Abstract
The nucleus geniculatus lateralis pars ventralis (GLv) is a prominent retinal target in all amniotes. In birds, it is in receipt of a dense and topographically organized retinal projection. The GLv is also the target of substantial and topographically organized projections from the optic tectum and the visual wulst (Hyperpallium). Tectal and retinal afferents terminate homotopically within the external GLv-neuropil. Efferents from the GLv follow a descending course through the tegmentum, and can be traced into the medial pontine nucleus. At present, the cells of origin of the Tecto-GLv projection are only partially described. Here we have characterized the laminar location, morphology, projection pattern and neurochemical identity of these cells, by means of neural tracer injections and intracellular fillings in slice preparations and extracellular tracer injections in-vivo. The Tecto-GLv projection arises from a distinct subset of layer 10 bipolar neurons, whose apical dendrites show a complex transverse arborization at the level of layer 7. Axons of these bipolar cells arise from the apical dendrites and follow a course through the optic tract to finally form very fine and restricted terminal endings inside the GLv-neuropil. Double label experiments showed that these bipolar cells were ChAT immunoreactive. Our results strongly suggest that Tecto-GLv neurons form a pathway by which integrated tectal activity rapidly feeds back to the GLv and exerts a focal cholinergic modulation of incoming retinal inputs.
Keywords: Optic tectum, GLv, Slice, Vine-neuron, ChAT, Birds
Introduction
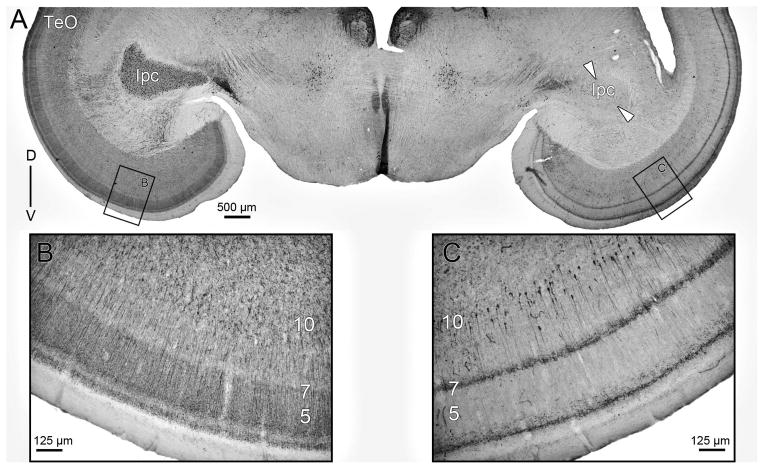
The optic tectum (TeO), corresponding to the superficial portion of the mammalian superior colliculus (SC), is a multi-laminar structure located in the roof of the mesencephalon. In birds, it is composed of 15 layers, each containing layer-specific cell populations (Ramón y Cajal, 1995). The avian tectum is in receipt of the majority of the retinal axons (Karten et al., 1997; Mpodozis et al., 1995), which terminate differentially into five specific layers within the superficial retino-recipient zone (Angaut and Repérant, 1976; Hayes and Webster, 1975; Ramón y Cajal, 1995). The TeO also receives afferents from several other sources, such as the ventral lateral thalamus (VLT), the tectal gray (GT), the isthmic nuclei pars parvocellularis (Ipc), pars magnocellularis (Imc) and pars semilunaris (SLu), the visual wulst (Hyperpallium) and the nucleus spiriformis lateralis, each exhibiting a particular pattern of arborization within specific tectal layers. In addition, the TeO is the locus of origin of several efferent ascending and descending visual pathways that arise from different layer-specific cell populations (Vanegas, 1984; Wylie et al., 2009). The tectal ganglion cells located in layer 13 are the source of the ascending tectofugal pathway (TeO → nucleus Rotundus → Entopallium), which is a main visual pathway in amniotes, including mammals (Benowitz and Karten, 1976; Dávila et al., 2002; Luksch et al., 1998; Major et al., 2000; Reiner, 1994). Deep and intermediate tectal layers are also the origin of two major descending projections to the hindbrain: the crossed tectobulbar pathway (CTB) and the ipsilateral tectoreticular-tectopontine pathway (ITP) (Hellmann et al., 2004; Reiner and Karten, 1982). Other significant efferent projections arise from the intermediate layers and proceed to several thalamic and tegmental targets, such as the isthmic nuclei pars magnocellularis (Imc), parvocellularis (Ipc) and semilunaris (SLu), the isthmo optic nucleus (ION), the nucleus lentiformis mesencephali (LM), the tectal gray (GT), the thalamic OPT complex (homologue of the mammalian dorsal geniculate), the VLT and the nucleus geniculatus lateralis pars ventralis (GLv) (Crossland and Hughes, 1978; Crossland and Uchwat, 1979; Gamlin and Cohen, 1988; Hunt and Künzle, 1976a; Vanegas, 1984; Wylie et al., 2009).
The GLv is one of the most prominent thalamic targets of retinal projections in all amniotes. In birds, the GLv is a distinctive structure nested in the optic tract in the ventrolateral diencephalon (Crossland and Uchwat, 1979). The avian GLv exhibits a characteristic cytoarchitecture composed of two main layers (as seen in figure 1): the lamina interna (GLv-li) which contains tightly packed somata of the projection cells, and the neuropil (GLv-ne) (Crossland and Uchwat, 1979; Guiloff et al., 1987; Tombol et al., 2004). The optic tectum is the main non-retinal source of GLv afferents. Retinal and tectal inputs end topographically and in homotopic correspondence inside the GLv-ne (Crossland and Uchwat, 1979). Other significant afferents originate from the visual wulst (homologous to the mammalian primary visual cortex) which produces a dense terminal field in the outer half of the GLv-ne, the tectal gray (GT), the nucleus lentiformis mesencephali pars magnocellularis (LMmc) and the ventrolateral thalamus (VLT) (Crossland and Uchwat, 1979; Gamlin and Cohen, 1988; Karten et al., 1973). Projections from the GLv follow a descending course through the pretectum and tegmentum (Hu et al., 2004), to finally target the nucleus pontis medialis (Pm) (Marín et al., 2001).
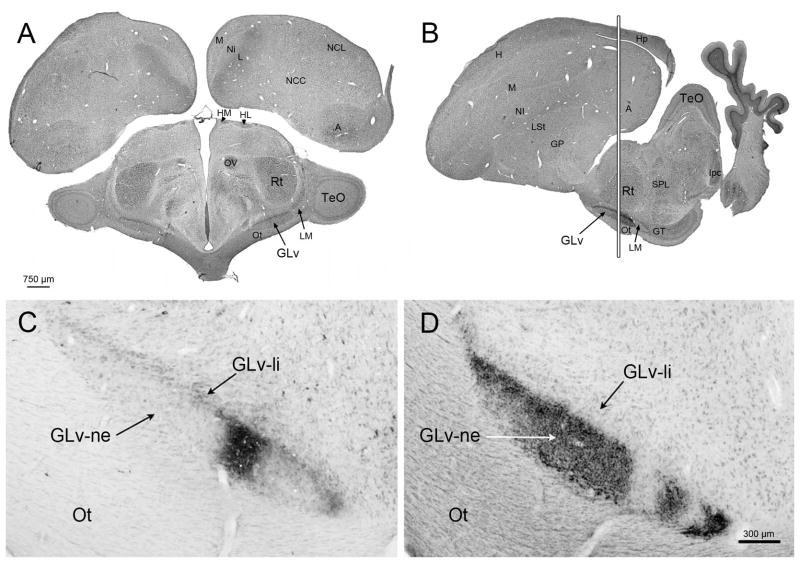
Figure 1. Tectal and retinal terminals in the GLv.
A) Transverse plane of a Giemsa staining showing the position of the GLv in a dorso-ventral orientation. B) Sagittal plane of the Giemsa staining showing the position of the GLv in a rostro-caudal orientation. White vertical line represent aproximatelly the location of the sections in C and D. C) Anterogradely labeled CTB-terminals in the GLv-ne after tectal injection into intermediate layers (injection site not shown). D) Labeled terminals in the Glv-ne after intraocular injection of CTB. Empty areas in the GLv-ne may probably due to uneven distribution of CTB into the vitreal chamber of the eye. Tectal and retinal afferents are topographic and coexist in close apposition into the GLv-ne.
The phylogenetic conservation of the GLv, together with its complex pattern of connections with the main structures of the visual system suggests that this nucleus plays a fundamental role within the neural structures subserving vision. However, in spite of several suggestions found in the literature such as chromatic discrimination (Maturana and Varela, 1982), circadian rhythm (Harrington, 1997), optokinetic reflex (Gioanni et al., 1991) and visuomotor responses (Guiloff, 1991; Pateromichelakis, 1979), the role of the GLv in vision remains unclear. In this context, a detailed survey of the TeO-GLv projection is of interest, as it may clarify which of the many visual operations taking place in the optic tectum are functionally linked to the GLv.
Previous work (Hunt and Künzle, 1976a) has shown that the tectal cells projecting to the GLv are located in layer 10, and correspond to the cell type previously described by Ramón y Cajal as “centrifugal neurons” (Ramón y Cajal, 1995). In addition, Medina and Reiner (1994) suggested that these cells could correspond to the ChAT immunoreactive neurons located in tectal layer 10. To delineate the functional contribution of this neuronal circuit to vision, the neuronal morphology, projection pattern and neurochemical identity of these cells require further assessment.
In order to clarify these essential issues, we performed in vivo experiments in pigeons and in vitro experiments in chicken brain slices (containing the TeO-GLv connection). We show that the TeO-GLv projection is indeed topographic, and originates from a population of radially oriented bipolar neurons with fusiform perikarya located in tectal layer 10. Conspicuous morphological characteristics of these cells are dense dendritic ramifications in layer 7 and an axon ascending in a vine-like fashion towards the ventral thalamus. In addition, we found that these cells are indeed ChAT positive and therefore the tectal influence over GLv might be cholinergic in nature.
Methods
Animals
Twelve adult feral pigeons (Columba livia) of either sex, obtained from an authorized local dealer were used in the in vivo experiments. All surgical procedures used on these animals were approved by the University of Chile’s Ethics Committee and conformed to National Institute of Health guidelines on the ethical use of the animals.
In addition, twenty-three White Leghorn chick hatchlings (Gallus gallus; P-1 until P-3) were used in the in vitro experiments. Fertilized eggs were obtained from local breeders (Hatchery Hoelzl, Moosburg, Germany) and incubated at 37°C and 70% humidity. All in vitro procedures were approved by the Munich Veterinary Animal Care Committee and conformed to National Institute of Health guidelines on the ethical use of the animals.
All efforts were made to minimize both the suffering and the number of animals used in these experiments.
In vivo extracellular injections
Injections of 3–10 nL of a 1% solution of CTB (List Labs, Campbell, CA) were performed in ten pigeons. Target of these injections were the eye chamber (2 cases), the GLv (5 cases), and the TeO (3 cases). In two additional pigeons, an injection of 200 nL of a 3% solution of kainic acid (Sigma) was placed unilaterally into the nucleus isthmi-parvocellularis (Ipc) for immunohistochemical experiments (see ChAT Immunohistochemistry section).
