Abstract
Background
Human immunodeficiency virus (HIV) has disproportionately affected African Americans. Couple-level interventions may be a promising intervention strategy.
Methods
To determine if a behavioral intervention can reduce HIV/sexually transmitted disease (STD) risk behaviors among African American HIV serodiscordant couples, a cluster randomized controlled trial (Eban) was conducted in Atlanta, Georgia; Los Angeles, California; New York, New York; and Philadelphia, Pennsylvania; with African American HIV serodiscordant heterosexual couples who were eligible if both partners were at least 18 years old and reported unprotected intercourse in the previous 90 days and awareness of each other's serostatus. One thousand seventy participants were enrolled (mean age, 43 years; 40% of male participants were HIV positive). Couples were randomized to 1 of 2 interventions: couple-focused Eban HIV/STD risk-reduction intervention or attention-matched individual-focused health promotion comparison. The primary outcomes were the proportion of condom-protected intercourse acts and cumulative incidence of STDs (chlamydia, gonorrhea, or trichomonas). Data were collected preintervention and postintervention, and at 6- and 12-month follow-ups.
Results
Data were analyzed for 535 randomized couples: 260 in the intervention group and 275 in the comparison group; 81.9% were retained at the 12-month follow-up. Generalized estimating equation analyses revealed that the proportion of condom-protected intercourse acts was larger among couples in the intervention group (0.77) than in the comparison group (0.47; risk ratio, 1.24; 95% confidence interval [CI], 1.09 to 1.41; P=.006) when adjusted for the baseline criterion measure. The adjusted percentage of couples using condoms consistently was higher in the intervention group (63%) than in the comparison group (48%; risk ratio, 1.45; 95% CI, 1.24 to 1.70; P<.001). The adjusted mean number of (log)unprotected intercourse acts was lower in the intervention group than in the comparison group (mean difference, –1.52; 95% CI, –2.07 to –0.98; P<.001). The cumulative STD incidence over the 12-month follow-up did not differ between couples in the intervention and comparison groups. The overall HIV sero-conversion at the 12-month follow-up was 5 (2 in the intervention group, 3 in the comparison group) of 535 individuals, which translates to 935 per 100 000 population.
Conclusion
To our knowledge, this is the first randomized controlled intervention trial to report significant reductions in HIV/STD risk behaviors among African American HIV serodiscordant couples.
The human immunodeficiency virus (HIV)/AIDS epidemic continues to have a severe impact on African Americans living in urban areas of the United States.1 Although African Americans represented only 12% of the US population in 2006, 45% of new HIV infections occurred among African Americans.1 Rates of new infections were 7 times higher among African Americans than among white individuals.2 Heterosexual exposure was the most common HIV transmission category for African American women and the second most common category for African American men.
Studies have documented low condom use among African Americans with steady partners.3-5 This low prevalence of condom use among couples and high rate of heterosexualtransmissionsuggestaneedfor couple-based HIV/sexually transmitted disease(STD) prevention interventions for African Americans. Several studies found that couple-based HIV counseling and testing increased condom use6-8 and reduced HIV/ STD transmission in international settings9-17 among heterosexual couples, including HIV serodiscordant heterosexual couples.7,8,12 Although these studies had encouraging findings, they had 1 or more methodologic limitations, including small samples and a lack of an attention-control group, a randomized control design, assessment of both biological and behavioral outcomes, generalizability across geographic areas, and culturally congruent values and beliefs, which can enhance interventions’ efficacy.
A meta-analysis18 found that most HIV prevention interventions were less effective for African Americans, highlighting the need for culturally congruent approaches. A few recent randomized controlled trials (RCTs) demonstrated the efficacy of culturally congruent, individual- or group-based HIV prevention interventions for African Americans in increasing condom use and reducing unprotected intercourse and STD rates.19-21 These studies identified several effective components of culturally congruent HIV prevention interventions with African Americans,22 including emphasizing African American familial norms of cooperation and unity, using African American facilitators to communicate reality-based and credible information,23,24 and using Afrocentric videos, songs, and poetry to inspire African Americans to protect themselves.24
We report an RCT focusing exclusively on African American HIV serodiscordant heterosexual couples. Building on HIV prevention research with couples4,5,25 and high-risk African Americans,20,21,26,27 a culturally congruent couple-focused HIV/STD risk-reduction intervention was designed. In a cluster RCT, African American HIV sero-discordant couples in 4 cities (Atlanta, Georgia; Los Angeles, California; New York, New York; and Philadelphia, Pennsylvania) were allocated to 1 of 2 interventions, the Eban HIV/STD risk reduction or the health promotion comparison (Table 1). We hypothesized that couples in the Eban HIV/STD risk-reduction intervention group would report a higher proportion of condom-protected intercourse acts, more consistent condom use, and fewer unprotected intercourse acts, and would be less likely to test positive for an STD (ie, chlamydia, gonorrhea, or trichomonas) over the 12-month follow-up period compared with those in the comparison intervention group. Characteristics of the participants are presented in Table 2.
Table 1.
Random Allocation to HIV/STD Risk Reduction (RR) and Health Promotion (HP) Interventions, Overall and by Clinical Site
| HIV-Positive Partner, No. (%) |
|||||
|---|---|---|---|---|---|
| Site | Total Participants, No. (%) | Total No. of Cohort Groups (%) | Total No. of Couples (RR-HP) | Male | Female |
| All sites | 1070 (100) | 110 (147) | 535 (260-275) | 212 (40) | 323 (60) |
| Columbia University, New York, New York | 442 (41.31) | 40 (58) | 221 (104-117) | 79 (36) | 142 (64) |
| Emory University, Atlanta, Georgia | 234 (21.87) | 27 (33) | 117 (57-60) | 49 (42) | 68 (58) |
| University of California, Los Angeles | 200 (18.69) | 24 (30) | 100 (52-48) | 42 (42) | 58 (58) |
| University of Pennsylvania, Philadelphia | 194 (18.13) | 19 (26) | 97 (47-50) | 42 (43) | 55 (57) |
Abbreviation: HIV, human immunodeficiency virus.
