Abstract
Introduction
Currently, evidence supports the use of adjuvant endocrine therapy with aromatase inhibitors in postmenopausal patients with hormone-receptor positive breast cancer. The goal of the current study is to understand the impact of patient age and health status on the decision of the oncologist to recommend adjuvant endocrine therapy (with or without chemotherapy) in older women with hormone-receptor positive breast cancer.
Methods
An on-line survey was conducted, with questions related to treatment of a hypothetical patient of varying age and health status with a T2N2 hormone-receptor positive, HER2-negative breast cancer. Treatment options included chemotherapy and endocrine therapy, endocrine therapy alone, or no therapy. With recommendation of endocrine therapy, respondents were further asked to specify use of either tamoxifen or aromatase inhibitors. A generalized linear mixed-effects model was used to determine the impact of age and health status on treatment recommendations.
Results
As the hypothetical patient’s age increased or health status deteriorated, oncologists were less likely to recommend a combination of chemotherapy and endocrine therapy (P<0.0001 for both). In contrast, oncologists were more likely to recommend endocrine therapy alone with advanced age (P<0.0001) and deteriorating health status (P<0.0001). With respect to the type of endocrine therapy selected, oncologists were more likely to choose treatment with aromatase inhibitors as opposed to tamoxifen (P<0.01), irrespective of age or health status. No therapy was infrequently recommended, constituting 2% of responses on average.
Conclusions
With increasing age and declining health status, oncologists were more likely to recommend treatment with endocrine therapy alone as opposed to the combination of chemotherapy with endocrine therapy. Oncologists were most likely to recommend use of aromatase inhibitors, irrespective of age or health status.
INTRODUCTION
Endocrine manipulation represents a critical component of breast cancer therapy, particularly in those patients with hormone receptor positive tumors.1 In the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis, tamoxifen therapy was shown to reduce the annual breast cancer death rate by 31% in estrogen receptor (ER)-positive patients.1 A number of recent and ongoing trials have compared the clinical benefit of tamoxifen to that of the third-generation aromatase inhibitors (AIs) anastrozole, letrozole and exemestane.2–7 The Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial provides a direct comparison adjuvant therapy with tamoxifen or anastrazole (or the combination of both), and represents the longest trial experience to date.4 The most recent analysis performed of the ATAC trial suggested a 15% benefit in disease-free survival (DFS) at 100 months median follow-up with anastrazole therapy as compared to tamoxifen. Hazard ratios were similar for patients above and below the age of 65. A comparison of 5 years of letrozole to 5 years of tamoxifen in the Breast International Group (BIG) 1–98 trial revealed similar results with respect to DFS, even amongst patients over the age of 75.7,8 As an alternative approach, several trials suggest switching from tamoxifen to anastrazole or exemestane after 2–3 years yields a benefit in DFS over tamoxifen therapy alone—furthermore, switching to exemestane may yield a benefit in OS in ER-positive patients.2,3,6 The collective clinical trial experience to date also suggests distinct toxicity considerations with both classes of agents; while tamoxifen leads to a higher incidence of thromboembolic events and endometrial malignancies, AIs may cause accelerated bone loss and arthralgias.9
Aside from the choice of endocrine therapy, a significant question remains regarding the benefit of combined chemotherapy and endocrine therapy in the older adult. The EBCTCG meta-analysis suggests an independent and additive benefit from chemotherapy and endocrine therapy in ER-positive patients.1 However, patients over the age of 70 only constituted roughly 5% of the population assessed, limiting the ability to confidently extrapolate these benefits to an older population. Two Survival, Epidemiology and End Results (SEER) database analyses suggested that certain subsets of older adults may derive greater benefit from adjuvant chemotherapy (i.e., those with lymph node-positive, ER-negative breast cancer).10,11 Prospective data to address the issue of combined chemotherapy and endocrine therapy in older adults is limited to one report from the French Adjuvant Study Group (FASG) 08 trial.12 In this trial, addition of anthracycline chemotherapy to 3 years of tamoxifen (30 mg oral daily) led to an improvement in DFS as compared to tamoxifen alone in patients with lymph node positive breast cancer over the age of 65. Trials comparing chemotherapeutic strategies in older adults are similarly lacking – to date, only one randomized, prospective analysis provides such a comparison. In Cancer and Leukemia Group B (CALGB) 49907, adjuvant therapy with capecitabine alone was shown to be inferior to standard therapies (including adriamycin and cylophosphamide, AC, and cyclophosphamide, methotrexate and 5-fluorouracil, CMF) in patients over the age of 65.13
The relative paucity of clinical trial data in older adults with breast cancer poses a substantial challenge to the oncologist with respect to medical decision-making.14,15 Guidelines from the National Comprehensive Cancer Network (NCCN) suggest that the decision to withhold chemotherapy to endocrine therapy in older adults should be individualized.16 Supplementing this, the Assessing Care of Vulnerable Elders (ACOVE)-derived guidelines suggest that life expectancy should strongly inform the decision to administer chemotherapy in locally advanced breast cancer.17 Both publications underscore the need to carefully evaluate health status and co-morbidities in the older adult. However, the lack of a standardized approach to stratify and assess the older patient renders critical importance to the attitudes of the oncologist in the determination of treatment choice. As such, the goal of the current study was to understand the impact of patient age and health status in the decision of the oncologist to recommend adjuvant endocrine therapy (either alone or in combination with chemotherapy) for high risk estrogen-receptor (ER) positive, HER2-negative breast cancer.