Pigeons were anesthetized with a combination of ketamine (40 mg/kg) and xylazine (12 mg/kg) injected intramuscularly. A single dose usually proved satisfactory for the duration of the surgical procedure. If necessary, a supplementary dose of anesthetic was administered (ketamine 10 mg/kg and xylazine 3mg/kg every two hours) Injections into the eye chamber were performed manually under a dissecting microscope, using an insulin syringe (30 gauge needle). Injections into the GLv, the Ipc and the TeO were achieved by stereotaxically (Karten and Hodos, 1967) lowering a micropipette filled with the CTB solution into the desired area. Once the target was reached, the tracer was injected applying pressure pulses using a picospritzer (Picospritzer II, General Valve, Fairfield, NJ). In order to enhance the accuracy of the injections, electrophysiological responses were monitored during the stereotaxic penetration. For a detailed review of these procedures, see Mpodozis et al. (1996).
After the injections the wounds were covered, the skin sutured, and treated with topical antibiotics. During the experiments the heart rate of the animals was continuously monitored and the body temperature was held at 40–42 C° by means of a thermoregulated electric blanket. During surgery and recovery all wounds and pressure points were treated with a commercial ointment of 5% lidocaine.
After 5–9 days of survival, and 30 days in the cases with the kainic acid injection, the animals were deeply anesthetized with an overdose of a mixture of ketamine and xylazine and perfused via the aorta with 500–800 mL of 0.75% saline, followed by 1,000 mL of an ice-cold solution of 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). After the perfusion, brains were excised, postfixed overnight in the paraformaldehyde solution, and then transferred for 2–3 days to a 30% sucrose solution (in 0.1 M PB) for cryoprotection. The brains were then mounted in the stereotaxic plane over the stage of a frozen sliding microtome and 30 μm sections were cut in the transverse plane.
In the cases with the CTB injection, sections were washed 30 minutes in 0.1 M phosphate-buffered saline (PBS, pH 7.4), and incubated in goat anti-CTB (1:15,000, List Labs) overnight at 4°C. The tissue was then processed using the avidin-biotin-peroxidase method (ABC Elite kit, Vector Labs, Burlingame, CA, U.S.A.) in 0.3% Triton X-100 in PB for 1 hour. Sections were then washed in PBS and reacted in 0.025% diaminobenzidine (DAB, Sigma, St. Louis, MO) and 0.3% hydrogen peroxide in PB for 10 minutes. The tissue was mounted on gelatinized slides, then dehydrated, cleared and cover-slipped using Permount (Fisher Scientific, Fair Lawn, NJ). Previous control experiments using the same protocol but without CTB injection in the brain were performed. The results showed no labeling in the brain of pigeons (data not shown).
In vitro extracellular injections
Injections targeting the GLv and the TeO in the chicken were made using 12 nL of a 10% solution of dextran biotin (BDA 10,000 MW, Molecular Probes) or dextran Alexa Fluor 546 10% (10,000 MW, Invitrogen, Molecular Probes) dissolved in PB 0.1 M.
Chick hatchlings were deeply anesthetized with a mixture (3:1) of Ketamine (50 mg/ml; Inresa Arzneimittel) and Rompun (2%; Bayer) at 37.5 and 5 mg/kg respectively and subsequently decapitated. The skull was opened at the midsagittal line and the dorsal surface of the brain exposed. After removal, the brain was transferred into ice-cooled (4°C) sucrose-substituted Krebs solution (210 mM sucrose, 3 mM KCl (Sigma, U.S.A.), 3 mM MgCl2•6H2O, 23 mM NaHCO3, 1.2 mM NaH2PO4•6H2O (Laborbedarf-Vertrieb GmbH, Germany), 11 mM D+-glucose).
The forebrain and cerebellum were removed with cuts through the junction with the diencephalon and the cerebellar peduncles. The remaining parts of the brain were cut midsagittally into two hemispheres. The brain tissue was then embedded in agar at 48 °C and rapidly cooled (1.5% Agar in HEPES solution: 290mM sucrose, 3mM KCl, 3mM MgCl2, and 5mM HEPES; Sigma Chemical Co., USA). The optic tectum of each hemisphere was aligned in an oblique transverse plane. The hemispheres were cut into slices between 500–900 μm with a vibratome (VF 200 Microtome, Precisionary Instruments Inc., USA). Slices containing the TeO-GLv were placed in standard ACSF solution (120 mM NaCl, 3 mM KCl, 1 mM MgCl2•6H2O, 23 mM NaHCO3, 1.2 mM NaH2PO4•1H2O, 11 mM D+-glucose) and kept submerged in a chamber that was bubbled continuously with Carbogen (95% oxygen, 5% CO2) at room temperature for at least 30 min.
Pipettes were fabricated from borosilicate glass (GB100-8P, 0.58 × 1.00 × 80 mm; Science products GmbH, Germany) with a one-stage microelectrode puller (Sutter Instrument Co., U.S.A.) to produce a tip opening around 30 μm. The electrodes were filled with mineral oil and attached to a Nanoliter 2000 injector (World Precision Instruments, U.S.A.). Electrodes were filled with dextran biotin 10% (BDA 10,000 MW, Molecular Probes) or dextran Alexa Fluor 546 10% (10,000 MW; invitrogen, Molecular Probes) dissolved in PB 0.1 M.
Single slices were submerged in a chamber with carbogenated ACSF solution. Under microscopic control and using a microdrive (Maerzhaeuser, West-Germany), injections of 13.8 nL tracer solution in three different positions along the GLv-ne were performed (medio-lateral axis; figure 3A).
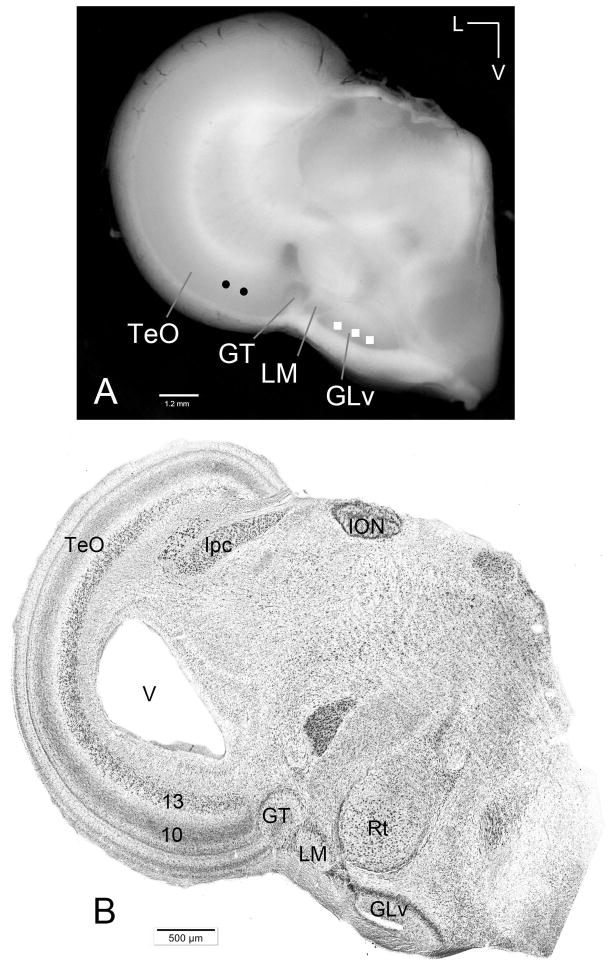
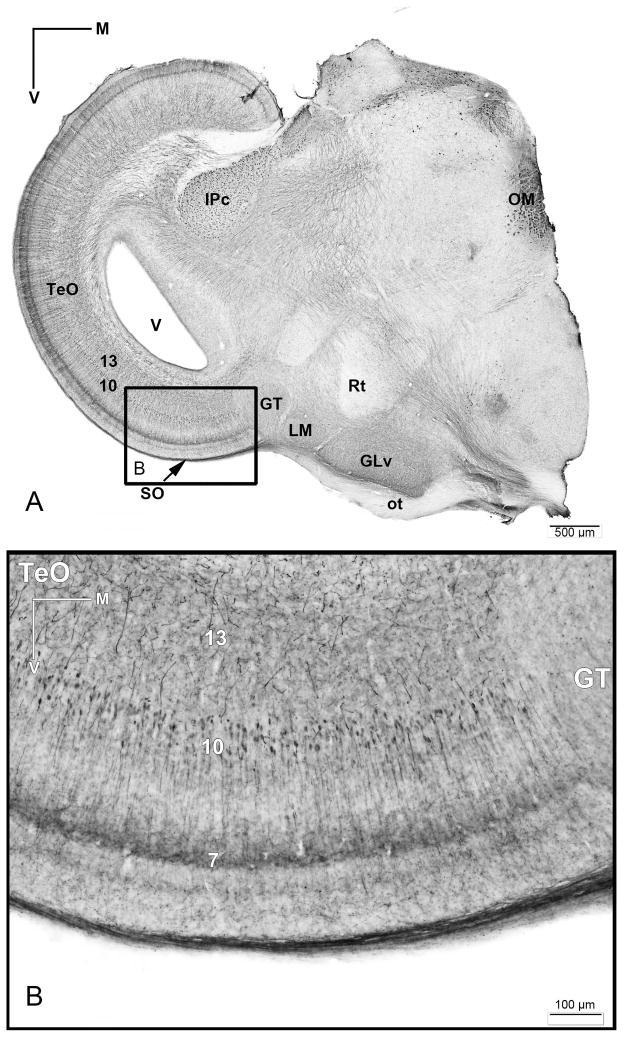
Figure 3. Chicken slice containing the TeO-GLv connection.
A) Macrophotography of a typical 500 μm slice used in this study. Circles and squares mark the location of extracellular injections of dextran amines into the TeO and GLv (performed in each slice) B) Giemsa staining of a 60 μm section showing tectum, pretectum and thalamus.. V = ventricle. Orientation: M = medial; V = ventral.
After injecting, slices were incubated four hours in carbogenated ACSF solution to allow transport of the tracer.
Slices containing BDA backfills were fixed overnight in 4% paraformaldehyde in 0.1M phosphate buffer (PFA/PB), transferred to 30% sucrose solution (in PB) for two hours, and cut to 60 μm sections with a cryotome (Kryostat 1720, Leitz, Germany). To visualize the filled neurons, a heavy-metal-intensified DAB protocol was applied. Sections were first incubated in H2O2 solution (3% (wt/vol) H2O2) for 30 minutes to block endogenous peroxidase. After washing eight times (8 × 10 minutes) in PB 0.1M until oxygen bubbles disappear, sections were incubated in 0.1 % (vol/vol) avidin-biotinylated HRP complex (ABC Elite kit, Vector Labs, Burlingame, CA, U.S.A.) solution containing 0.5% (vol/vol) Triton X-100 for two hours. Afterwards, samples were incubated in 0.026% diaminobenzidine Ni-Co with 0.03% H2O2 for 6 minutes. Sections were mounted onto gelatin-subbed slides, dehydrated in an ethanol series ending in Xylol and coverslipped with DPX (Sigma-Aldrich, Germany).
In vitro intracellular filling
Intracellular fillings were performed in chicken slices. The target of this experiment was to randomly fill bipolar cells located in layer 10 of the TeO.
