Abstract
The Saccharomyces cerevisiae PHO8 gene product, repressible alkaline phosphatase (ALP), is a glycoprotein enzyme that is localized to the yeast vacuole (lysosome). Using antibodies raised against synthetic peptides corresponding to two distinct hydrophilic sequences in ALP, we have been able to examine the biosynthesis, sorting and processing of this protein. ALP is synthesized as an inactive precursor containing a C-terminal propeptide that is cleaved from the protein in a PEP4-dependent manner. The precursor and mature protein are anchored in the membrane by an N-terminal hydrophobic domain that also appears to function as an uncleaved internal signal sequence. ALP has the topology of a type-II integral membrane protein containing a short basic N-terminal cytoplasmic tail that is accessible to exogenous protease when associated both with the endoplasmic reticulum and the vacuole. Similar to the soluble vacuolar hydrolases carboxypeptidase Y (CPY) and proteinase A (PrA), ALP transits through the early stages of the secretory pathway prior to vacuolar delivery. Two observations indicate, however, that ALP is localized to the vacuole by a mechanism which is in part different from that used by CPY and PrA: (i) maturation of proALP, which is indicative of vacuolar delivery, is less sensitive than CPY and PrA to the defects exhibited by certain of the vacuolar protein sorting (vps) mutants; and (ii) maturation of proALP proceeds normally in the presence of a potent vacuolar ATPase inhibitor, bafilomycin A1, which is known to block vacuole acidification and leads to the mis-sorting and secretion of precursor forms of CPY and PrA. These results indicate that ALP will be a useful model protein for studies of membrane protein sorting in yeast.
Full text
PDF


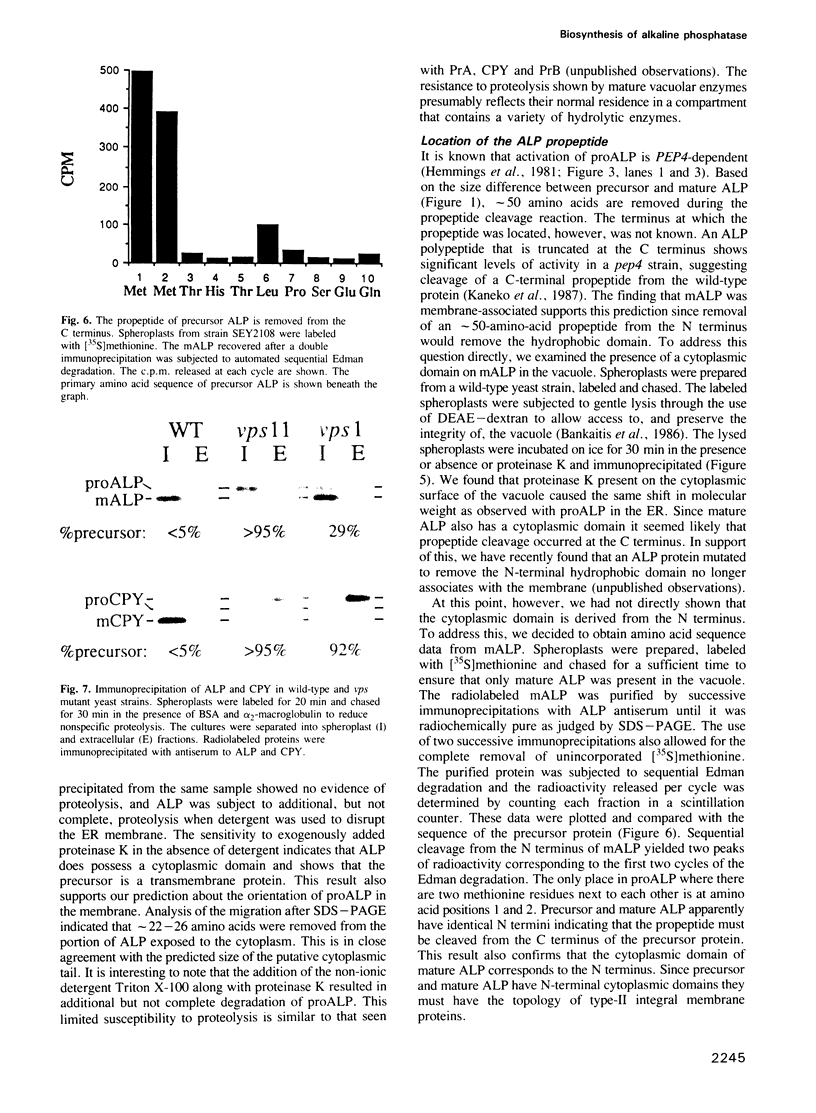

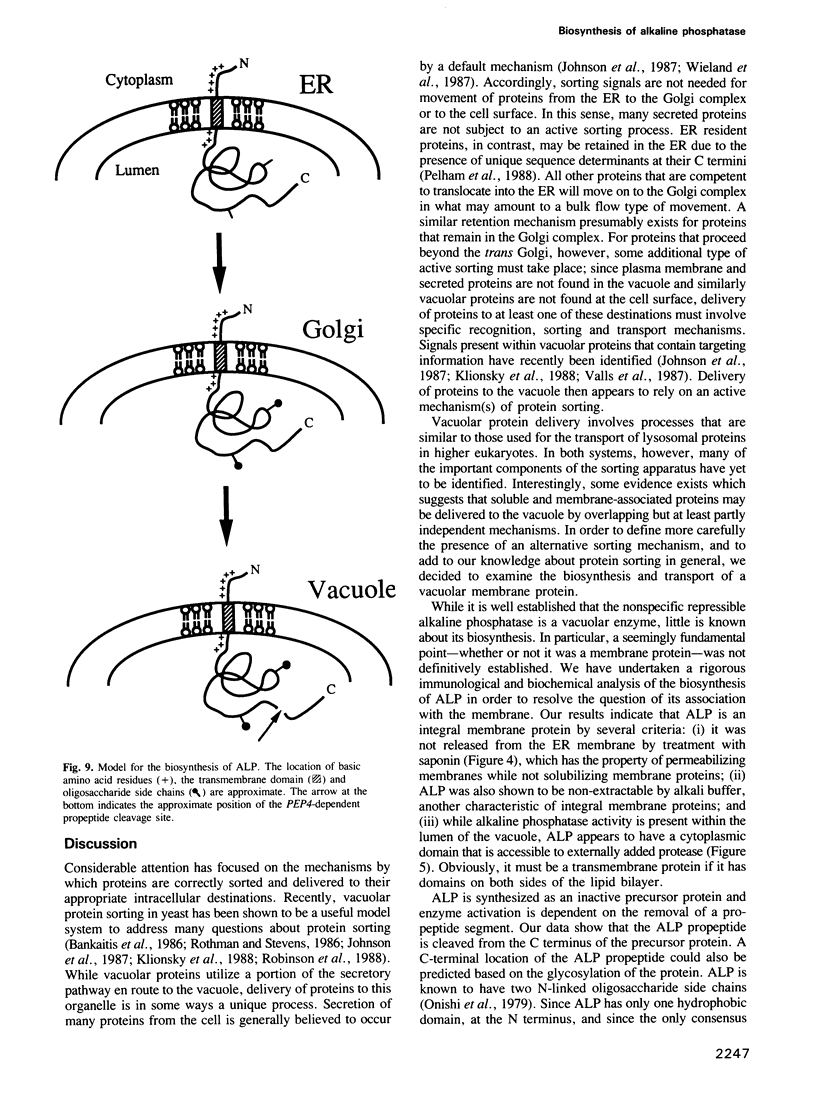



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G., Hunter C. P., Rothman J. H., Saari G. C., Valls L. A., Stevens T. H. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986 Jul;6(7):2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Johnson L. M., Emr S. D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta L. M., Robinson J. S., Klionsky D. J., Emr S. D. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988 Oct;107(4):1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriocanal J. G., Bonifacino J. S., Yuan L., Sandoval I. V. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986 Dec 15;261(35):16755–16763. [PubMed] [Google Scholar]
- Bauer H., Sigarlakie E. Localization of alkaline phosphatase in Saccharomyces cerevisiae by means of ultrathin frozen sections. J Ultrastruct Res. 1975 Feb;50(2):208–215. doi: 10.1016/s0022-5320(75)80052-9. [DOI] [PubMed] [Google Scholar]