Table 2.
Selected Characteristics at Baseline: All Randomized Participants and by Intervention Arma
| Characteristic | RR Group (n = 520) | HP Group (n = 550) | Overall (n = 1070) |
|---|---|---|---|
| Age, mean (SD), y | 43.25 (8.17) | 43.49 (8.16) | 43.41 (8.08) |
| Education, No. (%) | |||
| <HS graduate | 162 (31.52) | 164 (29.87) | 326 (30.67) |
| HS graduate/GED | 209 (40.66) | 228 (41.53) | 437 (41.11) |
| Some college | 143 (27.82) | 157 (28.60) | 300 (28.22) |
| Employed | 144 (28.07) | 158 (28.83) | 302 (28.46) |
| Monthly income, No. (%), $ | |||
| <400 | 156 (30.41) | 151 (27.61) | 307 (28.96) |
| 400-850 | 202 (39.38) | 244 (44.61) | 446 (42.08) |
| 851-1650 | 106 (20.66) | 99 (18.10) | 205 (19.34) |
| >1651 | 49 (9.55) | 53 (9.69) | 102 (9.62) |
| Insured, No. (%) | 377 (73.35) | 423 (77.33) | 800 (75.40) |
| Years lived in United States, mean (SD) | 41.91 (10.34) | 42.63 (9.45) | 42.29 (9.89) |
| Living arrangement, No. (%) | |||
| Live in own home/own apartment | 430 (83.66) | 468 (85.25) | 898 (84.48) |
| Live with nonrelative | 22 (4.28) | 27 (4.92) | 49 (4.61) |
| Rooming/welfare resident | 60 (11.67) | 51 (9.29) | 111 (10.44) |
| Homeless | 2 (0.39) | 3 (0.55) | 5 (0.47) |
| Living with study partner | 368 (71.88) | 438 (79.78) | 806 (75.97) |
| Time with study partner, mean (SD), y | 6.72 (7.31) | 7.45 (7.40) | 6.91 (6.56) |
| Married to study partner | 168 (32.68) | 177 (32.30) | 345 (32.49) |
| Previously incarcerated | 311 (60.86) | 350 (64.10) | 661 (62.54) |
| Alcohol dependence (CAGE questionnaire) | 80 (15.59) | 91 (16.58) | 171 (16.10) |
| Drug dependence (TCUDS) | 82 (15.98) | 100 (18.35) | 182 (17.20) |
| Outcomes | |||
| Proportion condom-protected sex, mean (SD) | 0.44 (0.43) | 0.44 (0.43) | 0.44 (0.43) |
| Unprotected sex, mean (SD) | 16.36 (28.93) | 14.83 (32.30) | 15.57 (30.71) |
| Consistent condom use, No. (%) | 111 (22.52) | 122 (23.28) | 233(22.91) |
| Concurrent partner, No. (%) | 98 (19.14) | 98 (18.01) | 196 (18.56) |
| Any STD, No. (%) | 79 (15.25) | 69 (12.64) | 148 (13.91) |
| HIV-positive participants only | |||
| Female, No. (%) | 158 (60.77) | 165 (60.00) | 323 (60.37) |
| Length of HIV diagnosis, mean (SD), mo | 9.62 (6.66) | 9.83 (7.84) | 9.73 (7.29) |
| CD4 lymphocyte count, mean (SD), cells/μL | 543.78 (325.42) | 510.74 (344.14) | 526.75 (335.14) |
| Don't know, No. (%) | 76 (29.23) | 87 (31.64) | 163 (31.47) |
| Viral load, No. (%), copies/mL | |||
| 0-50 | 61 (25.00) | 70 (25.93) | 131 (25.49) |
| >50 | 76 (31.15) | 73 (27.04) | 149 (28.99) |
| Don't know | 107 (43.85) | 127 (47.04) | 234 (45.53) |
Abbreviations: GED, General Educational Development test; HIV, human immunodeficiency virus; HP, health promotion; HS, high school; RR, risk reduction: STD, sexually transmitted disease; TCUDS, Texas Christian University Drug Screen.
SI conversion factor: To convert lymphocytes to cells × 109 L, multiply by 0.001.
Percentages do not sum to total because of missing data.
METHODS
Couples were enrolled at 4 sites, using a common recruitment protocol, from November 2003 through June 2007. The appropriate institutional review boards at each site approved the trial, and an independent National Institutes of Health– appointed data safety and monitoring board (DSMB) monitored it. Couples were eligible to participate if (1) each partner was at least 18 years old; (2) their relationship had existed for at least 6 months; (3) each partner intended to remain together for at least 12 months; (4) at least 1 partner reported having unprotected intercourse with the other in the previous 90 days; (5) each partner did not plan to relocate beyond a reasonable distance from the study site; (6) at least 1 partner self-identified as African American or black; (7) at least 1 partner reported that the couple was not planning a pregnancy within 18 months; (8) each partner was aware of the other's HIV serostatus; and (9) only 1 was HIV seropositive and had known that status for at least 3 months. To confirm the couples’ HIV serodiscordant status, we collected from both partners an oral specimen using OraSure test procedures (OraSure Technologies Inc, Bethlehem, Pennsylvania). Following an initial screening with an enzyme-linked immunosorbent assay, reactive specimens were confirmed using a Western blot assay. Using these same procedures, HIV-negative partners were tested for HIV at 12-month follow-up to determine the HIV seroconversion rate.
