.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Z, Thorek DL, Tsourkas A. Gadoliniumconjugated dendrimer nanoclusters as a tumor-targeted T1 magnetic resonance imaging contrast agent. Angew Chem Int Ed Engl. 2010;49:346–350. doi: 10.1002/anie.200905133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianolio E, Porto S, Napolitano R, Baroni S, Giovenzana GB, Aime S. Relaxometric investigations and MRI evaluation of a liposome-loaded pH-responsive gadolinium(III) complex. Inorg Chem. 2012;51:7210–7217. doi: 10.1021/ic300447n. [DOI] [PubMed] [Google Scholar]
- 24.Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M, Winter P, Sicard GA, Gaffney PJ, Wick-line SA, et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation. 2001;104:1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 25.Datta A, Hooker JM, Botta M, Francis MB, Aime S, Raymond KN. High relaxivity gadolinium hydroxypyridonate-viral capsid conjugates: nano-sized MRI contrast agents. J Am Chem Soc. 2008;130:2546–2552. doi: 10.1021/ja0765363. [DOI] [PubMed] [Google Scholar]
- 26.Richard C, Doan BT, Beloeil JC, Bessodes M, Toth E, Scherman D. Noncovalent functionalization of carbon nanotubes with amphiphilic gd3+ chelates: toward powerful t1 and t2 MRI contrast agents. Nano Lett. 2008;8:232–236. doi: 10.1021/nl072509z. [DOI] [PubMed] [Google Scholar]
- 27.Caravan P, Greenwood JM, Welch JT, Franklin SJ. Gadolinium-binding helix-turn-helix peptides: DNA-dependent MRI contrast agents. Chem Commun (Camb) 2003:2574–2575. doi: 10.1039/b307817e. [DOI] [PubMed] [Google Scholar]
- 28.Caravan P. Protein-targeted gadolinium-based magnetic resonance imaging (MRI) contrast agents: design and mechanism of action. Acc Chem Res. 2009;42:851–862. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 29.De Leon-Rodriguez LM, Lubag AJ, Malloy CR, Martinez GV, Gillies RJ, Sherry AD. Responsive MRI agents for sensing metabolism in vivo. Acc Chem Res. 2009;42:948–957. doi: 10.1021/ar800237f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Alternatives to gadolinium-based metal chelates for magnetic resonance imaging. Chem Rev. 2010;110:2960–3018. doi: 10.1021/cr900284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terreno E, Castelli DD, Viale A, Aime S. Challenges for molecular magnetic resonance imaging. Chem Rev. 2010;110:3019–3042. doi: 10.1021/cr100025t. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Jiang J, Zou J, Qiao J, Xue S, Wei L, Long R, Wang L, Castiblanco A, White N, et al. PEGylation of protein-based MRI contrast agents improves relaxivities and biocompatibilities. J Inorg Biochem. 2012;107:111–118. doi: 10.1016/j.jinorgbio.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toth E, Helm L, Merbach AE. Relaxivity of MRI contrast agents. In: Krause W, editor. Contrast Agents: Magnetic Resonance Imaging. Vol. 221. Springer-Verlag; New York: 2002. pp. 61–102. [Google Scholar]
- 34.Solomon I. Relaxation processes in a system of two spins. Phys Rev. 1955;99:559–565. [Google Scholar]
- 35.Bloembergen N. Proton relaxation times in paramagnetic solutions. J Chem Phys. 1957;27:572–573. [Google Scholar]
- 36.Bloembergen N, Morgan R. Theory of proton relaxation by Mn2+ ions in solution. J Chem Phys. 1961;34:842. [Google Scholar]
- 37.McLachlan AD. Line widths of electron resonance spectra in solution. Proc R Soc Lond A. 1964;280:271–288. [Google Scholar]
