Abstract
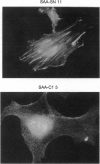
An increasingly large number of proteins involved in signal transduction have been identified in recent years and shown to control different steps of cell survival, proliferation, and differentiation. Among the genes recently identified at the tip of the long arm of the human X chromosome, a novel gene, C1, encodes a protein that appears to represent a newly discovered member of the group of signaling proteins involved in regulation of the small GTP binding proteins of the ras superfamily. The protein encoded by C1, p115, is synthesized predominantly in cells of hematopoietic origin. It is characterized by two regions of similarity to motifs present in known proteins: GAP and SH3 homologous regions. Its localization in a narrow cytoplasmic region just below the plasma membrane and its inhibitory effect on stress fiber organization indicate that p115 may down regulate rho-like GTPases in hematopoietic cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bione S., Maestrini E., Rivella S., Mancini M., Regis S., Romeo G., Toniolo D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994 Dec;8(4):323–327. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- Bione S., Tamanini F., Maestrini E., Tribioli C., Poustka A., Torri G., Rivella S., Toniolo D. Transcriptional organization of a 450-kb region of the human X chromosome in Xq28. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10977–10981. doi: 10.1073/pnas.90.23.10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguski M. S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993 Dec 16;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Cohen G. B., Ren R., Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995 Jan 27;80(2):237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Frohman M. A. Rapid amplification of complementary DNA ends for generation of full-length complementary DNAs: thermal RACE. Methods Enzymol. 1993;218:340–356. doi: 10.1016/0076-6879(93)18026-9. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Koide H. B., Hemmings B. A. Pleckstrin domain homology. Nature. 1993 May 27;363(6427):309–310. doi: 10.1038/363309b0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche N., Hall A. GAPs for rho-related GTPases. Trends Genet. 1994 Dec;10(12):436–440. doi: 10.1016/0168-9525(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Lancaster C. A., Taylor-Harris P. M., Self A. J., Brill S., van Erp H. E., Hall A. Characterization of rhoGAP. A GTPase-activating protein for rho-related small GTPases. J Biol Chem. 1994 Jan 14;269(2):1137–1142. [PubMed] [Google Scholar]
- Lanfrancone L., Pelicci G., Brizzi M. F., Aronica M. G., Casciari C., Giuli S., Pegoraro L., Pawson T., Pelicci P. G., Arouica M. G. Overexpression of Shc proteins potentiates the proliferative response to the granulocyte-macrophage colony-stimulating factor and recruitment of Grb2/SoS and Grb2/p140 complexes to the beta receptor subunit. Oncogene. 1995 Mar 2;10(5):907–917. [PubMed] [Google Scholar]
- Lim W. A., Richards F. M., Fox R. O. Structural determinants of peptide-binding orientation and of sequence specificity in SH3 domains. Nature. 1994 Nov 24;372(6504):375–379. doi: 10.1038/372375a0. [DOI] [PubMed] [Google Scholar]
- Maestrini E., Tamanini F., Kioschis P., Gimbo E., Marinelli P., Tribioli C., D'Urso M., Palmieri G., Poustka A., Toniolo D. An archipelago of CpG islands in Xq28: identification and fine mapping of 20 new CpG islands of the human X chromosome. Hum Mol Genet. 1992 Jul;1(4):275–280. doi: 10.1093/hmg/1.4.275. [DOI] [PubMed] [Google Scholar]
- McCormick F. Activators and effectors of ras p21 proteins. Curr Opin Genet Dev. 1994 Feb;4(1):71–76. doi: 10.1016/0959-437x(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Mellström K., Heldin C. H., Westermark B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp Cell Res. 1988 Aug;177(2):347–359. doi: 10.1016/0014-4827(88)90468-5. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Saraste M., Wilmanns M. High-resolution crystal structures of tyrosine kinase SH3 domains complexed with proline-rich peptides. Nat Struct Biol. 1994 Aug;1(8):546–551. doi: 10.1038/nsb0894-546. [DOI] [PubMed] [Google Scholar]
- Pawson T., Hunter T. Signal transduction and growth control in normal and cancer cells. Curr Opin Genet Dev. 1994 Feb;4(1):1–4. doi: 10.1016/0959-437x(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994 Feb;4(1):25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Tribioli C., Mancini M., Plassart E., Bione S., Rivella S., Sala C., Torri G., Toniolo D. Isolation of new genes in distal Xq28: transcriptional map and identification of a human homologue of the ARD1 N-acetyl transferase of Saccharomyces cerevisiae. Hum Mol Genet. 1994 Jul;3(7):1061–1067. doi: 10.1093/hmg/3.7.1061. [DOI] [PubMed] [Google Scholar]
- Tribioli C., Tamanini F., Patrosso C., Milanesi L., Villa A., Pergolizzi R., Maestrini E., Rivella S., Bione S., Mancini M. Methylation and sequence analysis around EagI sites: identification of 28 new CpG islands in XQ24-XQ28. Nucleic Acids Res. 1992 Feb 25;20(4):727–733. doi: 10.1093/nar/20.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]