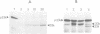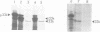Abstract
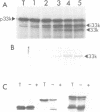
The 33 kd protein of the photosynthetic oxygen-evolving complex is synthesized in the cytoplasm as a larger precursor and transported into the thylakoid lumen via a stromal intermediate form. In this report we describe a reconstituted system in which the later stages of this import pathway can be studied in isolation. We demonstrate import of the 33 kd protein, probably as the intermediate form, into isolated pea thylakoids by a mechanism which is stimulated by the addition of ATP. The imported protein is processed to the mature size and is resistant to digestion by proteases. The thylakoidal protein transport system is specific in that non-chloroplast proteins and precursors of stromal proteins are not imported.
Keywords: chloroplast protein transport, precursor proteins, processing, thylakoid lumen proteins
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Straus J. W., Dudock B. S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cline K. Import of proteins into chloroplasts. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem. 1986 Nov 5;261(31):14804–14810. [PubMed] [Google Scholar]
- Flügge U. I., Hinz G. Energy dependence of protein translocation into chloroplasts. Eur J Biochem. 1986 Nov 3;160(3):563–570. doi: 10.1111/j.1432-1033.1986.tb10075.x. [DOI] [PubMed] [Google Scholar]
- Kirwin P. M., Elderfield P. D., Robinson C. Transport of proteins into chloroplasts. Partial purification of a thylakoidal processing peptidase involved in plastocyanin biogenesis. J Biol Chem. 1987 Dec 5;262(34):16386–16390. [PubMed] [Google Scholar]
- Kirwin P. M., Elderfield P. D., Williams R. S., Robinson C. Transport of proteins into chloroplasts. Organization, orientation, and lateral distribution of the plastocyanin processing peptidase in the thylakoid network. J Biol Chem. 1988 Dec 5;263(34):18128–18132. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamppa G. K. The chlorophyll a/b-binding protein inserts into the thylakoids independent of its cognate transit peptide. J Biol Chem. 1988 Oct 15;263(29):14996–14999. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. The effect of incorporation of amino acid analogues on the import and processing of chloroplast polypeptides. Eur J Biochem. 1985 Oct 1;152(1):67–73. doi: 10.1111/j.1432-1033.1985.tb09164.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Viitanen P. V., Doran E. R., Dunsmuir P. What is the role of the transit peptide in thylakoid integration of the light-harvesting chlorophyll a/b protein? J Biol Chem. 1988 Oct 15;263(29):15000–15007. [PubMed] [Google Scholar]