Electrodes were fabricated from borosilicate glass (GB150 F-8P, 0.86×1.50×80 mm, filament; Science products GmbH, Germany) with a two-stage microelectrode puller (DMZ Universal) to produce electrodes with a resistance of 60–80 MΩ. The electrodes were filled with a mixture of 3 % biocytin (hydrochloride, Sigma-Aldrich, Germany) and 0,5 % Sulforhodamine 101 (Sigma-Aldrich, Germany) dissolved in 0.05 M Tris-buffer (pH 7.4) with 0.5 M KCl.
After incubation in Acridin Orange (10 mM, Sigma-Aldrich GmbH, Germany) for at least 20 minutes, single slices were transferred into a custom-built submerged slice chamber on a microscope stage that was continuously perfused with ACSF solution (RT 21/22 °C); slices were stabilized with a U-shaped platinum holder.
Sharp electrodes were moved through the tissue using a micromanipulator (MWS-1A Narishige Co., Japan) under an epifluorescent microscope (NikonY-FL, Eclipse E600FN, Japan). Once a cell was penetrated, biocytin/rhodamine was injected into the cell with a positive current of 0.8 nA for 5 minutes. After a successful filling corroborated with rhodamine fluorescence, slices were incubated for three hours in carbogenated ACSF solution; then fixed overnight in 4% paraformaldehyde in 0.1M phosphate buffer (PFA/PB), transferred to 30% sucrose solution (in PB) and cut into 50 μm slices with a cryotome (Kryostat 1720, Leitz, Germany).
To visualize the neurons filled with biocytin, a slightly different heavy-metal-intensified DAB protocol was applied. Sections were first incubated in H2O2 solution (3% (wt/vol) H2O2) for 30 minutes to block endogenous peroxidase. After washing eight times (8 × 10 minutes) in PB 0.1M until oxygen bubbles disappear, slices were incubated in 0.5 % (vol/vol) avidin-biotinylated HRP complex (ABC) solution containing 0.5% (vol/vol) Triton X-100 overnight. Afterwards, samples were incubated in 0.026% diaminobenzidine Ni-Co for 10 minutes. The chromogenic reaction was done adding H2O2 (final concentration 0.01 %) for 60 seconds. Finally, sections were mounted onto gelatin-subbed slides, dehydrated and coverslipped in DPX as mentioned above.
ChAT Immunohistochemistry in vivo and in vitro
Choline acetyltransferase (ChAT) is a marker of cholinergic cells. The goat polyclonal anti-ChAT antibody (immunogen: human placental enzyme) has been well-characterized and previously demonstrated to recognize 68–70-kDa bands in Western blot analysis of brain extracts from rat and several species of fish that disappeared when the antibody was preincubated with human placental ChAT (Gaillard et al., 2008; Hoshi et al., 2011; Pérez et al., 2000; Sevigny et al., 2012).
ChAT immunohistochemistry was performed in both, pigeon and chicken tissue. Pigeon sections were obtained from the animals that received a kainic acid injection into the Ipc, according to the method described above. This procedure was performed in order to abolish the masking effect of the ChAT-li of the Ipc neuron-paintbrushes in the medial and superficial layers of the TeO.
Chicken sections were from chicken slices either containing D-Alexa-546 backfills or without backfills. These slices were fixed overnight in 4% paraformaldehyde in 0.1M phosphate buffer (PFA/PB), transferred to 30% sucrose solution (in PB) and re-sectioned at 30 μm with the cryotome. Sections of both types were incubated with a primary antibody against anti-choline acetyltransferase (goat, Chemicon: AB144P) 1:100 in a PBS 0.1M solution containing 0.5% Triton X-100 and normal horse serum) overnight (4°C). After washing, sections of pigeon and chicken without backfills were incubated in secondary biotinylated antibody (rabbit Anti-Goat 1:750, Vector Labs: BA-500) for two hours at room temperature. After washing in PB 0.1M, sections were incubated in 0.1 % (vol/vol) avidin-biotinylated HRP complex (ABC Elite kit, Vector Labs, Burlingame, CA, U.S.A.) solution containing 0.5% (vol/vol) Triton X-100 for one hour. Afterwards, samples were incubated in 0.026% diaminobenzidine Ni-Co with 0.03% H2O2 for 6 minutes. Sections were mounted onto gelatin-subbed slides, dehydrated and coverslipped in DPX as mentioned above.
Chicken sections with backfills were incubated in secondary antibody Alexa 488 (A11055 donkey anti goat 1:200, life technologies, Molecular Probes) (Berger et al., 2010) for two hours at room temperature. Control sections were treated accordingly but the primary antibody was omitted. Sections were mounted onto gelatin-subbed slides and coverslipped with a custom made anti-fade mounting medium (Propyl Gallat 0.2%, Sigma-Aldrich, Germany; DMSO 1 %, Sigma-Aldrich, Germany; Glycerol 90%; dissolved in PBS 1M, pH 7.4) (see table 1 for a summary of the methods used).
Table 1.
Summary table of the methods used
| Tectal injection | GLv injection | Intracellular filling | Anti- ChAT | Double labeling | |
|---|---|---|---|---|---|
| In Vivo Pigeon | Yes | Yes | No | Yes | No |
| CTB | CTB | with lesion in Ipc | |||
| In Vitro Chicken | Yes | Yes | Yes | Yes | Yes |
| D-Alexa 488 | BDA, D-Alexa 488 | Biocytin | D-Alexa 488 + ChAT-546 |
Results
In vivo experiments
Anterograde tectal tracing
Injections of CTB into the optic tectum resulted in the ipsilateral labeling of a well delimited, dense plexus of axonal processes confined to discrete regions of the GLv-ne (figure 1C). These tectal projections were topographically organized, as different injection sites into the optic tectum resulted in the labeling of fine axonal terminals in different locations within the GLv (data not shown). Tectal axonal terminals were of very fine caliber, and spanned the whole dorso-ventral extension of the GLv-ne, without noticeable invasion of the GLv-li. The distribution of the tectal terminals overlap fully with that of the more coarse retinal terminals, which also span densely through the whole extent of the GLv-ne until the limit with the GLv-li (figure 1D).
Injections of CTB into the optic tectum also resulted in the ipsilateral retrograde labeling of many cells within the IGL, the LMmc and the VLT (data not shown). We were unable to find any indication of a similar substantial projection from the GLv upon the optic tectum. Only very rarely were retrograde CTB labeled cells found in the GLv, even in cases with large tectal injections. These rare cells were located mostly in the GLv-li, with occasional ones appearing in the GLv-ne.
Retrograde GLv tracing
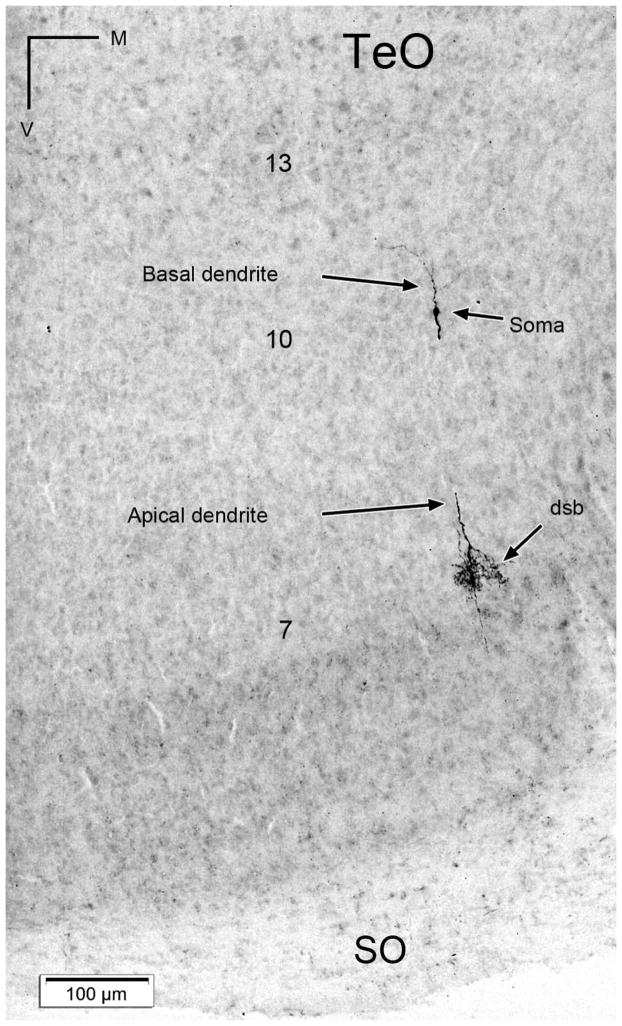
Due to the peculiar shape of the GLv, we were unable to obtain restricted tracer injections either confined to the limits of the nucleus, or resulting in a complete injection of the whole extent of the nucleus. The most consistent results (3 cases) were achieved by injecting the central portion of the GLv, at the level of the central Rt (figure 2C). In these cases, only minor leakage to the Rt, the medially adjacent VSOD, the dorsally adjacent VLT and the immediately ventral optic tract was observed. These injections consistently resulted in the ipsilateral retrograde labeling of a characteristic population of bipolar cells located in tectal layer 10 (figure 2A). These labeled cells had a very distinct morphology, featuring small fusiform somata and single apical and basal dendrites extending radially in opposite directions. The apical dendrite extended towards layer 2, and formed a conspicuous dense, transversely oriented specialization within the limits of layer 7 (figure 2B). The basal dendrite, of finer caliber, extended towards the deeper layers, and lacked evident specializations. In some instances, it was possible to trace the axon of these cells arising from the apical dendrite and following an ascending course towards layer 1.
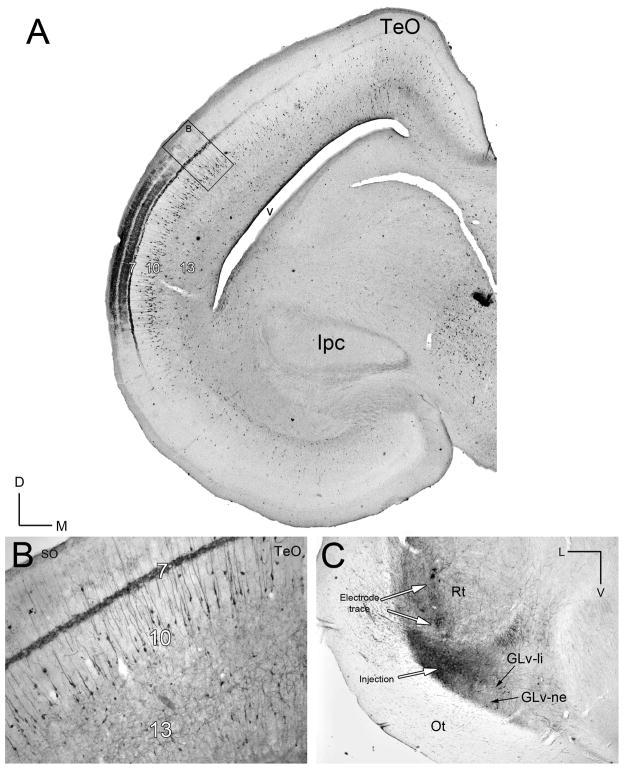
Figure 2. Retrograde tracing of the TeO-GLv projection in vivo.