- Bos T. J., Davis A. R., Nayak D. P. NH2-terminal hydrophobic region of influenza virus neuraminidase provides the signal function in translocation. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2327–2331. doi: 10.1073/pnas.81.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brada D., Schekman R. Coincident localization of secretory and plasma membrane proteins in organelles of the yeast secretory pathway. J Bacteriol. 1988 Jun;170(6):2775–2783. doi: 10.1128/jb.170.6.2775-2783.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli J. A., Golumbeski G. S., Dimond R. L. Lysosomal enzymes in Dictyostelium discoideum are transported to lysosomes at distinctly different rates. J Cell Biol. 1986 Apr;102(4):1264–1270. doi: 10.1083/jcb.102.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. W., Tkacz J. S., Lampen J. O. Asparagine-linked carbohydrate does not determine the cellular location of yeast vacuolar nonspecific alkaline phosphatase. J Bacteriol. 1982 Nov;152(2):865–873. doi: 10.1128/jb.152.2.865-873.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakle K. A., Bernstein M., Emr S. D. Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol Cell Biol. 1988 Oct;8(10):4098–4109. doi: 10.1128/mcb.8.10.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Schekman R., Flessel M. C., Thorner J. An MF alpha 1-SUC2 (alpha-factor-invertase) gene fusion for study of protein localization and gene expression in yeast. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7080–7084. doi: 10.1073/pnas.80.23.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Vassarotti A., Garrett J., Geller B. L., Takeda M., Douglas M. G. The amino terminus of the yeast F1-ATPase beta-subunit precursor functions as a mitochondrial import signal. J Cell Biol. 1986 Feb;102(2):523–533. doi: 10.1083/jcb.102.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel C. A., Goldberg D. E., Kornfeld S. Identification and characterization of cells deficient in the mannose 6-phosphate receptor: evidence for an alternate pathway for lysosomal enzyme targeting. Proc Natl Acad Sci U S A. 1983 Feb;80(3):775–779. doi: 10.1073/pnas.80.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. A., Zimmer K. P., Griffiths G., Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987 Sep;105(3):1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Haselbeck A., Schekman R. Interorganelle transfer and glycosylation of yeast invertase in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2017–2021. doi: 10.1073/pnas.83.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Biosynthesis of the vacuolar yeast glycoprotein carboxypeptidase Y. Conversion of precursor into the enzyme. Eur J Biochem. 1978 Apr 17;85(2):599–608. doi: 10.1111/j.1432-1033.1978.tb12275.x. [DOI] [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Hasilik A., Jones E. W. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jan;78(1):435–439. doi: 10.1073/pnas.78.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland E. C., Leung J. O., Drickamer K. Rat liver asialoglycoprotein receptor lacks a cleavable NH2-terminal signal sequence. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7338–7342. doi: 10.1073/pnas.81.23.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull J. D., Gilmore R., Lamb R. A. Integration of a small integral membrane protein, M2, of influenza virus into the endoplasmic reticulum: analysis of the internal signal-anchor domain of a protein with an ectoplasmic NH2 terminus. J Cell Biol. 1988 May;106(5):1489–1498. doi: 10.1083/jcb.106.5.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. M., Bankaitis V. A., Emr S. D. Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell. 1987 Mar 13;48(5):875–885. doi: 10.1016/0092-8674(87)90084-5. [DOI] [PubMed] [Google Scholar]
- Jones E. W., Zubenko G. S., Parker R. R. PEP4 gene function is required for expression of several vacuolar hydrolases in Saccharomyces cerevisiae. Genetics. 1982 Dec;102(4):665–677. doi: 10.1093/genetics/102.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma Y., Ohsumi Y., Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of SAccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10859–10863. [PubMed] [Google Scholar]
- Kaneko Y., Hayashi N., Toh-e A., Banno I., Oshima Y. Structural characteristics of the PHO8 gene encoding repressible alkaline phosphatase in Saccharomyces cerevisiae. Gene. 1987;58(1):137–148. doi: 10.1016/0378-1119(87)90036-9. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Banta L. M., Emr S. D. Intracellular sorting and processing of a yeast vacuolar hydrolase: proteinase A propeptide contains vacuolar targeting information. Mol Cell Biol. 1988 May;8(5):2105–2116. doi: 10.1128/mcb.8.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987 Dec;1(6):462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Matile P., Wiemken A., Schellenberg M., Meyer J. Activities and cellular localization of yeast proteases and their inhibitors. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1378–1383. doi: 10.1016/0006-291x(74)90350-7. [DOI] [PubMed] [Google Scholar]
- Lewis V., Green S. A., Marsh M., Vihko P., Helenius A., Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985 Jun;100(6):1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal and membrane anchor functions overlap in the type II membrane protein I gamma CAT. J Cell Biol. 1988 Jun;106(6):1813–1820. doi: 10.1083/jcb.106.6.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal recognition particle-dependent membrane insertion of mouse invariant chain: a membrane-spanning protein with a cytoplasmically exposed amino terminus. J Cell Biol. 1986 Jun;102(6):2169–2175. doi: 10.1083/jcb.102.