- 38.Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 39.Laurent S, Elst LV, Muller RN. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol Imaging. 2006;1:128–137. doi: 10.1002/cmmi.100. [DOI] [PubMed] [Google Scholar]
- 40.Villaraza AJ, Bumb A, Brechbiel MW. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: the interplay between size, function, and pharmacokinetics. Chem Rev. 2010;110:2921–2959. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karfeld-Sulzer LS, Waters EA, Davis NE, Meade TJ, Barron AE. Multivalent protein polymer MRI contrast agents: controlling relaxivity via modulation of amino acid sequence. Biomacromolecules. 2010;11:1429–1436. doi: 10.1021/bm901378a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wikstrom MG, Moseley ME, White DL, Dupon JW, Winkelhake JL, Kopplin J, Brasch RC. Contrast-enhanced MRI of tumors. Comparison of Gd-DTPA and a macromolecular agent. Invest Radiol. 1989;24:609–615. doi: 10.1097/00004424-198908000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Brasch R, Pham C, Shames D, Roberts T, van Dijke K, van Bruggen N, Mann J, Ostrowitzki S, Melnyk O. Assessing tumor angiogenesis using macromolecular MR imaging contrast media. J Magn Reson Imaging. 1997;7:68–74. doi: 10.1002/jmri.1880070110. [DOI] [PubMed] [Google Scholar]
- 44.Caravan P, Parigi G, Chasse JM, Cloutier NJ, Ellison JJ, Lauffer RB, Luchinat C, McDermid SA, Spiller M, McMurry TJ. Albumin binding, relaxivity, and water exchange kinetics of the diastereoisomers of MS-325, a gadolinium(III)-based magnetic resonance angiography contrast agent. Inorg Chem. 2007;46:6632–6639. doi: 10.1021/ic700686k. [DOI] [PubMed] [Google Scholar]
- 45.Bryant LH, Jr, Brechbiel MW, Wu C, Bulte JW, Herynek V, Frank JA. Synthesis and relaxometry of high-generation (G = 5, 7, 9, and 10) PAMAM dendrimer-DOTA-gadolinium chelates. J Magn Reson Imaging. 1999;9:348–352. doi: 10.1002/(sici)1522-2586(199902)9:2<348::aid-jmri30>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 46.Helm L, Merbach AE. Inorganic and bioinorganic solvent exchange mechanisms. Chem Rev. 2005;105:1923–1959. doi: 10.1021/cr030726o. [DOI] [PubMed] [Google Scholar]
- 47.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 48.Doble DM, Botta M, Wang J, Aime S, Barge A, Raymond KN. Optimization of the relaxivity of MRI contrast agents: effect of poly(ethylene glycol) chains on the water-exchange rates of Gd(III) complexes. J Am Chem Soc. 2001;123:10758–10759. doi: 10.1021/ja011085m. [DOI] [PubMed] [Google Scholar]
- 49.Caravan P, Greenfield MT, Li X, Sherry AD. The Gd(3+) complex of a fatty acid analogue of DOTP binds to multiple albumin sites with variable water relaxivities. Inorg Chem. 2001;40:6580–6587. doi: 10.1021/ic0102900. [DOI] [PubMed] [Google Scholar]
- 50.LaConte LE, Nitin N, Zurkiya O, Caruntu D, O'Connor CJ, Hu X, Bao G. Coating thickness of magnetic iron oxide nanoparticles affects R2 relaxivity. J Magn Reson Imaging. 2007;26:1634–1641. doi: 10.1002/jmri.21194. [DOI] [PubMed] [Google Scholar]
- 51.Tong S, Hou S, Zheng Z, Zhou J, Bao G. Coating optimization of superparamagnetic iron oxide nanoparticles for high T2 relaxivity. Nano Lett. 2010;10:4607–4613. doi: 10.1021/nl102623x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 53.Esqueda AC, Lopez JA, Andreu-de-Riquer G, Alvarado-Monzon JC, Ratnakar J, Lubag AJ, Sherry AD, De Leon-Rodriguez LM. A new gadolinium-based MRI zinc sensor. J Am Chem Soc. 2009;131:11387–11391. doi: 10.1021/ja901875v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhingra K, Maier ME, Beyerlein M, Angelovski G, Logothetis NK. Synthesis and characterization of a smart contrast agent sensitive to calcium. Chem Commun (Camb) 2008:3444–3446. doi: 10.1039/b801975d. [DOI] [PubMed] [Google Scholar]