A) Retrograde CTB-labeling of cells located mainly in layer 10 of the TeO after a GLv injection. B) Higher magnification of the labeled cells located in layer 10 of the TeO. Note the heavy labeled processes in the layer 7. C) Corresponding injection site in the lateral GLv. Orientation in A is the same as B. V = ventral; L = lateral.
In vitro experiments
The tecto-thalamic connection
To study the tecto-thalamic (TeO-GLv) connectivity with more detailed morphological features, oblique-transverse slices containing thalamus, pretectum and tectum (as seen in figure 3) were obtained. To ensure that the slices included the intact micro-circuitry between the TeO and the GLv, two localized extracellular injections of BDA-Alexa in the layers 9–10 of the ventral TeO were performed in four chickens (figure 4A). Tectal labeled neurons showed processes in both directions, apical and basal. The axons belonging to the labeled cells ascend to the superficial layers, proceed through the stratum opticum (SO) and optic tract (beneath GT and LM), and finally enter in an L-shape form into the GLv-ne, generating terminals in a topographic arrangement (figure 4B). The separation between the labeled terminals in GLv is 2.16 times smaller (107 μm) than the separation of the injections in the TeO (232 μm), indicating a scale reduction of the topographic map in the GLv.
Figure 4. Anterograde tracing of the TeO-GLv projection.
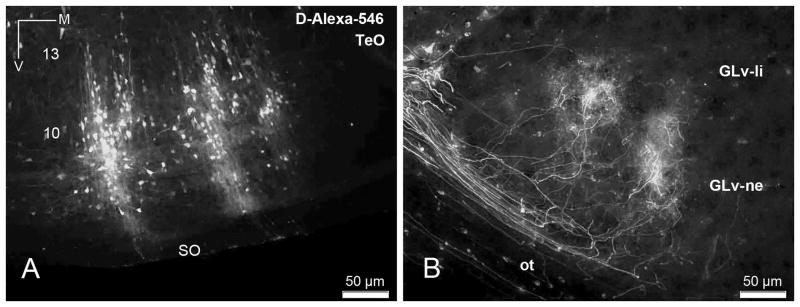
A) Two tectal injections of dextran-amine-alexa-546 (D-Alexa-546) in layer 9–10 of the TeO. B) Topographic terminals within the GLv-ne after the tectal injection. Orientation in B is the same as in A.
In order to corroborate the existence of the described TeO-GLv projection in the slice, and to label the neurons generating this projection, localized extracellular BDA-injections in the GLv-ne were made in four chickens (figure 5B). Only radial neurons were retrogadely labeled. All these cells were localized to layer 10 of the TeO. The labeled tectal neurons were of striking similar morphology to the ones labeled after CTb injection into the GLv in vivo and showed a characteristic high-density process in layer 7 of the TeO (figure 5A). Retrograde labeled neurons were never observed in lateral and dorsal regions of the TeO, indicating that in our preparation only the connection between the ventral part of the TeO with the GLv remained intact.
Figure 5. Retrograde tracing of the TeO-GLv projection.
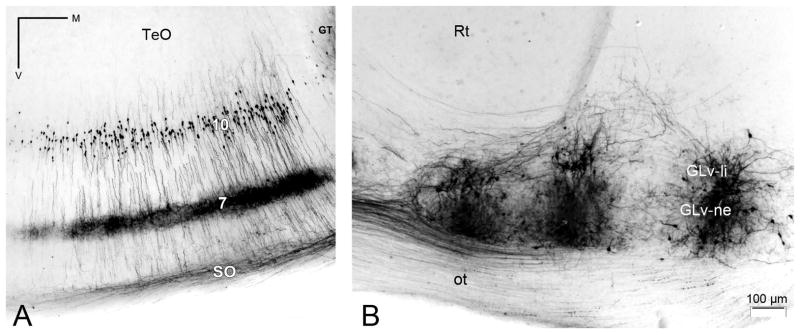
A) Composite image of five sections (separation between each section = 60μm) of retrogradely labeled cells with somata located in layer 10 of the TeO after three injections of BDA in the GLv-ne. B) Extracellular injections of BDA into the GLv-ne. Note how cells in GLv-li are also heavy labeled.
Morphology and projection pattern of the vine-neurons
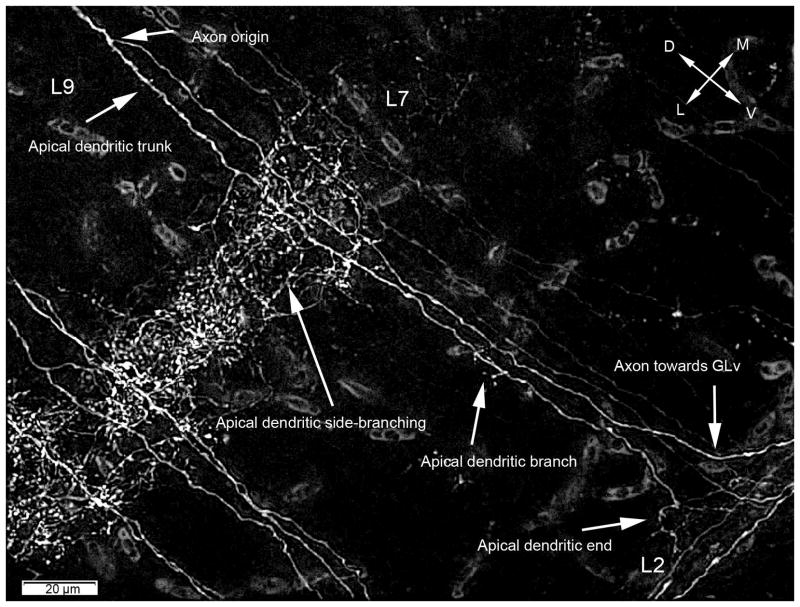
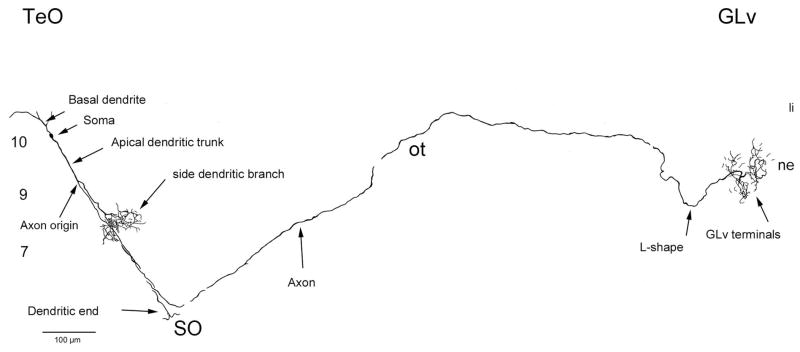
Extracellular retrograde tracings in the GLv-ne and intracellular fillings in layer 10 of the TeO showed bipolar neurons with an oval-shaped soma located in the layer 10 of the TeO (figure 6 and 8). The cells showed two primary dendritic branches: one rudimentary thin basal dendrite reaching into layer 13 (figure 6B), and a second thick apical dendritic trunk that extends through the superficial layers, giving rise to an extremely complex transverse arborization that spreads in layer 7 (figures 5A, 6 and 8). Finally, the dendrite thins, branches out in layer 4, and continues towards layer 2 where it ends in a tripod-shaped pattern (figure 6E and 7). Strikingly, the axon splits from the apical dendritic trunk at the level of layer 9, ascending almost parallel, in a characteristic vine-like fashion (figure 6, 7 and 10). Because of this conspicuous characteristic, we designated these cells as “vine-neurons”. At the level of layer 1 the axon turns 90 ° (as seen in figure 6E, 7 and 10) towards the GLv passing through stratum opticum (SO) and optic tract (Ot) subsequently. As shown in one complete intracellular filling, the terminal area is located in the GLv-ne (figure 9).
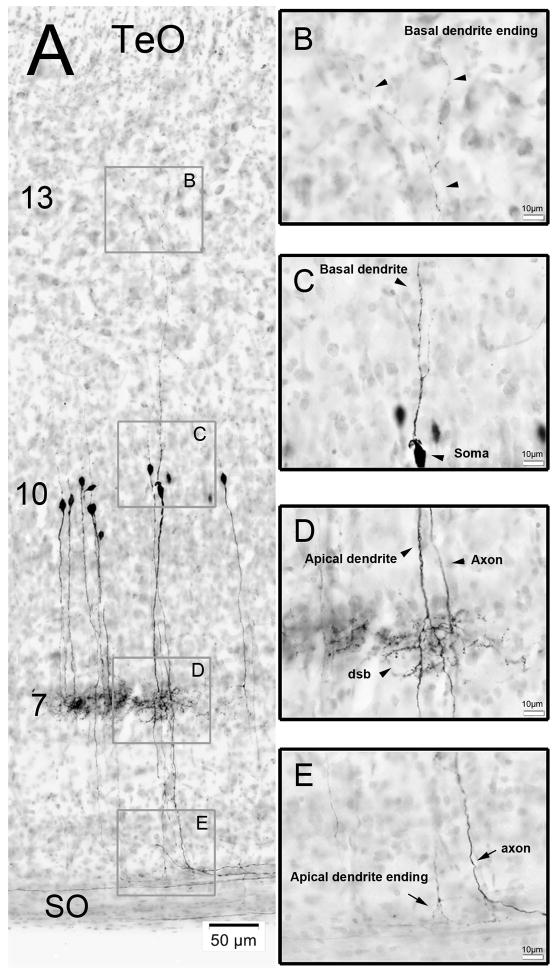
Figure 6. Retrograde labeling of cells in the TeO after BDA injections into GLv.
A) Retrogradely labeled cells with nissl counterstain. Basal dendrites extend until layer 13, somata are located in layer 10, dendritic side-branches lie in layer 7 and apical dendrites extend until layer 2 of the TeO. B) Inset showing the basal dendrite ending in layer 13 (black arrowheads). C) Inset showing the soma and the basal dendrite (black arrowheads). D) Inset showing the apical dendrite, the axon and the dendritic side-branch (dsb) of a neuron (black arrowheads). E) Inset showing the apical dendrite ending and the axon of one vine-neuron. Note the 90° bending of the axons between layer 2 and SO.
Figure 8. Intracellular filling of a vine-neuron with biocytin.
The soma is located in layer 10 and the dendritic side-branch (dsb) in layer 7 of the TeO. Soma width = 5.0 μm; dendritic side-branch width = 66 μm.
Figure 7. Deconvolution and extended focus projection of a retrogradely labeled cell in the TeO after GLv injection of D-Alexa-546.
Retrograde labeling shows that the axon splits from the apical denritic trunk at the level of layer 9. The apical dendrite side-branch massively in layer 7, continuing with a smaller branching in layer 4. Finally the apical dendrite ends in layer 2. Deconvolution used = Wiener. Orientation: L = lateral; V = ventral.
Figure 10. Reconstruction of an intracellularly filled vine-neuron with terminals in the GLv.
Figure shows a complete filled neuron with biocytin. Basal dendrite does not reach layer 13 as previously shown probably because it extend beyond the limits of the slice. The axon runs through the upper limit of the SO and the Ot before entering the GLv. Note the L-shape form of the axon entrance into the GLv and the butterfly-shape of the terminal in GLv-ne. 10, 9, 7 = layers of the TeO.