Couples were excluded if either partner (1) did not have a mailing address; (2) evidenced clinically significant psychiatric, physical, or neurological impairment that would limit effective participation as confirmed on a Mini-Mental State Examination; (3) reported victimization by severe violence perpetrated by the other in the past year, as assessed by the severe physical and sexual intimate partner violence subscales of the Revised Conflict Tactics Scale; (4) was unwilling or unable to commit to completing the study; or (5) was not fluent in English as determined by the consent process. Couples were also excluded if they had participated in a couple-based HIV/STD risk-reduction intervention in the past year.
To meet the sample size requirements and ensure a representative sample, we recruited participants from several sources, including HIV care clinics, AIDS service organizations, community-based organizations, targeted street out-reach, word-of-mouth, and the media, including radio, magazine, and newspaper advertisements. Recruiters informed potential participants about the study, obtained consent to be screened, and screened them for eligibility. People who seemed to be eligible were asked to invite their main sexual partner to participate. A letter to their partner that introduced the study was given to potential participants or mailed to their partner if the potential participants gave permission. Partners interested in participating were screened. If eligible, the recruiter scheduled the couple for baseline data collection. To permit comparisons between participants and eligible nonparticipants, the recruiter collected sociodemographic information and reasons for declining participation. Each participant was compensated.
Using a modified block randomization algorithm,28 we randomized groups of 3 to 5 couples to 1 of 2 interventions: couple-focused Eban HIV/STD risk reduction or individual-focused health promotion comparison. The sex of the HIV-positive partner was used as a blocking factor to ensure that couples with HIV-positive women were equally balanced across intervention arms. Randomized intervention assignments, generated and maintained by one of us (S.L.B, codirector of the Data Coordinating Center) were sent in sealed, confidential envelopes directly to the project director at each site, who executed the assignments.
The Eban HIV/STD risk-reduction intervention, described in detail elsewhere,29 incorporates Eban, a traditional African concept meaning “fence,” a symbol of safety, security, and love within one's family and relationship space. It was developed drawing on components from a previous couple-based HIV prevention intervention6,25 and group-based HIV prevention interventions14,21 that were found to be efficacious. It integrated components of social cognitive theory, historical and cultural beliefs about family and community preservation, and an Afrocentric paradigm into a relationship-oriented ecological frame work, described elsewhere.30 The focus was on multilevel risk and protective factors associated with HIV/STD risk reduction among African American HIV serodiscordant couples. (eAppendix, http://www.archinternmed.com).
Eban consisted of 8 weekly structured 2-hour sessions delivered by male and female African American cofacilitators who had at least a bachelor's degree and 2 years of clinical experience in HIV prevention or related fields. They received 40 hours of centralized facilitator training. The intervention included 4 sessions with individual couples and 4 with groups of couples. In the first half of session 1, a group of couples met with their cofacilitators; in the second half, participants met in single-sex groups with the same-sex facilitator. In sessions 2, 3, 4, and 8, each couple met separately with their cofacilitators. In sessions 5 to 7, group sessions were held.
Skills taught in individual couple sessions were reinforced in group sessions. Individual couple sessions were designed to address interpersonal factors associated with sexual risk reduction, including communication, problem solving, monogamy, and negotiation skills. Group sessions were designed to address community-level factors, including (1) increasing positive peer norms for condom use by emphasizing the threat of HIV to African American communities; (2) reducing the stigma associated with being African American couples affected by HIV; and (3) increasing social support for HIV risk reduction. The principles of Nguzu Saba (ie, unity, self-determination, collective work and responsibility, purpose, creativity, cooperative economics) were woven into the theme and content of the sessions and used to motivate couples to use condoms consistently to protect each other and their community.
The health promotion comparison intervention, described elsewhere,31 was designed to control for Hawthorne effects, to reduce the likelihood that effects of the Eban HIV/STD risk-reduction intervention could be attributed to nonspecific features, including group interaction and special attention. Guided by social cognitive theory, this intervention was structurally similar to the Eban HIV/STD risk-reduction intervention, containing the same number, type, duration, and sequencing of sessions implemented by African American male and female cofacilitators. It focused not on risk of STD, but on behaviors linked to risk of heart disease, hypertension, stroke, and certain cancers. It was designed to increase fruit and vegetable consumption, physical activity, and medical adherence, including HIV medication adherence. Unlike the Eban HIV/STD risk-reduction intervention, it focused on the participants as individuals, not as couples.
To ensure the fidelity of implementation for both interventions, as described elsewhere,29,31 facilitators used structured manuals with detailed implementation protocols, completed fidelity assessment forms after each session, met weekly with supervisors, and received reviews of audio-taped sessions and feedback from their supervisor. An independent quality assurance monitor also rated the fidelity of a random sample of 10% of sessions from each intervention.
Self-reported sexual behavior and biological specimens for STD assessments were collected independently from each partner at baseline, immediately postintervention, and 6 and 12 months postintervention. Facilitators were not involved in the data collection, and data collectors were blind to participants’ intervention. Individual-level responses were combined to form couple-level outcomes. Audio computer-assisted self-interviewing (ACASI) was used to collect self-reported sexual behaviors, including number of condom-protected vaginal and anal intercourse acts, number of unprotected vaginal or anal intercourse acts, and consistent condom use with study partner and incidence of concurrent partners in the past 90 days at baseline and follow-ups, and in the past 60 days at immediate postintervention. The timeline follow-back method was used to enhance recall of sexual behaviors.32
The primary behavioral outcome was the couple's reported proportion of condom-protected intercourse acts in the past 90 days, calculated as a weighted average of the partners’ responses. The denominator was the sum of vaginal and anal intercourse acts in the past 90 days reported by each partner (ie, 4 items); the numerator was the sum of male condom– and female condom–protected vaginal and anal intercourse acts in the past 90 days reported by each partner (ie, 8 items).