- 55.Li W-h, Fraser SE, Meade TJ. A calcium-sensitive magnetic resonance imaging contrast agent. J Am Chem Soc. 1999;121:1413–1414. [Google Scholar]
- 56.Pierre VC, Botta M, Aime S, Raymond KN. Tuning the coordination number of hydroxypyridonate-based gadolinium complexes: implications for MRI contrast agents. J Am Chem Soc. 2006;128:5344–5345. doi: 10.1021/ja057805x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liepold L, Anderson S, Willits D, Oltrogge L, Frank JA, Douglas T, Young M. Viral capsids as MRI contrast agents. Magn Reson Med. 2007;58:871–879. doi: 10.1002/mrm.21307. [DOI] [PubMed] [Google Scholar]
- 58.Yang W, Jones LM, Isley L, Ye Y, Lee HW, Wilkins A, Liu ZR, Hellinga HW, Malchow R, Ghazi M, et al. Rational design of a calcium-binding protein. J Am Chem Soc. 2003;125:6165–6171. doi: 10.1021/ja034724x. [DOI] [PubMed] [Google Scholar]
- 59.Yang W, Wilkins AL, Li S, Ye Y, Yang JJ. The effects of Ca2+ binding on the dynamic properties of a designed Ca2+-binding protein. Biochemistry. 2005;44:8267–8273. doi: 10.1021/bi050463n. [DOI] [PubMed] [Google Scholar]
- 60.Yang W, Wilkins AL, Ye Y, Liu ZR, Li SY, Urbauer JL, Hellinga HW, Kearney A, van der Merwe PA, Yang JJ. Design of a calcium-binding protein with desired structure in a cell adhesion molecule. J Am Chem Soc. 2005;127:2085–2093. doi: 10.1021/ja0431307. [DOI] [PubMed] [Google Scholar]
- 61.Maniccia AW, Yang W, Li SY, Johnson JA, Yang JJ. Using protein design to dissect the effect of charged residues on metal binding and protein stability. Biochemistry. 2006;45:5848–5856. doi: 10.1021/bi052508q. [DOI] [PubMed] [Google Scholar]
- 62.Li S, Yang W, Maniccia AW, Barrow D, Jr, Tjong H, Zhou HX, Yang JJ. Rational design of a conformationswitchable Ca2+- and Tb3+-binding protein without the use of multiple coupled metal-binding sites. FEBS J. 2008;275:5048–5061. doi: 10.1111/j.1742-4658.2008.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones LM, Yang W, Maniccia AW, Harrison A, van der Merwe PA, Yang JJ. Rational design of a novel calcium-binding site adjacent to the ligand-binding site on CD2 increases its CD48 affinity. Protein Sci. 2008;17:439–449. doi: 10.1110/ps.073328208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang JJ, Yang J, Wei L, Zurkiya O, Yang W, Li S, Zou J, Zhou Y, Maniccia AL, Mao H, et al. Rational design of protein-based MRI contrast agents. J Am Chem Soc. 2008;130:9260–9267. doi: 10.1021/ja800736h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pidcock E, Moore GR. Structural characteristics of protein binding sites for calcium and lanthanide ions. J Biol Inorg Chem. 2001;6:479–489. doi: 10.1007/s007750100214. [DOI] [PubMed] [Google Scholar]
- 66.Kirberger M, Wang X, Zhao K, Tang S, Chen G, Yang JJ. Integration of diverse research methods to analyze and engineer Ca-binding proteins: from prediction to production. Curr Bioinform. 2010;5:68–80. doi: 10.2174/157489310790596358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirberger M, Yang JJ. Structural differences between Pb2+- and Ca2+-binding sites in proteins: implications with respect to toxicity. J Inorg Biochem. 2008;102:1901–1909. doi: 10.1016/j.jinorgbio.