METHODS
Data utilized in the current study was obtained through an on-line survey of physicians conducted in 2007. The survey was distributed to two categories of respondents, namely (1) medical oncologists and (2) primary care providers. Previous analyses using the acquired dataset have explored differences in treatment recommendations amongst these two respondent categories, and have further addressed oncologists’ recommendations for patients with HER2-overexpressing disease.18,19 In the current analysis, responses from oncologists were assessed with respect to treatment recommendations for hormone receptor positive, non-HER2-overexpressing disease. Candidates for the survey study were identified using the American Medical Association (AMA) member database. A list of potential study participants was identified, with a distribution of years in practice, region, and gender representative of the database at large. Invitation letters were distributed to this group, and respondents who met eligibility criteria were enrolled until the target sample size was reached. It was determined that 150 respondents were required to yield 80% power for detecting a minimal difference of 9.7% in treatment recommendations with a Type I error of 5%. All respondents who completed the survey were compensated with $150 honoraria. Prior to distribution of invitation letters, the study had been approved by the Internal Review Boards of the University of California, Los Angeles and the City of Hope Comprehensive Cancer Center. Notably, participants were excluded if they worked for a market research company, manufacturer or distributor of pharmaceutical products, a pharmacy, a drug store, the Food and Drug Administration (FDA) or an advertising agency.
Procedure
The current analysis is focused on survey questions pertaining to a hypothetical patient with a 4 cm breast tumor with 4 positive axillary lymph nodes (T2N2). Respondents received pathologic data pertaining to the patient including hormone receptor status (ER and PR positive) and HER2 status (HER2 negative). The age and health status of the hypothetical patient were varied. Specifically, age was varied on five-year increments between 70 and 85 years. Health status was categorized as either “good”, “average”, or “poor”. “Good” health status was represented by an individual with no medical co-morbidities and a schedule including 3 days of exercise per week. In contrast, “average” health status was represented by a patient with non-insulin dependent diabetes and hypertension who requires assistance with housework but lives independently. Finally, “poor” health status was represented by a patient who lives independently but requires a home health aid for tasks such as dressing, housework and shopping. Furthermore, this hypothetical patient had a history of transient ischemic attack, coronary artery bypass grafting, and severe osteoarthritis. An anticipated longevity was provided in association with each gradation of health status, with a range of 6.1 to 15.8 years for “good” health status, 5.9 to 14.8 years for “average” health status and 4.5 to 8.6 years for “poor” health status.
Hypothetical case scenarios were presented to survey respondents in random order of health status and age of the patient. For each hypothetical patient, the respondent chose between treatment options including chemotherapy and endocrine therapy in combination, endocrine therapy alone, no therapy, or “other”. The choice of “other” was re-coded appropriately if the text entry corresponded to an available treatment choice. The choice of endocrine therapy was further sub-divided into tamoxifen or one of three aromatase inhibitors for adjuvant breast cancer treatment (letrozole, anastrozole, or exemestane).
Statistical Analysis
Four treatment choices were presented to the respondents initially, which included (1) anthracycline-based chemotherapy and endocrine therapy, (2) non-anthracycline-based chemotherapy and endocrine therapy, (3) endocrine therapy alone, and (4) no therapy. To assess the recommendation of chemotherapy irrespective of type (i.e., anthracycline versus non-anthracycline), responses were consolidated into three categories: (1) chemotherapy with endocrine therapy, (2) endocrine therapy alone, and (3) no therapy. We applied the generalized linear mixed-effects model (GLMM) for multinomial outcomes to examine the effects of patient age and health and therapeutic choice while adjusting for oncologist-level covariates. We adopted the estimation method of Kuss and McLerran using SAS PROC GLIMMIX, which reformulates a hierarchical multinomial to a hierarchical multivariate binomial problem. This estimation method uses marginal quasi-likelihood estimators in the random effects model to approximate solutions for the Generalized Estimation Equation (GEE) for marginal models. We applied this method to perform log-binomial regression for estimating rate ratios. Rate ratio is the ratio of the proportion of oncologists recommending a treatment at a particular patient condition, such as poor health at age 85, relative to the proportion recommending the treatment at another patient condition, such as good health at age 85. The GLMM multinomial model was estimated by setting chemotherapy with endocrine therapy as the referent category. The expected multinomial probabilities for each of the three response categories were used for calculating rate ratios. However, determining the significance of a covariate effect on the specific choice of adjuvant therapy was based on dichotomizing the outcomes, and applying the GLMM log-binomial regression using GEE with exchangeable working correlation matrix. Among oncologists (except those who selected no therapy), the expected probabilities and the effects of covariates on the choice of either tamoxifen or AI (combining exemestane, letrozole, and anastrozole) were estimated using the GLMM log-binomial regression.