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler B., Hirsch H. H., Müller H., Wolf D. H. Biogenesis of the yeast lysosome (vacuole): biosynthesis and maturation of proteinase yscB. EMBO J. 1988 Jun;7(6):1705–1710. doi: 10.1002/j.1460-2075.1988.tb02999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler B., Müller H., Wolf D. H. Maturation of vacuolar (lysosomal) enzymes in yeast: proteinase yscA and proteinase yscB are catalysts of the processing and activation event of carboxypeptidase yscY. EMBO J. 1987 Jul;6(7):2157–2163. doi: 10.1002/j.1460-2075.1987.tb02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler B., Müller M., Müller H., Meussdoerffer F., Wolf D. H. In vivo biosynthesis of the vacuolar proteinases A and B in the yeast Saccharomyces cerevisiae. J Biol Chem. 1982 Oct 10;257(19):11203–11206. [PubMed] [Google Scholar]
- Mechler B., Wolf D. H. Analysis of proteinase A function in yeast. Eur J Biochem. 1981 Dec;121(1):47–52. doi: 10.1111/j.1432-1033.1981.tb06427.x. [DOI] [PubMed] [Google Scholar]
- Mitchell J. K., Fonzi W. A., Wilkerson J., Opheim D. J. A particulate form of alkaline phosphatase in the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1981 Feb 13;657(2):482–494. doi: 10.1016/0005-2744(81)90333-8. [DOI] [PubMed] [Google Scholar]
- Moehle C. M., Dixon C. K., Jones E. W. Processing pathway for protease B of Saccharomyces cerevisiae. J Cell Biol. 1989 Feb;108(2):309–325. doi: 10.1083/jcb.108.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2079–2082. [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1983 May 10;258(9):5614–5617. [PubMed] [Google Scholar]
- Onishi H. R., Tkacz J. S., Lampen J. O. Glycoprotein nature of yeast alkaline phosphatase. Formation of active enzyme in the presence of tunicamycin. J Biol Chem. 1979 Dec 10;254(23):11943–11952. [PubMed] [Google Scholar]
- Owada M., Neufeld E. F. Is there a mechanism for introducing acid hydrolases into liver lysosomes that is independent of mannose 6-phosphate recognition? Evidence from I-cell disease. Biochem Biophys Res Commun. 1982 Apr 14;105(3):814–820. doi: 10.1016/0006-291x(82)91042-7. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Hardwick K. G., Lewis M. J. Sorting of soluble ER proteins in yeast. EMBO J. 1988 Jun;7(6):1757–1762. doi: 10.1002/j.1460-2075.1988.tb03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Pohlig G., Rothman J. H., Stevens T. H. Structure, biosynthesis, and localization of dipeptidyl aminopeptidase B, an integral membrane glycoprotein of the yeast vacuole. J Cell Biol. 1989 Apr;108(4):1363–1373. doi: 10.1083/jcb.108.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. S., Klionsky D. J., Banta L. M., Emr S. D. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988 Nov;8(11):4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. H., Stevens T. H. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 1986 Dec 26;47(6):1041–1051. doi: 10.1016/0092-8674(86)90819-6. [DOI] [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984 Sep 25;259(18):11505–11508. [PubMed] [Google Scholar]