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 69.Qiao J, Li S, Wei L, Jiang J, Long R, Mao H, Wang L, Yang H, Grossniklaus HE, Liu ZR, et al. HER2 targeted molecular MR imaging using a de novo designed protein contrast agent. PLoS One. 2011;6:e18103. doi: 10.1371/journal.pone.0018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu W, Okollie B, Bhujwalla ZM, Artemov D. PAMAM dendrimer-based contrast agents for MR imaging of Her-2/neu receptors by a three-step pretargeting approach. Magn Reson Med. 2008;59:679–685. doi: 10.1002/mrm.21508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wild JM, Woodrow J, van Beek EJ, Misselwitz B, Johnson R. Evaluation of rHA labeled with Gd-DTPA for blood pool imaging and targeted contrast delivery. Contrast Media Mol Imaging. 2010;5:39–43. doi: 10.1002/cmmi.366. [DOI] [PubMed] [Google Scholar]
- 72.Schneider G, Pasowicz M, Vymazal J, Seidl Z, Aschauer M, Konopka M, Bilecen D, Iezzi R, Ballarati C. Gadobenate dimeglumine and gadofosveset trisodium for MR angiography of the renal arteries: multicenter intraindividual crossover comparison. AJR Am J Roentgenol. 2010;195:476–485. doi: 10.2214/AJR.09.3868. [DOI] [PubMed] [Google Scholar]
- 73.Misselwitz B, Schmitt-Willich H, Ebert W, Frenzel T, Weinmann HJ. Pharmacokinetics of Gadomer-17, a new dendritic magnetic resonance contrast agent. MAGMA. 2001;12:128–134. doi: 10.1007/BF02668094. [DOI] [PubMed] [Google Scholar]
- 74.Kaneshiro TL, Jeong EK, Morrell G, Parker DL, Lu ZR. Synthesis and evaluation of globular Gd-DOTA-monoamide conjugates with precisely controlled nano-sizes for magnetic resonance angiography. Biomacro-molecules. 2008;9:2742–2748. doi: 10.1021/bm800486c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alric C, Taleb J, Le Duc G, Mandon C, Billotey C, Le Meur-Herland A, Brochard T, Vocanson F, Janier M, Perriat P, et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J Am Chem Soc. 2008;130:5908–5915. doi: 10.1021/ja078176p. [DOI] [PubMed] [Google Scholar]
- 76.Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun WC, et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew Chem Int Ed Engl. 2007;46:8171–8173. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 77.Polasek M, Fuchs BC, Uppal R, Schuhle DT, Alford JK, Loving GS, Yamada S, Wei L, Lauwers GY, Guimaraes AR, et al. Molecular MR imaging of liver fibrosis: a feasibility study using rat and mouse models. J Hepatol. 2012 doi: 10.1016/j.jhep.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spuentrup E, Ruhl KM, Botnar RM, Wiethoff AJ, Buhl A, Jacques V, Greenfield MT, Krombach GA, Gunther RW, Vangel MG, et al. Molecular magnetic resonance imaging of myocardial perfusion with EP-3600, a collagen-specific contrast agent: initial feasibility study in a swine model. Circulation. 2009;119:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.826388. [DOI] [PubMed] [Google Scholar]
- 79.Burtea C, Laurent S, Port M, Lancelot E, Ballet S, Rousseaux O, Toubeau G, Vander Elst L, Corot C, Muller RN. Magnetic resonance molecular imaging of vascular cell adhesion molecule-1 expression in inflammatory lesions using a peptide-vectorized paramagnetic imaging probe. J Med Chem. 2009;52:4725–4742. doi: 10.1021/jm9002654. [DOI] [PubMed] [Google Scholar]
- 80.De Leon-Rodriguez LM, Lubag A, Udugamasooriya DG, Proneth B, Brekken RA, Sun X, Kodadek T, Dean Sherry A. MRI detection of VEGFR2 in vivo using a low molecular weight peptoid-(Gd)8-dendron for targeting. J Am Chem Soc. 2010;132:12829–12831. doi: 10.1021/ja105563a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmieder AH, Winter PM, Caruthers SD, Harris TD, Williams TA, Allen JS, Lacy EK, Zhang H, Scott MJ, Hu G, et al. Molecular MR imaging of melanoma angiogenesis with αvβ3-targeted paramagnetic nanoparticles. Magn Reson Med. 2005;53:621–627. doi: 10.1002/mrm.20391. [DOI] [PubMed] [Google Scholar]