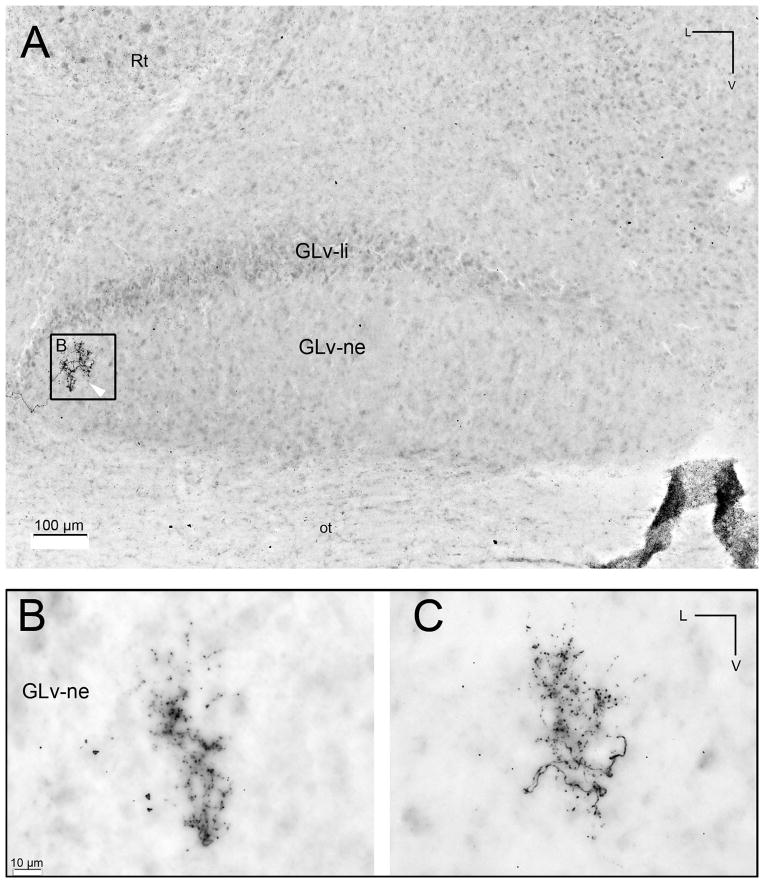
Figure 9. Intracellular filling showing a tectal terminal in the GLv-ne.
A) Composite image of two contiguous 60 μm sections of the terminal of a vine-neuron in the lateral part of the GLv-ne. B) and C) Inset showing the two sections where the terminal was observed.
Neurochemical identity of the vine-neurons
Previous reports of ChAT immunoreactivity in the TeO of birds (Medina and Reiner, 1994; Sorenson et al., 1989) consistently showed that the only ChAT positive tectal cell bodies are located in layer 10, and correspond to a subpopulation of bipolar neurons whose apical dendrites seems to ramify in layer 7. In order to assess whether these cholinergic cells correspond to the vine-neurons, we first replicated these immunohistochemical studies in both pigeons (two cases) and chicks (two cases). Since in pigeons the afferent ChAT positive axons from the nucleus Ipc densely ramify across tectal layers, masking the intrinsic tectal ChAT immunoreactivity, we performed this study in tissue obtained from animals that had previously been subjected to an unilateral chemical ablation of the Ipc using injections of Kainic acid (see methods and figure 11). Thus, the typical cholinergic “paintbrush endings” derived from the Ipc, were eliminated by the destruction of the corresponding region of the Ipc.
Figure 11. AntiChAT immunohistochemistry in the pigeon.
A) Overview of antiChAT-DAB labeling. Left side shows the intact Ipc nucleus and the characteristic ChAT-li of the paintbrush endings radially oriented in the TeO. Arrowheads on the right side outline the neurochemically ablated Ipc. B) Inset of the ventral part of the optic tectum showing mainly anti-ChAT positive paintbrushes from the IPc neurons in layer 5. C) Anti-ChAT positive somata located in layer 10 of the TeO are observed after chemical ablation of the Ipc nucleus. Note that the heavy staining previously observed in layer 5 (panel B) is no longer observed.
In both species, anti-ChAT DAB-immunohistochemistry showed specific labeling of cells located in the layer 10 of the TeO, the Ipc and the nucleus nervi oculomotori (OM), among other structures (figure 11, 12A). The labeled neurons of the layer 10 were distributed radially and closely resembled the size, morphology and distribution of the vine-neurons described above (A-ChAT-488 Soma size = 7.6 μm ± 0.80; A-546-Dextran = 7.2 μm ±0.6. Quantitative data (n = 34 cells) of the cell measurements were obtained from double labeling experiments not shown here. See also figure 11C, 12B and 13B).
Figure 12. AntiChAT immunohistochemistry in chicken slice.
A) Overview of diencephalic and mesencephalic structures showing antiChAT-DAB labeling. B) Inset of the ventral part of the optic tectum showing anti-ChAT positive cells with the somata located in layer 10 of the TeO. Note that Layer 7 shows neurite immunoreactivity. V = ventricle.
Figure 13. Double labeling experiments showing cells located in layer 10 of the TeO.
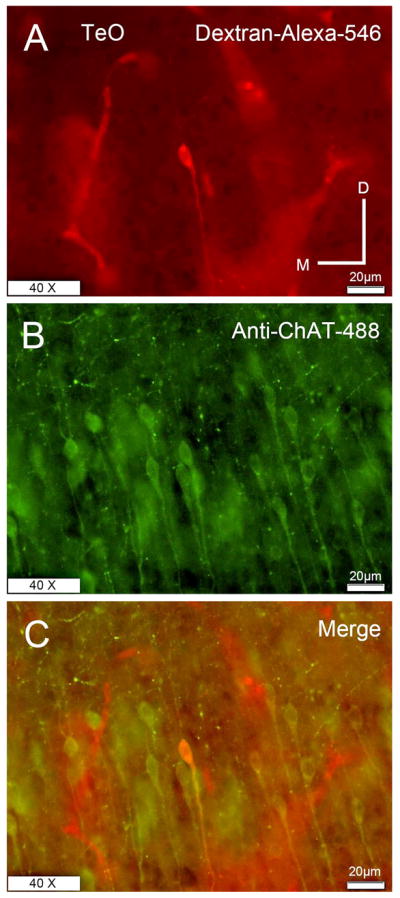
A) Retrograde labeling of a vine-neuron with D-Alexa-546 located in layer 10 of the TeO after a GLv-ne injection. B) ChAT Immunohistochemistry with alexa-488 of the same section showing labeled cells in layer 10 of the TeO. C) Merge of both images showing ChAT labeling of the vine-neuron. All retrograde cells labeled with A-546-Dextran were positive for ChAT (data not shown). Orientation: M = medial; D = dorsal.
Finally, to ensure that these anti-ChAT cells correspond to the tectal cells projecting to the GLv, double-labeling experiments were performed in three chickens. The results showed that all retrograde cells, marked after injections of BDA-Alexa in the GLv, positively colocalize with anti-ChAT immunoreactivity (Figure 13), indicating a cholinergic neurochemical identity for the vine-neurons.
Discussion
We were able to characterize the morphology, projection pattern and neurochemical identity of the cells of origin of the TeO-GLv projection in two different avian species, pigeons and chickens, using complementary in vivo and in vitro approaches. Our data indicate that neurons located in layer 10 of the TeO, corresponding to Cajal’s “centrifugal neurons”, project in a topographic manner to the GLv, without giving collaterals to any of the pretectal structures near the axonal course. In addition, these cells are positive for ChAT immunoreactivity. Therefore, the TeO-GLv synapses are likely to be cholinergic in nature.
Slice limitations
Long and middle range projections containing somata and terminals of the same neurons are rarely preserved in slices preparations, because they can easily course beyond the physical limits of the slice. Notwithstanding this, the results obtained in the in vivo experiments helped us to devise an unconventional plane of sectioning that resulted in slices containing, in its ventral aspect, the integrity of the TeO-GLv projection. Such slice preparation, together with the relatively rapid transport of dextran tracers (2mm/h) (Fritzsch, 1993), allowed us to perform a detailed morphological analysis of this projection in the living slice. Specifically, we were able to label the TeO-GLv projecting cells with Golgi-like resolution (Reiner et al., 2000; Sebestény et al., 2002). The results obtained in the slice were fully consistent with those obtained in vivo, further confirming the validity of the in vitro approach utilized in this study.
Morphology and projection pattern of the vine-neurons
Tectal cells projecting to the GLv were first morphologically identified by Hunt and Künzle (1976b). They showed that these cells, a subpopulation of layer 10 bipolar cells featuring small fusiform somata, correspond to the ones previously described by Ramón y Cajal (1891) as “centrifugal neurons” (Ramón y Cajal, 1995). The name “centrifugal” was given because Ramón y Cajal assumed that the conspicuous axons of these cells, that originated in layer 9 and left the tectum through the stratum opticum, would project to the retina. However, it has been repeatedly shown that in birds centrifugal neurons innervating the retina do not originate from the tectum, but from the isthmo-optic nucleus (ION) (Repérant et al., 2006). Thus, to avoid the confusion between the “true” centrifugal cells and the TeO-GLv ones, and given that these latter neurons show a conspicuous axon ascending in a vine-like fashion in the TeO, we suggest calling these cells “vine-neurons”.
Our backfills confirmed that the somata of these cells are located in layer 10 of the TeO, mainly in layer 10b. This specific location suggests that vine-neurons may be positive for the transcription factor Brn3a, a specific marker for layer 10b, as Wang et. al (2006) showed for shepherd-crook neurons. Interestingly, in this latter study Brn3a labeled more cells than shepherd crook neurons, suggesting the existence of another cell group in this sublayer.
In addition, our results corroborate that the horizontal branching expansion that these cells exhibit in layer 7 originates from the apical dendritic trunk (figure 6 and 7) as Van Gehuchten suggested in 1892, and not from the axon as Ramón y Cajal pointed out in 1896 (Ramón y Cajal, 1995). These dendritic expansions may likely be the postsynaptic target of the subpopulation of retinal cells that give rise to the coarse, horizontally oriented terminals that densely cover layer 7. In addition, using the intracellular filling method we were able to label five vine-neurons; in two cases we could label the axon leaving the tectum, and in one case we could follow the whole trajectory of the axon as it enters and ramifies into the GLv-ne. As expected from the in vivo and in vitro results, this axonal terminal was very restricted and spanned through the dorso/ventral extent of the GLv-ne without reaching the GLv-li. We did not find any evidence of axon collaterals entering into the pretectal and thalamic structures located nearby the axonal course, such as LM, GT, and VLT.
Neurochemical identity of the vine-neurons
Immunohistochemical localization of different markers such as GABA, GAD, Substance P and choline acetyltransferase (ChAT) has been extensively studied in the avian brain (Bagnoli et al., 1992; Britto et al., 1992; Güntürkün and Karten, 1991; Medina and Reiner, 1994; Sorenson et al., 1989; Veenman et al., 1994; Veenman and Reiner, 1994). Previous report of ChAT immunoreactivity showed that in the TeO the only ChAT positive cell population is a subset of layer 10 bipolar neurons featuring an apical dendrite that posses a characteristic expansion in layer 7. These previous studies have also shown that the GLv-ne is the only thalamic structure exhibiting a distinct plexus of cholinergic processes. Given these results, Medina and Reiner (1994) suggested that the source of such cholinergic processes were the tectal cells of layer 10.