Medical oncologist characteristics considered as covariates included physician age, gender, geographic region, number of years in practice, number of patients with any type of disease treated in the month prior to the survey, percentage of patients aged 65 years or older, number of breast cancer patients in the current patient population, number and percent of current breast cancer patients who are post-menopausal, number and percent of current breast cancer patients who are on adjuvant hormonal therapy, and the number of post-menopausal breast cancer patients ever treated. Covariates were treated as both continuous and categorical. Patient age and health status were treated as categorical variables. The significance of the effects of age, health, and their interaction were assessed using a two-sided Wald’s test with Type I error of 0.05. Proc GLIMMIX and proc GENMOD (SAS 9.1, SAS Institute, Cary, NC) were used.
RESULTS
Oncologist Characteristics
A total of 151 medical oncologists participated in the survey; their characteristics are listed in Table 1. The mean age of oncologists surveyed was 50.1. The majority of respondents were male (81.5%) and 47% had been in practice for more than 20 years. Furthermore, oncologists were treating a median of 200 patients with breast cancer, with the majority of these patients being postmenopausal.
Table 1.
Characteristics of 151 medical oncologists participating in the current survey.
| Physician Characteristics | No. Oncologists (%) | P Valuea |
|---|---|---|
| Age, y | ||
| 30–39 | 23 (15.2) | |
| 40–49 | 39 (25.8) | <.0001 |
| 50–59 | 70 (46.4) | |
| 60+ | 19 (12.6) | |
| Mean ± SD | 50.1 ± 8.6 | |
|
| ||
| Sex | ||
| Male | 123 (81.5) | <.0001 |
| Female | 28 (18.5) | |
|
| ||
| Regionb | ||
| Northeast | 32 (21.2) | |
| Midwest | 35 (23.2) | .34 |
| South | 47 (31.1) | |
| West | 37 (24.5) | |
|
| ||
| No. years in practice | ||
| <10 | 29 (19.2) | |
| 10–19 | 51 (33.8) | .0002 |
| 20+ | 71 (47.0) | |
| Mean ± SD | 17.3 ± 7.8 | |
|
| ||
| No. patients treated (last mo.) | ||
| <200 | 26 (17.2) | |
| 200–299 | 47 (31.1) | .09 |
| 300–399 | 36 (23.8) | |
| 400+ | 42 (27.8) | |
| Median | 300 | |
|
| ||
| Percentage of patients over the age of 65 | ||
| 0% – 25% | 6 (4.0%) | |
| 26% – 50% | 64 (42.4%) | |
| 51% – 75% | 72 (47.7%) | < .0001 |
| 76% – 100% | 9 (6.0%) | |
| Median | 55% | |
|
| ||
| Number of breast cancer patients (current) | ||
| <100 | 27 (17.9) | |
| 100–199 | 46 (30.5) | .056 |
| 200–299 | 32 (21.2) | |
| 300+ | 46 (30.5) | |
| Median | 200 | |
|
| ||
| Percentage of current breast cancer patients who are post-menopausal | ||
| < 25% | 3 (2.0) | |
| 25% – 49% | 14 (9.3) | |
| 50% – 74% | 98 (64.9) | |
| 75%+ | 36 (23.8) | |
| Median | 66% | |
|
| ||
| Number of post-menopausal breast cancer patients ever treated (q468) | ||
| <250 | 30 (19.9) | |
| 250–499 | 28 (18.5) | |
| 500–999 | 32 (21.2) | .28 |
| 1000–1999 | 39 (25.8) | |
| 2000+ | 22 (14.6) | |
| Median | 550 | |
Based on chi-square test.