- Schauer I., Emr S., Gross C., Schekman R. Invertase signal and mature sequence substitutions that delay intercompartmental transport of active enzyme. J Cell Biol. 1985 May;100(5):1664–1675. doi: 10.1083/jcb.100.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. An internal signal sequence: the asialoglycoprotein receptor membrane anchor. Cell. 1986 Jan 17;44(1):177–185. doi: 10.1016/0092-8674(86)90496-4. [DOI] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. The use of endo-beta-N-acetylglucosaminidase H in characterizing the structure and function of glycoproteins. Biochem Biophys Res Commun. 1977 Oct 10;78(3):935–944. doi: 10.1016/0006-291x(77)90512-5. [DOI] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Sequence of a yeast DNA fragment containing a chromosomal replicator and the TRP1 gene. Gene. 1980 Jul;10(2):157–166. doi: 10.1016/0378-1119(80)90133-x. [DOI] [PubMed] [Google Scholar]
- Uchida E., Ohsumi Y., Anraku Y. Purification and properties of H+-translocating, Mg2+-adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1985 Jan 25;260(2):1090–1095. [PubMed] [Google Scholar]
- Valls L. A., Hunter C. P., Rothman J. H., Stevens T. H. Protein sorting in yeast: the localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell. 1987 Mar 13;48(5):887–897. doi: 10.1016/0092-8674(87)90085-7. [DOI] [PubMed] [Google Scholar]
- Waheed A., Gottschalk S., Hille A., Krentler C., Pohlmann R., Braulke T., Hauser H., Geuze H., von Figura K. Human lysosomal acid phosphatase is transported as a transmembrane protein to lysosomes in transfected baby hamster kidney cells. EMBO J. 1988 Aug;7(8):2351–2358. doi: 10.1002/j.1460-2075.1988.tb03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Pohlmann R., Hasilik A., von Figura K., van Elsen A., Leroy J. G. Deficiency of UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase in organs of I-cell patients. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1052–1058. doi: 10.1016/0006-291x(82)91076-2. [DOI] [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Woolford C. A., Daniels L. B., Park F. J., Jones E. W., Van Arsdell J. N., Innis M. A. The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation of Saccharomyces cerevisiae vacuolar hydrolases. Mol Cell Biol. 1986 Jul;6(7):2500–2510. doi: 10.1128/mcb.6.7.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa T., Ohsumi Y., Anraku Y. Solubilization and purification of alpha-mannosidase, a marker enzyme of vacuolar membranes in Saccharomyces cerevisiae. J Biol Chem. 1988 Apr 15;263(11):5158–5163. [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]