Consistent condom use, defined as condom use during every vaginal and anal intercourse act, was constructed by dichotomizing the proportion of condom-protected intercourse into 2 categories at unity. Specifically, couples in which both partners independently reported 100% condom use were considered consistent condom users, and all others were considered inconsistent condom users.
The total number of unprotected vaginal and anal intercourse acts was first constructed for each partner by subtracting the sum of the male condom– and female condom–protected vaginal and anal intercourse acts from the total number of intercourse acts with study partners in the past 90 days. In dividuals reporting no sexual activity in the past 90 days were assigned a value equal to zero for this outcome. Couple-level outcomes were then constructed by averaging the partners’ responses. Consistency of male and female partners’ reports for each of the shared behaviors was relatively high.33 The Spear-man correlation coefficient ranged from 0.42 to 0.65 (P<.001).
Concurrent partnerships were defined by individuals’ reports of intercourse with someone other than their study partner in the past 90 days. Couples were defined as having concurrent partners if at least 1 partner reported having a concurrent partner.
The couple-level cumulative incidence of STD was the primary biological outcome. We first constructed incidence measures for each partner at each postintervention visit. Women provided 2 self-collected vaginal swab specimens and men provided a urine specimen after completing the ACASI. Specimens were delivered to the Emory University pathology laboratory and assayed for Chlamydia trachomatis and Neisseria gonorrhoeae using the Becton Dickinson Probe ET Amplified DNA Assay (Becton, Dickinson and Co, Sparks, Maryland) and for Trichomonas vaginalis using a noncommercial real-time polymerase chain reaction assay.34 Participants with positive STD test results received directly observable single-dose antimicrobial treatment and risk-reduction counseling per Centers for Disease Control and Prevention recommendations. If a participant tested positive for an STD at baseline, the couple was treated within 7 to 14 days postbaseline; thus, both the participant and his or her partner were treated for the STD before collection of postintervention specimens. Participants were considered an incident STD case if at any of the 3 postintervention assessments they tested positive for any of the 3 STDs. Couples were incident cases if either partner was an incident case.
Participants also completed measures of sociodemo-graphic and relationship characteristics, including age, education, marital status, employment status, income, type of health insurance, incarceration history, length of relationship, quality of relationship, and cohabitation with the study partner. Partners who were HIV positive reported their length of diagnosis, CD4 lymphocyte count, and viral load. The CAGE questionnaire35 was used to assess lifetime alcohol dependence and the Texas Christian University Drug Screen (TCUDS)36 to identify individuals with a history of heavy drug use and dependence. Alcohol and drug problems were denoted by CAGE scores (α=0.73) greater than or equal to 2 and TCUDS scores (α=0.89) greater than or equal to 3, respectively.
This study was originally powered to detect an 8-percentage-point difference in STD incidence between the HIV/STD risk-reduction and health promotion interventions. Power was computed for a 2-sided, α=.05 level test, assuming a binomial model with the couple as the unit of analysis, controlling for the intraclass correlation coefficient (ICC) among responses of the 3 to 5 couples per group. Assuming 20% incidence in the health promotion group, compared with a 12% incidence in the Eban HIV/STD risk-reduction intervention group, it was determined that a sample of 800 couples (400 per arm) would yield a statistical power of 81%, accounting for an attrition rate of 20% at 12 months and an ICC of 0.01. On the basis of an interim analysis presented to the DSMB, a reduced target sample size was selected that would yield an estimated 80% power to detect the specified effect size for the primary behavioral end point. Site principal investigators, the National Institute of Mental Health (NIMH) staff collaborator, and site staff were blinded to the results of the interim analysis. Sample size calculations using the observed effect size for the biological end point suggested that even with 800 couples we would still have much lower power than originally anticipated. Considering time and funding constraints, the DSMB advised continuation of the final recruitment phase targeting the reduced sample size.
The primary analyses used standard intent-to-treat methods in which all available data on all randomized participants were included. To assess intervention effects, generalized estimating equation models were constructed, controlling for the correlations among repeated measures for couples over time and among responses of couples treated together as a group. Models for behavioral outcomes were adjusted for baseline response. Models for STD incidence were adjusted for baseline STD status, sex of the HIV-positive partner, and length of HIV diagnosis. We report unadjusted and adjusted data for baseline responses, estimated risk ratios for binary outcomes, and estimated mean differences for continuous outcomes at the immediate postintervention assessment, at 6-month and 12-month assessments, and over the postintervention period as a whole, corresponding 95% confidence intervals (CIs), and significance probabilities.
RESULTS
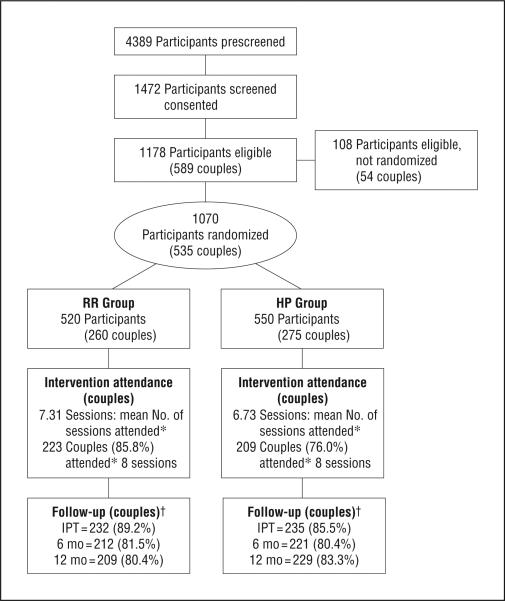
The Figure presents the flow of participants in the trial. Of the 589 couples that were eligible, 535 (90.8%) were randomized and included in primary analyses. A total of 72 groups with 260 couples were allocated to the Eban HIV/STD risk-reduction intervention; 75 groups with 275 couples were allocated to the health promotion intervention. The HIV-positive partner was female in most of the couples, and the percentage of couples with sero-positive female participants was comparable at the 4 sites, ranging from 57% to 64%. Attendance at the sessions of both interventions was very high. On average, couples in the Eban HIV/STD risk-reduction intervention attended 7.31 (SD, 1.88), or 91.4% of the sessions, and couples in the health promotion intervention attended 6.73 (SD,2.49), or 84.1% of the sessions (P=.003). The retention rate at immediate postintervention and 6- and 12-month postintervention assessments was 87.3% (467 couples), 80.9% (433 couples), and 81.9% (432 couples), respectively, and did not differ significantly between arms.
