Abstract
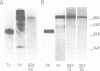
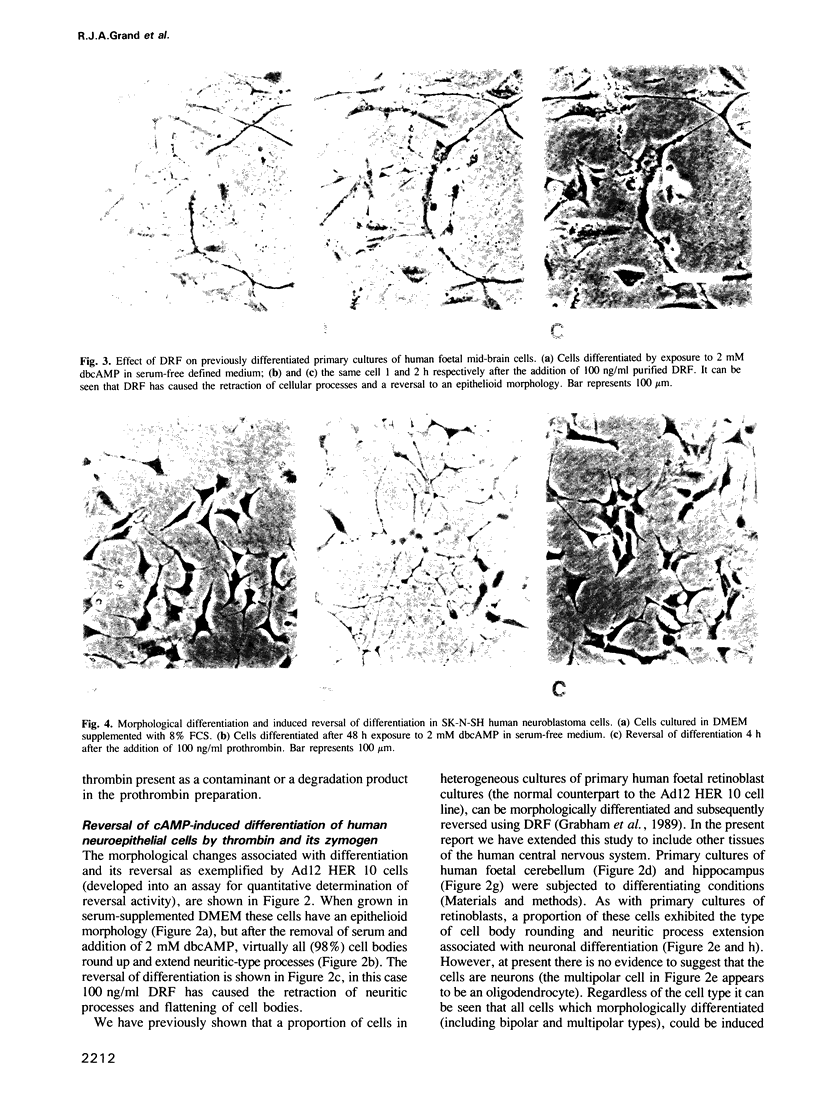
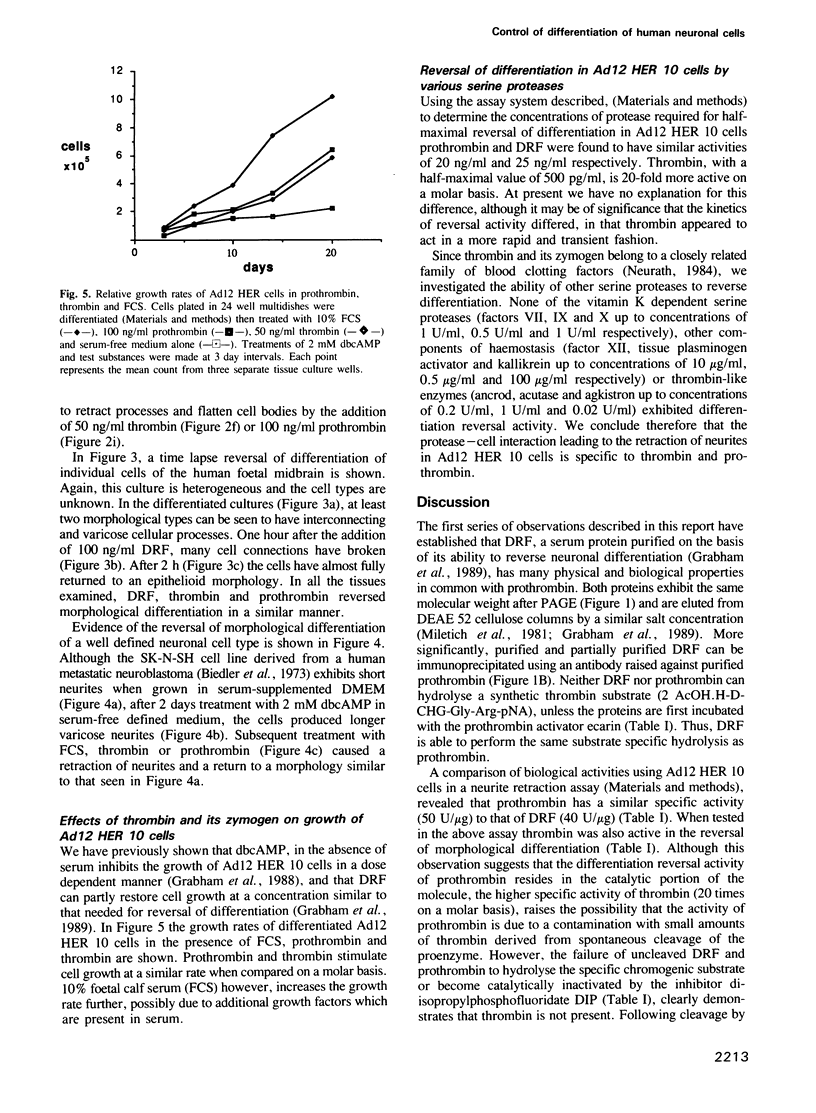
We have previously shown that a serum protein, termed differentiation reversal factor (DRF), is responsible for neurite retraction in differentiated cultures of an adenovirus 12 (Ad12) transformed human retinoblast cell line. Data is presented here to show that DRF is identical to the serine protease prothrombin. Both proteins have been immunoprecipitated using an antibody raised against purified prothrombin and have been shown to hydrolyse a specific thrombin substrate only after activation by the snake venom ecarin. Following addition to Ad12 HER 10 cells, which had previously been differentiated by culture in the presence of 2 mM dibutyryl cAMP in serum-free medium, thrombin and prothrombin caused half-maximal retraction of neurites at concentrations of 0.5 ng/ml and 20 ng/ml respectively. Interestingly, activation of prothrombin was shown to be unnecessary for biological activity. Using the inhibitor di-isopropylfluorophosphate (DIP), we have shown that abrogation of the proteolytic activity of thrombin also results in a loss (greater than 2000 fold) of differentiation reversal activity. Thrombin and its zymogen both stimulated the mitosis of differentiated Ad12 HER 10 cells to a similar extent. In addition, differentiation reversal was highly specific since, at physiologically significant concentrations, closely related serine proteases did not cause neurite retraction. Prothrombin and thrombin also reversed morphological differentiation in the SK-N-SH neuroblastoma cell line and in heterogeneous cultures of cells from various regions in the human foetal brain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Walker P. S., Fellows R. E. Properties of neurons from dissociated fetal rat brain in serum-free culture. J Neurosci. 1983 Dec;3(12):2448–2462. doi: 10.1523/JNEUROSCI.03-12-02448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Bruhn H. D., Bernsmeier R., Lück P., Zurborn K. H., Christophers E. Influences of thrombin, factor XIII and fibronectin on the growth of tumor cells and leukemic cells in vitro. Klin Wochenschr. 1983 Feb 15;61(4):209–211. doi: 10.1007/BF01488977. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Glenn K. C., Cunningham D. D. Conditions which affect initiation of animal cell division by trypsin and thrombin. J Cell Physiol. 1978 Apr;95(1):13–22. doi: 10.1002/jcp.1040950103. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Scott D. L., Gordon E. A., LaBelle E. F. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985 Sep;42(2):479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. D., Farrell D. H. Thrombin interactions with cultured fibroblasts: relationship to mitogenic stimulation. Ann N Y Acad Sci. 1986;485:240–248. doi: 10.1111/j.1749-6632.1986.tb34586.x. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D., Gurwitz D. Proteolytic regulation of neurite outgrowth from neuroblastoma cells by thrombin and protease nexin-1. J Cell Biochem. 1989 Jan;39(1):55–64. doi: 10.1002/jcb.240390107. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Glenn K. C., Cunningham D. D. Thrombin-stimulated cell division involves proteolysis of its cell surface receptor. Nature. 1979 Apr 19;278(5706):711–714. doi: 10.1038/278711a0. [DOI] [PubMed] [Google Scholar]
- Grabham P. W., Grand R. J., Byrd P. J., Gallimore P. H. Differentiation of normal and adenovirus-12 E1 transformed human embryo retinal cells. Exp Eye Res. 1988 Jul;47(1):123–133. doi: 10.1016/0014-4835(88)90029-2. [DOI] [PubMed] [Google Scholar]
- Grabham P. W., Grand R. J., Gallimore P. H. Purification of a serum factor which reverses dibutyryl cAMP induced differentiation. Cell Signal. 1989;1(3):269–281. doi: 10.1016/0898-6568(89)90044-2. [DOI] [PubMed] [Google Scholar]
- Guenther J., Nick H., Monard D. A glia-derived neurite-promoting factor with protease inhibitory activity. EMBO J. 1985 Aug;4(8):1963–1966. doi: 10.1002/j.1460-2075.1985.tb03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci U S A. 1988 May;85(10):3440–3444. doi: 10.1073/pnas.85.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hawkins R. L., Seeds N. W. Effect of proteases and their inhibitors on neurite outgrowth from neonatal mouse sensory ganglia in culture. Brain Res. 1986 Nov 19;398(1):63–70. doi: 10.1016/0006-8993(86)91250-3. [DOI] [PubMed] [Google Scholar]
- Kyritsis A. P., Tsokos M., Triche T. J., Chader G. J. Retinoblastoma--origin from a primitive neuroectodermal cell? Nature. 1984 Feb 2;307(5950):471–473. doi: 10.1038/307471a0. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Elion J., Butkowski R. J., Downing M., Nesheim M. E. Prothrombin. Methods Enzymol. 1981;80(Pt 100):286–302. doi: 10.1016/s0076-6879(81)80025-0. [DOI] [PubMed] [Google Scholar]
- Massacrier A., Nègre-Aminou P., Couraud F., Cau P. Quantitative analysis of fetal rat brain neurons developing in primary cultures. I. Stereological study of the neuronal differentiation. Brain Res. 1988 May 16;468(2):161–170. doi: 10.1016/0165-3806(88)90128-9. [DOI] [PubMed] [Google Scholar]
- McKinney M., Snider R. M., Richelson E. Thrombin binding to human brain and spinal cord. Mayo Clin Proc. 1983 Dec;58(12):829–831. [PubMed] [Google Scholar]
- Means E. D., Anderson D. K. Thrombin interactions with central nervous system tissue and implications of these interactions. Ann N Y Acad Sci. 1986;485:314–322. doi: 10.1111/j.1749-6632.1986.tb34593.x. [DOI] [PubMed] [Google Scholar]