We readily confirmed these previous observations and suggestions by means of anti-ChAT immune essays in pigeons and chickens. We found that the morphology and distribution of the ChAT immunoreactive cells closely resemble that of the vine-neurons, indicating that both cell types are identical (figure 13). Our double-labeling experiments provide a direct confirmation of this identity, as we observed that all vine-neurons retrogradely labeled from the GLv were also ChAT positive. We have also observed that the intensity of cholinergic label in the GLv-ne appears greatly reduced after an excitotoxic lesion of the TeO (results not shown), confirming that the TeO is a source of cholinergic terminals upon the GLv. In addition, further physiological evidence shows that micro-stimulation in layer 10 of the TeO elicited cholinergic EPSP in the GLv (unpublished results). Thus, our results strongly suggest that the TeO-GLv synapse is cholinergic in nature.
Contrary to what we show here, Hunt and Künzle (1976b) suggested that vine-neurons have a GABAergic identity. Such conclusion was based on tracing studies using 3H-GABA. However, what this method reveals is the uptake of GABA and not necessarily the neurochemical identity of these cells. In this regard, literature shows the existence of GABA uptake in hippocampal cholinergic nerve endings (Bonanno and Raiteri, 1987). Moreover, in pigeon and chicken, first the GLv-ne contains a dense plexus of GABA-containing processes, presumably caused by intrinsic GABAergic interneurons (Tombol et al., 2004; Veenman et al., 1994), and second, the GLv-li projection cells are presumably GABAergic (Sun et al., 2005). On the other hand, Veeman and Reiner (1994) and Sun et al. (2005) have shown that cells located in layer 10 of the TeO are GABA positive. In addition, previous experiments have shown the coexistence of ChAT-li and GABA in motor neurons in the hypoglossal nucleus in the rat (Davidoff and Schulze, 1988).
Hence, the presence of GABA in the vine-neurons (in the older studies) may likely be due to the reuptake of GABA at the axonal terminal in the GLv. However, further studies will be needed in order to rule out the GABAergic neurochemical identity of the vine-neurons.
GLv-TeO projection
Several reports suggest the existence of a reciprocal projection between the GLv and the TeO (Guiloff et al., 1987; Maturana and Varela, 1982). This alleged projection originates from a small population of cells and seems to be non topographic (Crossland and Uchwat, 1979; Hunt and Künzle, 1976a; Wylie et al., 2009). In our in vivo experiments we found that the GLv cells retrogadely labeled from the TeO were always very scant, even after large tectal injections. Most of these cells were located in the GLv-li, but occasionally some of them were located in GLv-ne as well. In addition, the distribution of these rare labeled cells was very irregular, and showed no evidence of topographic arrangement. On the other hand, in our in vitro approach no retrograde labeled cells were found, probably due to the fact that the slice contains only part of the intact TeO-GLv connection. Thus, our results indicate that the population of GLv-tectum projecting cells is rather small and heterogeneous, and do not form a substantial organized projection as the TeO-GLv projection. This issue is relevant to clarify the TeO/GLv functional interactions.
Synaptic interactions in the Retino-Tecto-GLv network
Retinal fibers ending in the superficial layers (2–5 and 7) of the optic tectum are glutamatergic (Atoji, 2011; Henke et al., 1976; Morino et al., 1991; Theiss et al., 1998). At the same time, retino-recipient layers, including layer 7, express high levels of AMPA receptors Glu-R1 (Batista et al., 2002; Pires and Britto, 1997). We suggest that layer 7 labeling of Glu-R1 may correspond to the expression of these receptors in the dendritic side-branch of vine-neurons. If this is the case, retinal inputs may influence the activity of the vine-neurons. Following this notion, the axon that emerges from the apical dendritic trunk in layer 9 may generate a direct fast response (without necessarily the influence of the soma) that will arrive onto the GLv just a few milliseconds after the retinal input in both tectum and GLv. Furthermore, the apical dendrite of the vine-neurons proceeds beyond layer 7, crossing all tectal retino-recipient region until layer 2, thus possibly allowing for synaptic contacts with a myriad of different intrinsic or external sources, including other retinal subpopulations. Additionally, the basal dendrites of these cells, which extend until tectal layer 13, might interact with various types of processes present in these deep layers, such as the isthmic and arcopallial endings that terminate diffusely between layers 10–13. On the other hand, retinal terminals in tectal layer 7 also express high levels of nicotinic beta-2 receptors (Britto et al., 1992; Britto et al., 1994), suggesting a cholinergic presynaptic modulation of its glutamatergic release. Vine-neurons might be a possible candidate for modulation of these retinal terminals. In fact, dendritic release of several neurotransmitters has been widely shown to occur in the central nervous system of mammals (Cheramy et al., 1981; Isaacson, 2001; Ludwig and Pittman, 2003). Alternatively, the cholinergic paintbrush endings coming from the neurons of nucleus isthmo parvocellularis (Ipc) may enhance the retino-vine-neuron synapses at selected visually active loci. This is an interesting possibility that would link the activity of the retino-GLv projection with the presumed attentional function of the isthmo-tectal network (Asadollahi et al., 2011; Marín et al., 2007; Marín et al., 2012; Wang et al., 2006).
Retinal afferents to the GLv have been suggested to be collateral branches of axons that end in the superficial layer of the optic tectum (Cowan et al., 1961; Guiloff et al., 1987; Tombol et al., 2004). Retino-GLv terminals are known to be glutamatergic and, as the retino-tectal endings, endowed with nicotinic receptors (Guo et al., 2005; Guo et al., 1998). Previous physiological work in chick brain slices has shown a nicotinic-dependent presynaptic enhancement of the synaptic transmission between the retinal terminals and the projection cells of the GLv-li (Guo et al., 2005; Guo et al., 2010). Given their cholinergic character, vine-neurons are the most likely candidates to be the source of such modulation, since as we have shown here they end in the GLv-ne homotopically and in close apposition with the retinal terminals. If that were the case, the “centrifugal” effect hypothesized by Ramón y Cajal for the vine-neurons would indeed be carried out, not in the retina, but at the level of the retino-GLv terminals. Interestingly, physiological studies have also shown that the activation of nicotinic receptors enhance GABA release in the GLv-ne and muscarinic receptors inhibits the synaptic retino-GLv transmission (Guo and Chiappinelli, 2002; Guo et al., 2010). Thus, a complex mechanism of excitatory and inhibitory cholinergic effects seems to be taking place in the GLv-ne. We suggest that tectal afferents could be acting upon the GLv projection cells in two ways: excitatory, through the gating of the retinal terminals that drive these cells, and inhibitory, through nicotinic and muscarinic receptors possibly located at the GABAergic intrinsic cells and the projecting cells respectively (or in a yet unknown element). Taking all together, in a scenario in which several GLv loci were simultaneously active by independent visual stimuli, the concurrence of tectal activity upon a given retinal-activated GLv locus could selectively enhance the activity of such locus, depressing, at the same time, via GABAergic enhancement, the activity of the neighboring loci.
The avian and mammalian GLv complex
A common feature of the visual system in amniotes is the presence of a set of retinorecipient structures located adjacent to the lateral wall of the ventral thalamus that could be recognized as a “ventrothalamic complex”. These structures have in common an absence of subsequent projections to the telencephalon, together with descendent efferents directed towards pretectal, tegmental and pontine targets (Butler and Hodos, 2005). In mammals, this ventrothalamic complex is called the GLv complex (in primates pregeniculate nucleus) and is composed of several subnuclei or subdivisions, clustered in a large structure containing two or more retinorecipient neuropils and several nuclear layers (Conley and Friederich-Ecsy, 1993a; Harrington, 1997). A main characteristic of this complex is the presence of retinal, collicular, cortical and cerebellar afferents (Conley and Friederich-Ecsy, 1993b). In turn, projections from this complex to the medial pons, along with projections to the optic tectum, the zona incerta and other hypothalamic targets, the pretectum, the accessory optic nuclei and the motor tegmentum, have been confirmed in a number of mammalian species (Harrington, 1997).
In birds, the GLv is also part of a complex constituted by a collection of several distinct structures that succeed each other in a rostro-caudal order. Following the nomenclature of Karten and Hodos (1967), this complex includes the nucleus LA, IGL, VLT and GLv. The literature regarding these nuclei is scant and somewhat confusing, thus the anatomical and functional reasons to group these structures together are at present unclear. It can be said, however, that the overall pattern of connections of this complex is remarkably similar to that of the mammalian GLv complex. Interestingly, none of the single components of this group exhibit a pattern of connections as diverse as the one attributed to the mammalian complex. The most conspicuous and better known member of the avian ventro-thalamic complex, the GLv, lacks of cerebellar afferents, and does not emit a significant topographic projection to the tectum or to the accessory optic nuclei (unpublished observations).
A collicular projection upon the GLv complex has been described in many mammalian species such as the cat, the rat and the tree shrew (Conley and Friederich-Ecsy, 1993b; Cosenza and Moore, 1984; Graham, 1977; Taylor et al., 1986). This projection terminates in the external-most neuropil of the complex (thus, a putative homologue for the GLv-ne in birds) and, as in the avian projection, it follows a topographic arrangement. The cells forming this projection are located in the retinorecipient tectal zone, SGS, without a clear layer arrangement. Unfortunately, the labeling of these cells does not show a fine detailed morphology of dendrites and axons, making it difficult to establish any clear comparison with the vine-neurons described in this study. Moreover, the neurochemical identity of these collicular cells is far from resolved. Therefore, more detailed surveys regarding the morphology and neurochemistry of the tectal cells projecting to the GLv in mammals will be necessary in order to establish a consistent comparative approach. In any case, the avian GLv nucleus should not be considered homologous to the mammalian GLv complex as a whole. To be more specific, we propose to rename the avian GLv nucleus as “nucleus geniculatus lateralis, pars ventralis principalis” (GLv-pri).
Summary
We have used tracer and immunohistochemical techniques to characterize the morphology, projection pattern and neurochemical identity of the neurons originating the TeO-GLv projection. Our data indicate that these neurons project to the GLv. Furthermore, the ChAT immunoreactivity of these cells strongly suggests that the TeO-GLv synapses are cholinergic. Nevertheless, other neurotransmitters involvement cannot be ruled out. The information presented will be valuable for future physiological studies regarding the visual role of the GLv and of the TeO-GLv connection.