Figure.
Eban participant couple CONSORT diagram. HP indicates health promotion; IPT, immediate posttest; RR, risk reduction. *Attendance (full, partial, or make-up session completed by both partners of each couple). †Participants lost to follow-up: 18 in the RR group (7 deaths, 6 incarcerations, 2 no longer interested in participation, and 3 for other reasons) and 17 in the HP group (5 deaths, 5 incarcerations, 2 no longer interested, and 5 for other reasons).
Table 3 presents the descriptive statistics for outcomes by intervention condition and time. Table 4 presents effect size estimates and significance tests for the intervention effect at each postintervention assessment and over the postintervention period. In the unadjusted analyses, over the postintervention period, and at the immediate postintervention and 6- and 12-month assessments, the proportion of condom-protected acts of intercourse and the percentage reporting consistent condom use were greater and the number of unprotected intercourse acts was smaller among couples in the Eban HIV/STD risk-reduction intervention group than among couples in the health promotion intervention group. The adjusted analyses revealed similar results, with 1 exception. Couples in the Eban HIV/STD risk-reduction intervention group reported a similar proportion of condom-protected sex compared with couples in the health promotion intervention group at the 12-month assessment. There were no significant differences in incidence of concurrent partners between the 2 interventions in either analysis (unadjusted P =.81; adjusted P=.95).
Table 3.
Summary of Sexual Behavior Outcomes at Baseline, Immediate Postintervention Test (IPT), and 6- and 12-Month Follow-ups
| Follow-up |
||||
|---|---|---|---|---|
| Outcome | Baseline | IPT | 6 mo | 12 mo |
| Proportion of condom-protected sex, mean (SD) | ||||
| HIV/STD RR group | 0.44 (0.38) | 0.82 (0.28) | 0.75 (0.36) | 0.72 (0.38) |
| HP group | 0.44 (0.40) | 0.55 (0.43) | 0.56 (0.43) | 0.56 (0.43) |
| Consistent (100%) condom use, No. (%) | ||||
| HIV/STD RR group | 29 (11.15) | 110 (42.31) | 94 (36.15) | 95 (36.54) |
| HP group | 38 (13.82) | 75 (27.27) | 72 (26.18) | 73 (26.55) |
| Unprotected sex, mean (SD) | ||||
| HIV/STD RR group | 16.38 (23.66) | 2.80 (6.82) | 5.05 (20.75) | 5.92 (20.10) |
| HP group | 14.82 (25.24) | 8.52 (24.59) | 8.03 (17.42) | 7.25 (15.22) |
| Concurrent partners, No. (%) | ||||
| HIV/STD RR group | 49 (18.85) | 39 (15.00) | 48 (18.46) | 67 (25.77) |
| HP group | 49 (17.82) | 42 (15.27) | 47 (17.09) | 64 (23.27) |
Abbreviations: HIV/STD, human immunodeficiency virus/sexually transmitted disease; HP, health promotion; RR, risk reduction.
Table 4.
Longitudinal Analysis of HIV/STD Risk Behaviors, Adjusting for Clustering Within Randomized Group (Unadjusted and Adjusted for Baseline Response)a
| Treatment Effects | Proportion of Condom-Protected Sex |
Consistent (100%) Condom Use |
(log)Unprotected Sex |
Concurrent Partners |
||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI)b | P Value | RR (95% CI)b | P Value | Difference (95%CI)c | P Value | RR (95% CI)b | P Value | |
| Unadjusted for Baseline Response | ||||||||
| Baseline | 0.98 (0.77 to 1.24) | .84 | 0.81 (0.52 to1.27) | .36 | 0.35 (–0.21 to 0.90) | .22 | 1.06 (0.74 to 1.52) | .74 |
| Over entire FU | 1.36 (1.16 to 1.59) | <.001 | 1.23 (1.02 to 1.50) | .03 | –0.93 (–1.46 to –0.41) | <.001 | 1.04 (0.80 to 1.34) | .81 |
| IPT | 1.89 (1.49 to 2.40) | <.001 | 1.47 (1.17 to 1.85) | .003 | –1.44 (–2.18 to –0.70) | <.001 | 0.95 (0.64 to 1.42) | .81 |
| 6 mo | 1.37 (1.10 to 1.72) | .008 | 1.44 (1.13 to 1.83) | .006 | –1.65 (–2.41 to –0.90) | <.001 | 1.05 (0.73 to 1.49) | .81 |
| 12 mo | 1.34 (1.04 to 1.72) | .02 | 1.35 (1.07 to 1.71) | .02 | –0.99 (–1.76 to –0.22) | .01 | 1.09 (0.82 to 1.46) | .81 |
| ICC | 0.48 | 0.31 | 0.42 | 0.42 | ||||
|
Adjusted for Baseline Response | ||||||||
| Over entire FU | 1.24 (1.09 to 1.41) | .006 | 1.45 (1.24 to 1.70) | <.001 | –1.52 (–2.07 to –0.98) | <.001 | 1.01 (0.81 to 1.25) | .95 |
| IPT | 1.49 (1.13 to 1.95) | .009 | 1.39 (1.13 to 1.71) | .002 | –1.63 (–2.30 to –0.95) | <.001 | 1.06 (0.76 to 1.49) | .95 |
| 6 mo | 1.22 (1.05 to 1.41) | .01 | 1.57 (1.27 to 1.94) | <.001 | –1.79 (–2.50 to –1.08) | <.001 | 0.96 (0.71 to 1.29) | .95 |
| 12 mo | 1.05 (0.85 to 1.30) | .64 | 1.40 (1.13 to 1.75) | .003 | –1.15 (–1.88 to –0.42) | .002 | 1.01 (0.78 to 1.30) | .95 |
| ICC | 0.34 | 0.37 | 0.41 | 0.31 | ||||
Abbreviations: CI, confidence interval; ICC, estimated intraclass correlation coefficient from exchangeable working correlation matrix; FU, follow-up; HIV/STD, human immunodeficiency virus/sexually transmitted disease; IPT, immediate posttest; RR, risk ratio.