- Medrano E. E., Cafferata E. G., Larcher F. Role of thrombin in the proliferative response of T-47D mammary tumor cells. Mitogenic action and pleiotropic modifications induced together with epidermal growth factor and insulin. Exp Cell Res. 1987 Oct;172(2):354–364. doi: 10.1016/0014-4827(87)90393-4. [DOI] [PubMed] [Google Scholar]
- Monard D. Cell-derived proteases and protease inhibitors as regulators of neurite outgrowth. Trends Neurosci. 1988 Dec;11(12):541–544. doi: 10.1016/0166-2236(88)90182-8. [DOI] [PubMed] [Google Scholar]
- Monard D., Niday E., Limat A., Solomon F. Inhibition of protease activity can lead to neurite extension in neuroblastoma cells. Prog Brain Res. 1983;58:359–364. doi: 10.1016/S0079-6123(08)60037-0. [DOI] [PubMed] [Google Scholar]
- Neurath H. Evolution of proteolytic enzymes. Science. 1984 Apr 27;224(4647):350–357. doi: 10.1126/science.6369538. [DOI] [PubMed] [Google Scholar]
- Paraskeva C., Brown K. W., Gallimore P. H. Adenovirus-cell interactions early after infection: in vitro characteristics and tumourigenicity of adenovirus type 2-transformed rat liver epithelial cells. J Gen Virol. 1982 Jan;58(Pt 1):73–81. doi: 10.1099/0022-1317-58-1-73. [DOI] [PubMed] [Google Scholar]
- Påhlman S., Odelstad L., Larsson E., Grotte G., Nilsson K. Phenotypic changes of human neuroblastoma cells in culture induced by 12-O-tetradecanoyl-phorbol-13-acetate. Int J Cancer. 1981 Nov 15;28(5):583–589. doi: 10.1002/ijc.2910280509. [DOI] [PubMed] [Google Scholar]
- Reddan J. R., Dziedzic D. C., McGee S. J. Thrombin induces cell division in rabbit lenses cultured in a completely defined serum-free medium. Invest Ophthalmol Vis Sci. 1982 Apr;22(4):486–493. [PubMed] [Google Scholar]
- Rupniak H. T., Rein G., Powell J. F., Ryder T. A., Carson S., Povey S., Hill B. T. Characteristics of a new human neuroblastoma cell line which differentiates in response to cyclic adenosine 3':5'-monophosphate. Cancer Res. 1984 Jun;44(6):2600–2607. [PubMed] [Google Scholar]
- Snider R. M. Thrombin effects on cultured nerve cells: clinical implications and evidence for a novel mechanism of neuronal activation. Ann N Y Acad Sci. 1986;485:310–313. doi: 10.1111/j.1749-6632.1986.tb34592.x. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Nick H., Hofsteenge J., Monard D. Glial-derived neurite-promoting factor is a slow-binding inhibitor of trypsin, thrombin, and urokinase. Arch Biochem Biophys. 1987 Jan;252(1):237–244. doi: 10.1016/0003-9861(87)90028-2. [DOI] [PubMed] [Google Scholar]
- Ziller C., Dupin E., Brazeau P., Paulin D., Le Douarin N. M. Early segregation of a neuronal precursor cell line in the neural crest as revealed by culture in a chemically defined medium. Cell. 1983 Feb;32(2):627–638. doi: 10.1016/0092-8674(83)90482-8. [DOI] [PubMed] [Google Scholar]