- 82.Boles KS, Schmieder AH, Koch AW, Carano RA, Wu Y, Caruthers SD, Tong RK, Stawicki S, Hu G, Scott MJ, et al. MR angiogenesis imaging with Robo4-vs. αVβ3-targeted nanoparticles in a B16/F10 mouse melanoma model. FASEB J. 2010;24:4262–4270. doi: 10.1096/fj.10-157933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Winter PM, Caruthers SD, Kassner A, Harris TD, Chinen LK, Allen JS, Lacy EK, Zhang H, Robertson JD, Wickline SA, et al. Molecular imaging of angio-genesis in nascent Vx-2 rabbit tumors using a novel α(v)β3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res. 2003;63:5838–5843. [PubMed] [Google Scholar]
- 84.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with α(v)β3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 85.Ouimet T, Lancelot E, Hyafil F, Rienzo M, Deux F, Lemaitre M, Duquesnoy S, Garot J, Roques BP, Michel JB, et al. Molecular and cellular targets of the MRI contrast agent P947 for atherosclerosis imaging. Mol Pharm. 2012;9:850–861. doi: 10.1021/mp2003863. [DOI] [PubMed] [Google Scholar]
- 86.Artemov D, Mori N, Ravi R, Bhujwalla ZM. Magnetic resonance molecular imaging of the HER-2/neu receptor. Cancer Res. 2003;63:2723–2727. [PubMed] [Google Scholar]
- 87.Morse DL, Gillies RJ. Molecular imaging and targeted therapies. Biochem Pharmacol. 2010;80:731–738. doi: 10.1016/j.bcp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allison M. The HER2 testing conundrum. Nat Biotechnol. 2010;28:117–119. doi: 10.1038/nbt0210-117. [DOI] [PubMed] [Google Scholar]
- 89.Orlova A, Magnusson M, Eriksson TL, Nilsson M, Larsson B, Hoiden-Guthenberg I, Widstrom C, Carlsson J, Tolmachev V, Stahl S, et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006;66:4339–4348. doi: 10.1158/0008-5472.CAN-05-3521. [DOI] [PubMed] [Google Scholar]
- 90.Wei L, Li S, Yang J, Ye Y, Zou J, Wang L, Long R, Zurkiya O, Zhao T, Johnson J, et al. Protein-based MRI contrast agents for molecular imaging of prostate cancer. Mol Imaging Biol. 2011;13:416–423. doi: 10.1007/s11307-010-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Emonds KM, Swinnen JV, Mortelmans L, Mottaghy FM. Molecular imaging of prostate cancer. Methods. 2009;48:193–199. doi: 10.1016/j.ymeth.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 92.Reubi JC, Maecke HR. Peptide-based probes for cancer imaging. J Nucl Med. 2008;49:1735–1738. doi: 10.2967/jnumed.108.053041. [DOI] [PubMed] [Google Scholar]
- 93.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jensen JA, Carroll RE, Benya RV. The case for gastrin-releasing peptide acting as a morphogen when it and its receptor are aberrantly expressed in cancer. Peptides. 2001;22:689–699. doi: 10.1016/s0196-9781(01)00380-1. [DOI] [PubMed] [Google Scholar]
- 95.Yoshimura T, Fujita K, Kawakami S, Takeda K, Chan S, Beligere G, Dowell B. Stability of pro-gastrin-releasing peptide in serum versus plasma. Tumour Biol. 2008;29:224–230. doi: 10.1159/000152940. [DOI] [PubMed] [Google Scholar]
- 96.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 97.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heldin CH, Rubin K, Pietras K, Ostman A. High inter-stitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 99.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]