All P values were adjusted.
Empirical RR (risk reduction vs health promotion) estimates examining treatment effects for behavioral outcomes of interest with “independence” working correlation specified.
Difference (risk reduction minus health promotion) estimates examining treatment effects for behavioral outcomes of interest with “independence” working correlation specified.
In the unadjusted analyses and adjusted analyses, the cumulative STD incidence did not significantly differ in the Eban HIV/STD risk-reduction intervention group compared with the health promotion intervention group over the postintervention period (risk ratio, 0.98; 95% CI, 0.62-1.56;P=.93)or at any postintervention assessment (P>.35). The overall HIV seroconversion at 12-month follow-up was 5 (2 in the risk-reduction intervention group, 3 in the health promotion group) of 535 individuals, which translates to 935 per 100 000 population.
COMMENT
This trial demonstrated that a theory-based culturally congruent intervention can reduce self-reported sexual risk behavior among African American HIV serodiscordant couples. The intervention had significant effects, averaged over the 1-year follow-up period, on the primary behavioral outcome, the proportion of condom-protected sex, and the percentage of couples practicing consistent condom use, and the number of unprotected sex acts in which couples engaged. The overall magnitude and consistency of findings across the sexual behavior outcomes strengthen confidence in the intervention's efficacy.
Public health scientists have urged a shift beyond individual-level HIV interventions to prevention strategies that have an impact on social structures and context to curb the epidemic among African Americans.37,38 The intervention used here, in structure and content, was relationship based and redirected the focus to changing the relationship factors that influence sexual decision making and increasing the likelihood that risk reduction will be stable over time. Individual, couple, and group formats were used to maximize discussions of relationships and communication about risk reduction. Male and female cofacilitators led the intervention and modeled the communication and transparency needed when 2 individuals need to share responsibility for safer sex practices along with relationship maintenance. Cultural congruence was achieved by integrating concepts of Nguzu Saba39 into each session. The findings strengthen the accumulating evidence on the efficacy of couple-based HIV/ STD prevention strategies5,15,25 and expand the repertoire of efficacious interventions for couples.
In contrast to the significant effects on the primary and secondary sexual behavior outcomes, the intervention did not influence the incidence of STDs. This may have occurred because the intervention did not affect concur rency. Recall that if a participant tested positive for an STD, both partners were treated. Thus, participants had to have unprotected intercourse with a concurrent partner who had an STD to contract an STD after the intervention. The Eban HIV/STD risk-reduction intervention did not reduce rates of concurrency. Future research should examine strategies to reduce concurrent partnerships in HIV serodiscordant couples.
To our knowledge, this is the first study designed for African American HIV serodiscordant couples to publish HIV seroconversion rates. The observed HIV seroconversion rate, 935 per 100 000, was substantially larger than the annual HIV incidence estimate overall for African Americans2 of 83.8 per 100 000. Thus, HIV negative African Americans in HIV serodiscordant relationships, even relatively stable relationships, are at substantially high risk for HIV acquisition.
This study has a number of strengths. It used a randomized controlled design and a dose and modality equivalent comparison group, controlling for group interaction and special attention. Sampling couples in 4 geographical areas of the United States increased generalizability. The study also had limitations. The sample may not be representative of all African American HIV serodiscordant couples. The participating couples knew they were in an HIV serodiscordant relationship, whereas many people in such relationships do not realize it. The findings may not generalize to such people. The primary behavioral outcome was measured with self-reports, which can be influenced by socially desirable responding. However, the use of ACASI, testing participants for STDs, and collection of data on shared behaviors from partners may have mitigated potential problems with self-report validity.
In conclusion, to our knowledge, this is the first study to demonstrate the efficacy of an HIV/STD intervention in reducing sexual risk behavior among African American HIV serodiscordant couples. It shows that couples at high risk of transmitting HIV can be recruited for such interventions, are willing to attend multiple intervention sessions, and can be retained for follow-up efficacy assessments. The findings draw attention to an effective intervention strategy that may be scaled up to curb the magnitude and continued spread of HIV and other STDs. Future studies must explore the generalizability of the findings to couples irrespective of serostatus and in settings where individuals and couples are not aware of their risks for HIV transmission2,40,41 but whose relationships can be supported as they learn to minimize risks for themselves and each other. Moreover, the approach of engaging couples should be tested elsewhere in the United States and in other parts of the world, including sub-Saharan Africa, where sex-based power imbalances make it especially difficult for women in couples to reduce their risk of heterosexual exposure to HIV and other STDs.
Acknowledgments
Funding/Support: This trial was funded by research grants from the National Institute of Mental Health (NIMH) of the US National Institutes of Health. This trial was supported by NIMH funds to Dr El-Bassel (U10 MH064395), Dr Jemmott (2 U10 MH64394), Dr Landis (U10 MH078819), Dr Wingood (U10 MH064393), and Dr Wyatt (5 U10 MH064404). Dr Pequegnat was the federal principal investigator (the NIMH staff collaborator) and her involvement on the study did not present a financial conflict.