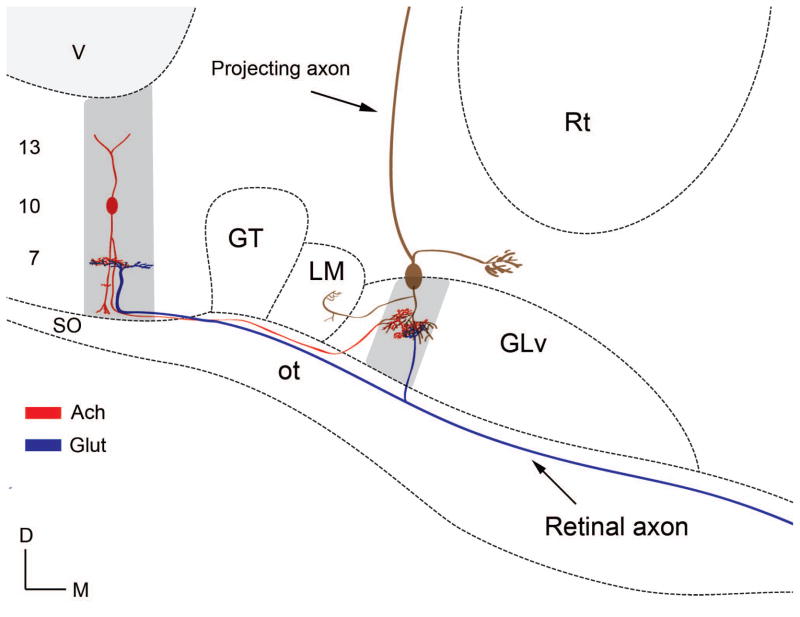
Figure 14. Schematic drawing of proposed neural circuitry of the TeO-GLv projection.
Cholinergic vine-neuron located in layer 10 of the TeO (red) projects topographically onto a presumable GABAergic GLv projection cell (brown). Glutamatergic retinal input from a retinal ganglion cell ends in GLv and TeO (blue). The gray shadings indicate the activation of a specific locus in the TeO and the concurrent activation of a homotopic locus in the GLv. V= ventricle
Acknowledgments
Grant Sponsor: Fondo Nacional de Desarrollo Científico y Tecnológico, Grant numbers: 1110281, 1120124, 1080220
We wish to thank Birgit Seibel, Yvonne Schwarz, Gaby Schwabedissen, Agnieszka Brzozowska-Prechtl, Elisa Sentis and Solano Henriquez for excellent technical assistance. Sebastián Tapia Pino for experimental help. Dr. Camilo Libedinsky for critically reading the manuscript. Dr. Prasad Vaddepalli for microscopy assistance. Máximo Fernández, Dr. Cristián Gutiérrez and Quirin Krabichler for helpful comments during the conduct of this research.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
Author contribution: All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: TVZ, HL, JM, HJK, GM. Acquisition of data: TVZ, JM, HJK, GM, SH. Analysis and interpretation of data: TVZ, HL, JM, HJK, GM, SH. Drafting of the manuscript: TVZ, HL, JM, HJK, GM. Critical revision of the manuscript for important intellectual content: HL, JM, HJK, GM. Statistical analysis: TVZ. Administrative, technical, and material support: HL, JM, HJK, GM. Study supervision: HL.
References
- Angaut P, Repérant J. Fine structure of the optic fibre termination layers in the pigeon optic tectum: A golgi and electron microscope study. Neuroscience. 1976;1(2):93-IN13. doi: 10.1016/0306-4522(76)90003-8. [DOI] [PubMed] [Google Scholar]
- Asadollahi A, Mysore SP, Knudsen EI. Rules of competitive stimulus selection in a cholinergic isthmic nucleus of the owl midbrain. The Journal of Neuroscience. 2011;31(16):6088–6097. doi: 10.1523/JNEUROSCI.0023-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoji Y. Immunohistochemical localization of vesicular glutamate transporter 2 (vGluT2) in the central nervous system of the pigeon (Columba livia) The Journal of Comparative Neurology. 2011;519(14):2887–2905. doi: 10.1002/cne.22663. [DOI] [PubMed] [Google Scholar]
- Bagnoli P, Fontanesi G, Alesci R, Erichsen JT. Distribution of neuropeptide Y, substance P, and Choline acetyltransferase in the developing visual system of the pigeon and effects of unilateral retina removal. The Journal of Comparative Neurology. 1992;318(4):392–414. doi: 10.1002/cne.903180405. [DOI] [PubMed] [Google Scholar]
- Batista SS, Pires RS, Britto LR. Differential expression of AMPA-type glutamate receptor subunits during development of the chick optic tectum. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 2002;35(8):973–978. doi: 10.1590/s0100-879x2002000800015. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Karten HJ. Organization of the tectofugal visual pathway in the pigeon: a retrograde transport study. Journal of Comparative Neurology. 1976;167(4):503–520. doi: 10.1002/cne.901670407. [DOI] [PubMed] [Google Scholar]
- Berger SB, Romero X, Ma C, Wang G, Faubion WA, Liao G, Compeer E, Keszei M, Rameh L, Wang N, Boes M, Regueiro JR, Reinecker H-C, Terhorst C. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat Immunol. 2010;11(10):920–927. doi: 10.1038/ni.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno G, Raiteri M. Presence of a gamma-aminobutyric acid (GABA) uptake system on cholinergic terminals of rat hippocampus: evidence for neuronal coexistence of acetylcholine and GABA? Journal of Pharmacology and Experimental Therapeutics. 1987;240(1):294–297. [PubMed] [Google Scholar]
- Britto LRG, Keyser KT, Lindstrom JM, Karten HJ. Immunohistochemical localization of nicotinic acetylcholine receptor subunits in the mesencephalon and diencephalon of the chick (Gallus gallus) The Journal of Comparative Neurology. 1992;317(4):325–340. doi: 10.1002/cne.903170402. [DOI] [PubMed] [Google Scholar]
- Britto LRG, Torrao AS, Hamassaki-Britto DE, Mpodozis J, Keyser KT, Lindstrom JM, Karten HJ. Effects of retinal lesions upon the distribution of nicotinic acetylcholine receptor subunits in the chick visual system. The Journal of Comparative Neurology. 1994;350(3):473–484. doi: 10.1002/cne.903500311. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative vertebrate neuroanatomy: evolution and adaptation. 2005 Wiley.com.
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289(5798):537–543. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- Conley M, Friederich-Ecsy B. Functional organization of the ventral lateral geniculate complex of the tree shrew (Tupaia belangeri): I. Nuclear subdivisions and retinal projections. The Journal of Comparative Neurology. 1993a;328(1):1–20. doi: 10.1002/cne.903280102. [DOI] [PubMed] [Google Scholar]
- Conley M, Friederich-Ecsy B. Functional organization of the ventral lateral geniculate complex of the tree shrew (Tupaia belangeri): II. Connections with the cortex, thalamus, and brainstem. The Journal of Comparative Neurology. 1993b;328(1):21–42. doi: 10.1002/cne.903280103. [DOI] [PubMed] [Google Scholar]
- Cosenza RM, Moore RY. Afferent connections of the ventral lateral geniculate nucleus in the rat: an HRP study. Brain Research. 1984;310(2):367–370. doi: 10.1016/0006-8993(84)90162-8. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Adamson L, Powell TP. An experimental study of the avian visual system. J Anat. 1961;95:545–563. [PMC free article] [PubMed] [Google Scholar]
- Crossland WJ, Hughes CP. Observations on the afferent and efferent connections of the avian isthmo-optic nucleus. Brain Research. 1978;145(2):239–256. doi: 10.1016/0006-8993(78)90860-0. [DOI] [PubMed] [Google Scholar]
- Crossland WJ, Uchwat CJ. Topographic projections of the retina and optic tectum upon the ventral lateral geniculate nucleus in the chick. The Journal of Comparative Neurology. 1979;185(1):87–106. doi: 10.1002/cne.901850106. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Schulze W. Coexistence of GABA-and choline acetyltransferase (ChAT)-like immunoreactivity in the hypoglossal nucleus of the rat. Histochemistry. 1988;89(1):25–33. doi: 10.1007/BF00496580. [DOI] [PubMed] [Google Scholar]
- Dávila JC, Andreu MJ, Real MÁ, Puelles L, Guirado S. Mesencephalic and diencephalic afferent connections to the thalamic nucleus rotundus in the lizard, Psammodromus algirus. European Journal of Neuroscience. 2002;16(2):267–282. doi: 10.1046/j.1460-9568.2002.02091.x. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Fast axonal diffusion of 3000 molecular weight dextran amines. Journal of Neuroscience Methods. 1993;50(1):95–103. doi: 10.1016/0165-0270(93)90060-5. [DOI] [PubMed] [Google Scholar]
- Gaillard F, Bonfield S, Gilmour GS, Kuny S, Mema SC, Martin BT, Smale L, Crowder N, Stell WK, Sauvé Y. Retinal anatomy and visual performance in a diurnal cone-rich laboratory rodent, the Nile grass rat (Arvicanthis niloticus) The Journal of Comparative Neurology. 2008;510(5):525–538. doi: 10.1002/cne.21798. [DOI] [PubMed] [Google Scholar]
- Gamlin PDR, Cohen DH. Projections of the retinorecipient pretectal nuclei in the pigeon (columba livia) The Journal of Comparative Neurology. 1988;269(1):18–46. doi: 10.1002/cne.902690103. [DOI] [PubMed] [Google Scholar]
- Gioanni H, Palacios A, Sansonetti A, Varela F. Role of the nucleus geniculatus lateralis ventralis (GLv) in the optokinetic reflex: a lesion study in the pigeon. Experimental Brain Research. 1991;86(3):601–607. doi: 10.1007/BF00230533. [DOI] [PubMed] [Google Scholar]
- Graham J. An autoradiographic study of the efferent connections of the superior colliculus in the cat. The Journal of Comparative Neurology. 1977;173(4):629–654. doi: 10.1002/cne.901730403. [DOI] [PubMed] [Google Scholar]
- Guiloff GD. Ultrastructural study of the avian ventral lateral geniculate nucleus. Visual Neuroscience. 1991;6(02):119–134. doi: 10.1017/s0952523800010518. [DOI] [PubMed] [Google Scholar]
- Guiloff GD, Maturana HR, Varela FJ. Cytoarchitecture of the avian ventral lateral geniculate nucleus. The Journal of Comparative Neurology. 1987;264(4):509–526. doi: 10.1002/cne.902640406. [DOI] [PubMed] [Google Scholar]
- Güntürkün O, Karten HJ. An immunocytochemical analysis of the lateral geniculate complex in the pigeon (Columba livia) The Journal of Comparative Neurology. 1991;314(4):721–749. doi: 10.1002/cne.903140407. [DOI] [PubMed] [Google Scholar]
- Guo J-Z, Liu Y, Sorenson EM, Chiappinelli VA. Synaptically Released and Exogenous ACh Activates Different Nicotinic Receptors to Enhance Evoked Glutamatergic Transmission in the Lateral Geniculate Nucleus. Journal of Neurophysiology. 2005;94(4):2549–2560. doi: 10.1152/jn.00339.2005. [DOI] [PubMed] [Google Scholar]
- Guo JZ, Chiappinelli VA. A novel choline-sensitive nicotinic receptor subtype that mediates enhanced GABA release in the chick ventral lateral geniculate nucleus. Neuroscience. 2002;110(3):505–513. doi: 10.1016/s0306-4522(01)00579-6. [DOI] [PubMed] [Google Scholar]
- Guo JZ, Sorenson EM, Chiappinelli VA. Cholinergic modulation of non-N-methyl-d-aspartic acid glutamatergic transmission in the chick ventral lateral geniculate nucleus. Neuroscience. 2010;166(2):604–614. doi: 10.1016/j.neuroscience.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JZ, Tredway TL, Chiappinelli VA. Glutamate and GABA release are enhanced by different subtypes of presynaptic nicotinic receptors in the lateral geniculate nucleus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18(6):1963–1969. doi: 10.1523/JNEUROSCI.18-06-01963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington ME. The Ventral Lateral Geniculate Nucleus and the Intergeniculate Leaflet: Interrelated Structures in the Visual and Circadian Systems. Neuroscience & Biobehavioral Reviews. 1997;21(5):705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- Hayes BP, Webster KE. An electron microscope study of the retino-receptive layers of the pigeon optic tectum. The Journal of Comparative Neurology. 1975;162(4):447–465. doi: 10.1002/cne.901620404. [DOI] [PubMed] [Google Scholar]
- Hellmann B, Güntürkün O, Manns M. Tectal mosaic: Organization of the descending tectal projections in comparison to the ascending tectofugal pathway in the pigeon. The Journal of Comparative Neurology. 2004;472(4):395–410. doi: 10.1002/cne.20056. [DOI] [PubMed] [Google Scholar]