Footnotes
Author Contributions: The first 6 authors are the scientific steering committee for the trial, and their names are listed in alphabetical order. Study concept and design: El-Bassel, Jemmott, Landis, Pequegnat, Wingood, and Bellamy. Acquisition of data: Jemmott, Landis, Pequegnat, Wingood, Wyatt, and Bellamy. Analysis and interpretation of data: El-Bassel, Jemmott, Landis, Wingood, and Bellamy. Drafting of the manuscript: El-Bassel, Jemmott, Landis, Pequegnat, Wingood, Wyatt, and Bellamy. Critical revision of the manuscript for important intellectual content: Jemmott, Landis, Pequegnat, Wingood, Wyatt, and Bellamy. Statistical analysis: Jemmott, Landis, and Bellamy. Obtained funding: El-Bassel, Jemmott, Landis, Wingood, and Wyatt. Administrative, technical, and material support: Landis, Pequegnat, Wingood, and Wyatt. Study supervision: Jemmott, Landis, Pequegnat, Wingood, and Wyatt.
Trial Registration: clinicaltrials.gov Identifier: NCT00644163
Online-Only Material: An eAppendix is available at http://www.archinternmed.com.
Financial Disclosure: None reported.
REFERENCES
- 1.OMHD AMH Factsheets HIVAIDS: eliminate disparities in HIV and AIDS [November 4, 2008];Office of Minority Health and Health Disparities (OMHD) 2007 http://www.cdc.gov /omhd/amh/factsheets/hiv.htm.
- 2.Centers for Disease Control and Prevention (CDC) Subpopulation estimates from the HIV incidence surveillance system: United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(36):985–989. [PubMed] [Google Scholar]
- 3.Catania JA, Coates TJ, Stall R, et al. Prevalence of AIDS-related risk factors and condom use in the United States. Science. 1992;258(5085):1101–1106. doi: 10.1126/science.1439818. [DOI] [PubMed] [Google Scholar]
- 4.Hunt WK, Myers HF, Dyche M. Living with risk: male partners of HIV-positive women. Cult Diversity Ethnic Minor Psychol. 1999;5(3):276–286. doi:10.1037/1099-9809.5.3.276. [Google Scholar]
- 5.Wyatt GE, Moe A, Guthrie D. The gynecological, reproductive, and sexual health of HIV-positive women. Cult Diversity Ethnic Minor Psychol. 1999;5(3):183–196. doi:10.1037/1099-9809.5.3.183. [Google Scholar]
- 6.El-Bassel N, Witte SS, Gilbert L, et al. The efficacy of a relationship-based HIV/STD prevention program for heterosexual couples. Am J Public Health. 2003;93(6):963–969. doi: 10.2105/ajph.93.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deschamps MM, Pape JW, Haffner A, Hyppolite R, Johnson WD. Heterosexual activity in at risk couples for HIV infection [abstract W.C.3089.]. Int Conf AIDS. 1991;(7):318. [Google Scholar]
- 8.Higgins DL, Galavotti C, O'Reilly KR, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA. 1991;266(17):2419–2429. [PubMed] [Google Scholar]
- 9.Allen S, Tice J, Van de Perre P, et al. Effect of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. BMJ. 1992;304(6842):1605–1609. doi: 10.1136/bmj.304.6842.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glick P. Scaling up HIV voluntary counseling and testing in Africa: what can evaluation studies tell us about potential prevention impacts? Eval Rev. 2005;29(4):331–357. doi: 10.1177/0193841X05276437. [DOI] [PubMed] [Google Scholar]
- 11.van der Straten A, Vernon KA, Knight KR, Gomez CA, Padian NS. Managing HIV among serodiscordant heterosexual couples: serostatus, stigma and sex. AIDS Care. 1998;10(5):533–548. doi: 10.1080/09540129848406. [DOI] [PubMed] [Google Scholar]
- 12.Kamenga M, Ryder RW, Jingu M, et al. Evidence of marked sexual behavior change associated with low HIV-1 seroconversion in 149 married couples with discordant HIV-1 serostatus: experience at an HIV counselling center in Zaire. AIDS. 1991;5(1):61–67. doi: 10.1097/00002030-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt GE, Myers HF, Loeb TB. Women, trauma, and HIV: an overview. AIDS Behav. 2004;8(4):401–403. doi: 10.1007/s10461-004-7324-3. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt GE, Longshore D, Chin D, et al. The efficacy of an integrated risk reduction intervention for HIV-positive women with child sexual abuse histories. AIDS Behav. 2004;8(4):453–462. doi: 10.1007/s10461-004-7329-y. [DOI] [PubMed] [Google Scholar]
- 15.Voluntary HIV-1 Counseling and Testing Efficacy Study Group Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000;356(9224):103–112. [PubMed] [Google Scholar]
- 16.Allen S, Serufilira A, Bogaerts J, et al. Confidential HIV testing and condom promotion in Africa: impact on HIV and gonorrhea rates. JAMA. 1992;268(23):3338–3343. [PubMed] [Google Scholar]
- 17.Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985-1997. Am J Public Health. 1999;89(9):1397–1405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mize SJ, Robinson BE, Bockting WO, Scheltema KE. Meta-analysis of the effectiveness of HIV prevention interventions for women. AIDS Care. 2002;14(2):163–180. doi: 10.1080/09540120220104686. [DOI] [PubMed] [Google Scholar]
- 19.Myers HF, Wyatt GE, Loeb TB, et al. Severity of child sexual abuse, post-traumatic stress and risky sexual behaviors among HIV-positive women. AIDS Behav. 2006;10(2):191–199. doi: 10.1007/s10461-005-9054-6. [DOI] [PubMed] [Google Scholar]
- 20.Jemmott LS, Jemmott JB, III, O'Leary A. Effects on sexual risk behavior and STD rate of brief HIV/STD prevention interventions for African American women in primary care settings. Am J Public Health. 2007;97(6):1034–1040. doi: 10.2105/AJPH.2003.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiClemente RJ, Wingood GM, Harrington KF, et al. Efficacy of an HIV prevention intervention for African American adolescent girls: a randomized controlled trial. JAMA. 2004;292(2):171–179. doi: 10.1001/jama.292.2.171. [DOI] [PubMed] [Google Scholar]
- 22.Wyatt GE. Enhancing cultural and contextual intervention strategies to reduce HIV/AIDS among African Americans. Am J Public Health. 2009;99(11):1941–1945. doi: 10.2105/AJPH.2008.152181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyatt GE, Williams JK, Myers HF. African-American sexuality and HIV/AIDS: recommendations for future research. J Natl Med Assoc. 2008;100(1):44–48, 50-51. doi: 10.1016/s0027-9684(15)31173-1. [DOI] [PubMed] [Google Scholar]
- 24.Scott KD, Gilliam A, Braxton K. Culturally competent HIV prevention strategies for women of color in the United States. Health Care Women Int. 2005;26(1):17–45. doi: 10.1080/07399330590885795. [DOI] [PubMed] [Google Scholar]
- 25.El-Bassel N, Witte SS, Gilbert L, et al. Long-term effects of an HIV/STI sexual risk reduction intervention for heterosexual couples. AIDS Behav. 2005;9(1):1–13. doi: 10.1007/s10461-005-1677-0. [DOI] [PubMed] [Google Scholar]
- 26.Jemmott LS, Jemmott JB, Hutchinson MK, Cederbaum JA, O'Leary A. Sexually transmitted infection/HIV risk reduction interventions in clinical practice settings. J Obstet Gynecol Neonatal Nurs. 2008;37(2):137–145. doi: 10.1111/j.1552-6909.2008.00221.x. [DOI] [PubMed] [Google Scholar]
- 27.Jemmott JB, III, Jemmott LS, Braverman PK, Fong GT. HIV/STD risk reduction interventions for African American and Latino adolescent girls at an adolescent medicine clinic: a randomized controlled trial. Arch Pediatr Adolesc Med. 2005;159(5):440–449. doi: 10.1001/archpedi.159.5.440. [DOI] [PubMed] [Google Scholar]
- 28.Bellamy SL. NIMH Multisite HIV/STD Prevention Trial for African American Couples Study Group. A dynamic block-randomization algorithm for group-randomized clinical trials when the composition of blocking factors is not known in advance. Contemp Clin Trials. 2005;26(4):469–479. doi: 10.1016/j.cct.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 29.NIMH Multisite HIV/STD Prevention Trial for African American Couples Group Eban HIV/STD risk reduction intervention: conceptual basis and procedures. J Acquir Immune Defic Syndr. 2008;49(suppl 1):S15–S27. doi: 10.1097/QAI.0b013e318184255d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronfenbrenner U. Toward an experimental ecology of human development. Am Psychol. 1977;32(7):513–531. doi:10.1037/0003-066X.32.7.513. [Google Scholar]
- 31.NIMH Multisite HIV/STD Prevention Trial for African American Couples Group Eban health promotion intervention: conceptual basis and procedures. J Acquir Immune Defic Syndr. 2008;49(suppl 1):S28–S34. doi: 10.1097/QAI.0b013e3181842548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carey MP, Carey KB, Maisto SA, Gordon CM, Weinhardt LS. Assessing sexual risk behaviour with the timeline followback (TLFB) approach: continued development and psychometric evaluation with psychiatric outpatients. Int J STD AIDS. 2001;12(6):365–375. doi: 10.1258/0956462011923309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NIMH Multisite HIV/STD Prevention Trial for African American Couples Group Concordant and discordant reports on sexual behaviors and condom use. AIDS Behav. doi: 10.1007/s10461-010-9699-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caliendo AM, Jordan JA, Green AM, Ingersoll J, Diclemente RJ, Wingood GM. Real-time PCR improves detection of trichomonas vaginalis infection compared with culture using self-collected vaginal swabs. Infect Dis Obstet Gynecol. 2005;13(3):145–150. doi: 10.1080/10647440500068248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252(14):1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 36.Peters RH, Greenbaum PE, Steinberg ML, et al. Effectiveness of screening instruments in detecting substance use disorders among prisoners. J Subst Abuse Treat. 2000;18(4):349–358. doi: 10.1016/s0740-5472(99)00081-1. [DOI] [PubMed] [Google Scholar]
- 37.Friedman SR, Cooper HL, Osborne AH. Structural and social contexts of HIV risk among African Americans. Am J Public Health. 2009;99(6):1002–1008. doi: 10.2105/AJPH.2008.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adimora AA, Schoenbach VJ, Doherty IA. HIV and African Americans in the southern United States: sexual networks and social context. Sex Transm Dis. 2006;33(7)(suppl):S39–S45. doi: 10.1097/01.olq.0000228298.07826.68. [DOI] [PubMed] [Google Scholar]
- 39.Karenga M, Kwanzaa . A Celebration of Family, Community, & Culture. University of Sankore Press; Timbuktu, Mali: 1998. [Google Scholar]
- 40.Centers for Disease Control and Prevention [January 14, 2009];Data, statistics, and reports. 2008 http://www.cdc.gov/std/stats08/default.htm.
- 41.O'Leary A, Jemmott LS, Jemmott JB. Mediation analysis of an effective sexual risk-reduction intervention for women: the importance of self-efficacy. Health Psychol. 2008;27(2)(suppl):S180–S184. doi: 10.1037/0278-6133.27.2(Suppl.).S180. [DOI] [PubMed] [Google Scholar]