- Henke H, Schenker T, Cuénod M. Effects of retinal ablation on uptake of glutamate, glycine, GABA, proline and choline in pigeon tectum. Journal of neurochemistry. 1976;26(1):131–139. [PubMed] [Google Scholar]
- Hoshi H, Tian L-M, Massey SC, Mills SL. Two distinct types of ON directionally selective ganglion cells in the rabbit retina. The Journal of Comparative Neurology. 2011;519(13):2509–2521. doi: 10.1002/cne.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Naito J, Chen Y, Ohmori Y, Fukuta K. Afferent and Efferent Connections of the Nucleus Geniculatus Lateralis Ventralis Demonstrated by WGA-HRP in the Chick. Anatomia, Histologia, Embryologia. 2004;33(4):192–195. doi: 10.1111/j.1439-0264.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Künzle H. Observations on the projections and intrinsic organization of the pigeon optic tectum: An autoradiographic study based on anterograde and retrograde, axonal and dendritic flow. The Journal of Comparative Neurology. 1976a;170(2):153–172. doi: 10.1002/cne.901700203. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Künzle H. Selective uptake and transport of label within three identified neuronal systems after injection of 3H-GABA into the pigeon optic tectum: An autoradiographic and golgi study. The Journal of Comparative Neurology. 1976b;170(2):173–189. doi: 10.1002/cne.901700204. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Mechanisms governing dendritic γ-aminobutyric acid (GABA) release in the rat olfactory bulb. Proceedings of the National Academy of Sciences. 2001;98(1):337–342. doi: 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ, Cox K, Mpodozis J. Two distinct populations of tectal neurons have unique connections within the retinotectorotundal pathway of the pigeon (Columba livia) Journal of Comparative Neurology. 1997;387(3):449–465. [PubMed] [Google Scholar]
- Karten HJ, Hodos W. A Stereotaxic Atlas of the Brain of the Pigeon:(Columba Livia) Johns Hopkins Press; Baltimore: 1967. [Google Scholar]
- Karten HJ, Hodos W, Nauta WJH, Revzin AM. Neural connections of the “visual wulst” of the avian telencephalon. Experimental studies in the pigeon (Columba livia) and owl (Speotyto cunicularia) The Journal of Comparative Neurology. 1973;150(3):253–277. doi: 10.1002/cne.901500303. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends in Neurosciences. 2003;26(5):255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Luksch H, Cox K, Karten HJ. Bottlebrush dendritic endings and large dendritic fields: Motion-detecting neurons in the tectofugal pathway. The Journal of Comparative Neurology. 1998;396(3):399–414. [PubMed] [Google Scholar]
- Major DE, Luksch H, Karten HJ. Bottlebrush dendritic endings and large dendritic fields: Motion-detecting neurons in the mammalian tectum. The Journal of Comparative Neurology. 2000;423(2):243–260. [PubMed] [Google Scholar]
- Marín G, Henny P, Letelier JC, Sentis E, Karten H, Mrosko B, Mpodozis J. A simple method to microinject solid neural tracers into deep structures of the brain. Journal of Neuroscience Methods. 2001;106(2):121–129. doi: 10.1016/s0165-0270(01)00332-6. [DOI] [PubMed] [Google Scholar]
- Marín G, Salas C, Sentis E, Rojas X, Letelier JC, Mpodozis J. A Cholinergic Gating Mechanism Controlled by Competitive Interactions in the Optic Tectum of the Pigeon. The Journal of Neuroscience. 2007;27(30):8112–8121. doi: 10.1523/JNEUROSCI.1420-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín GJ, Durán E, Morales C, González-Cabrera C, Sentis E, Mpodozis J, Letelier JC. Attentional capture? Synchronized feedback signals from the isthmi boost retinal signals to higher visual areas. The Journal of Neuroscience. 2012;32(3):1110–1122. doi: 10.1523/JNEUROSCI.4151-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana HR, Varela FJ. Color-opponent responses in the avian lateral geniculate: A study in the quail (Coturnix coturnix japonica) Brain Research. 1982;247(2):227–241. doi: 10.1016/0006-8993(82)91248-3. [DOI] [PubMed] [Google Scholar]
- Medina L, Reiner A. Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. The Journal of Comparative Neurology. 1994;342(4):497–537. doi: 10.1002/cne.903420403. [DOI] [PubMed] [Google Scholar]
- Morino P, Bahro M, Cuénod M, Streit P. Glutamate-like Immunoreactivity in the Pigeon Optic Tectum and Effects of Retinal Ablation. European Journal of Neuroscience. 1991;3(4):366–378. doi: 10.1111/j.1460-9568.1991.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Mpodozis J, Cox K, Shimizu T, Bischof H-J, Woodson W, Karten HJ. GABAergic inputs to the nucleus rotundus (pulvinar inferior) of the pigeon (Columba livia) The Journal of Comparative Neurology. 1996;374(2):204–222. doi: 10.1002/(SICI)1096-9861(19961014)374:2<204::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mpodozis J, Letelier J-C, Concha ML, Maturana H. Conduction velocity groups in the retino-tectal and retino-thalamic visual pathways of the pigeon (Columba livia) International Journal of Neuroscience. 1995;81(3–4):123–136. doi: 10.3109/00207459509015304. [DOI] [PubMed] [Google Scholar]
- Pateromichelakis S. Response properties of units in the lateral geniculate nucleus of the domestic chick (Gallus domesticus) Brain Research. 1979;167(2):281–296. doi: 10.1016/0006-8993(79)90823-0. [DOI] [PubMed] [Google Scholar]
- Pérez SE, Yáñez J, Marín O, Anadón R, González A, Rodríguez-Moldes I. Distribution of choline acetyltransferase (ChAT) immunoreactivity in the brain of the adult trout and tract-tracing observations on the connections of the nuclei of the isthmus. The Journal of Comparative Neurology. 2000;428(3):450–474. doi: 10.1002/1096-9861(20001218)428:3<450::aid-cne5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Pires RS, Britto LR. Distribution of AMPA-type glutamate receptor subunits in the chick visual system. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 1997;30(1):73–77. doi: 10.1590/s0100-879x1997000100012. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histology of the Nervous System. Oxford University Press; 1995. [Google Scholar]
- Reiner A. Laminar Distribution of the Cells of Origin of Ascending and Descending Tectofugal Pathways in Turtles: Implications for the Evolution of Tectal Lamination;ppabcxyzpp274–283. Brain, Behavior and Evolution. 1994;43(4–5):274–283. doi: 10.1159/000113639. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karten HJ. Laminar distribution of the cells of origin of the descending tectofugal pathways in the pigeon (Columba livia) The Journal of Comparative Neurology. 1982;204(2):165–187. doi: 10.1002/cne.902040206. [DOI] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. Journal of Neuroscience Methods. 2000;103(1):23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Repérant J, Ward R, Miceli D, Rio J, Médina M, Kenigfest N, Vesselkin N. The centrifugal visual system of vertebrates: a comparative analysis of its functional anatomical organization. Brain research reviews. 2006;52(1):1–57. doi: 10.1016/j.brainresrev.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Sebestény T, Davies DC, Zayats N, Németh A, Tömböl T. The ramification and connections of retinal fibres in layer 7 of the domestic chick optic tectum: a Golgi impregnation, anterograde tracer and GABA-immunogold study. Journal of Anatomy. 2002;200(2):169–183. doi: 10.1046/j.0021-8782.2001.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny CP, Bassi J, Williams DA, Anderson CR, Thomas WG, Allen AM. Efferent projections of C3 adrenergic neurons in the rat central nervous system. The Journal of Comparative Neurology. 2012;520(11):2352–2368. doi: 10.1002/cne.23041. [DOI] [PubMed] [Google Scholar]
- Sorenson EM, Parkinson D, Dahl JL, Chiappinelli VA. Immunohistochemical localization of choline acetyltransferase in the chicken mesencephalon. The Journal of Comparative Neurology. 1989;281(4):641–657. doi: 10.1002/cne.902810412. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wang H, Laverghetta A, Yamamoto K, Reiner A. The distribution and cellular localization of glutamic acid decarboxylase-65 (GAD65) mRNA in the forebrain and midbrain of domestic chick. Journal of chemical neuroanatomy. 2005;29(4):265–281. doi: 10.1016/j.jchemneu.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Jeffery G, Lieberman AR. Subcortical afferent and efferent connections of the superior colliculus in the rat and comparisons between albino and pigmented strains. Experimental Brain Research. 1986;62(1):131–142. doi: 10.1007/BF00237409. [DOI] [PubMed] [Google Scholar]
- Theiss C, Hellmann B, Güntürkün O. The differential distribution of AMPA-receptor subunits in the tectofugal system of the pigeon. Brain Research. 1998;785(1):114–128. doi: 10.1016/s0006-8993(97)01395-4. [DOI] [PubMed] [Google Scholar]
- Tombol T, Eyre M, Zayats N, Nemeth A. The internal structure of the nucleus geniculatus lateralis ventralis in the avian brain: a Golgi study and electron microscopic investigation. Cells, tissues, organs. 2004;177(4):237–256. doi: 10.1159/000080137. [DOI] [PubMed] [Google Scholar]
- Vanegas H. Comparative Neurology of the Optic Tectum. New York: Plenum Press, New York and London; 1984. [Google Scholar]
- Veenman CL, Albin RL, Richfield EK, Reiner A. Distributions of GABAA, GABAB, and benzodiazepine receptors in the forebrain and midbrain of pigeons. The Journal of Comparative Neurology. 1994;344(2):161–189. doi: 10.1002/cne.903440202. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Reiner A. The distribution of GABA-containing perikarya, fibers, and terminals in the forebrain and midbrain of pigeons, with particular reference to the basal ganglia and its projection targets. The Journal of Comparative Neurology. 1994;339(2):209–250. doi: 10.1002/cne.903390205. [DOI] [PubMed] [Google Scholar]
- Wang Y, Luksch H, Brecha NC, Karten HJ. Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (Gallus gallus): A possible substrate for synchronizing tectal channels. The Journal of Comparative Neurology. 2006;494(1):7–35. doi: 10.1002/cne.20821. [DOI] [PubMed] [Google Scholar]
- Wylie DR, Gutierrez-Ibanez C, Pakan JM, Iwaniuk AN. The optic tectum of birds: mapping our way to understanding visual processing. Canadian journal of experimental psychology = Revue canadienne de psychologie experimentale. 2009;63(4):328–338. doi: 10.1037/a0016826. [DOI] [PubMed] [Google Scholar]