Regions:
Northeast= CT, MA, ME, NH, NJ, NY, PA, RI, VT
Midwest= IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI
South= AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV
West= AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY
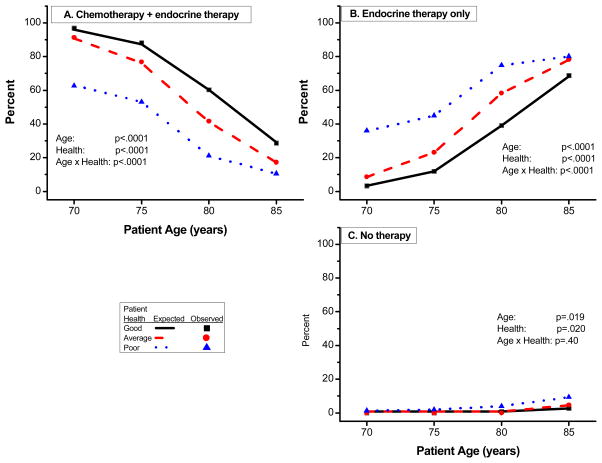
Overall Therapeutic Recommendations by Age and Health Status
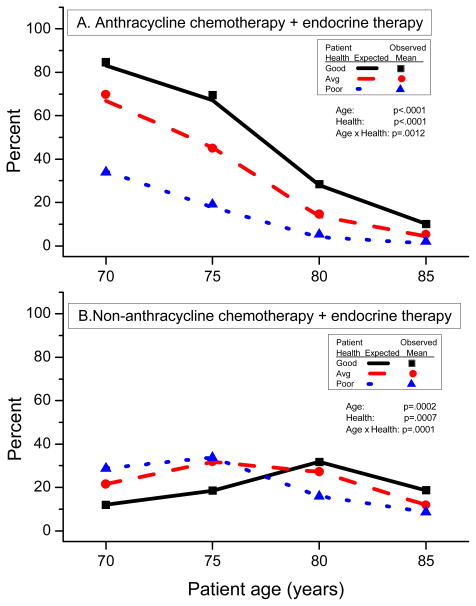
Patient age and health status significantly influenced oncologists’ choice of therapy (P<0.0001 for both). Figure 1 shows the estimated percentages of oncologists recommending each of the three therapies according to age and health status (chemotherapy with endocrine therapy, endocrine therapy alone, and no therapy). With increasing patient age, oncologists were less likely to select the combination of chemotherapy and endocrine therapy, regardless of health (P<0.0001) (Figure 1A, Table 2). Likewise, with further deterioration of health status, oncologists were less likely to select the combination therapy (P<0.0001), irrespective of age. Paralleling these trends, recommendation for anthracycline-based chemotherapy with endocrine therapy decreased with increasing age (P<0.0001) and worsening health (P<0.0001) (Figure 2A). Non-anthracycline-based chemotherapy with endocrine therapy was also dependent upon patient age (P=0.0002) and patient health (P=0.0007), but a consistent trend was not observed (Figure 2B).
Figure 1.
Effects of patient age and health status on therapeutic recommendations by medical oncologists treating a standardized patient with T2N2 hormone-receptor positive, HER2-negative breast cancer. Lines are model estimates for Good Health (solid lines), Average Health (dashed lines), and Poor Health (dotted lines). Symbols are observed percents for Good Health (square), Average Health (circle), and Poor Health (triangle).
Table 2.
Therapeutic recommendations of medical oncologists treating a standardized patient with T2N2 hormone-receptor positive, HER2-negative breast cancer with varying age and health status.
| Chemotherapy + Endocrine therapy | Endocrine therapy only | No therapy | Total* | |
|---|---|---|---|---|
| Good health | ||||
| Age | ||||
| 70 | 145 (96.7%) | 5 (3.3%) | 0 (0%) | 150 (100%) |
| 75 | 133 (88.1%) | 18 (11.9%) | 0 (0%) | 151 (100%) |
| 80 | 91 (60.3%) | 59 (39.1%) | 1 (0.7%) | 151 (100%) |
| 85 | 43 (28.7%) | 103 (68.7%) | 4 (2.7%) | 150 (100%) |
| Average health | ||||
| Age | ||||
| 70 | 136 (91.3%) | 13 (8.7%) | 0 (0%) | 149 (100%) |
| 75 | 116 (76.8%) | 35 (23.2%) | 0 (0%) | 151 (100%) |
| 80 | 63 (41.7%) | 88 (58.3%) | 0 (0%) | 151 (100%) |
| 85 | 26 (17.2%) | 118 (78.2%) | 7 (4.6%) | 151 (100%) |
| Poor health | ||||
| Age | ||||
| 70 | 94 (62.7%) | 54 (36.0%) | 2 (1.3%) | 150 (100%) |
| 75 | 80 (53.0%) | 68 (45.0%) | 3 (2.0%) | 151 (100%) |
| 80 | 32 (21.2%) | 113 (74.8%) | 6 (4.0%) | 151 (100%) |
| 85 | 16 (10.6%) | 121 (80.1%) | 14 (9.3%) | 151 (100%) |
Total may be less than 151 because of missing responses.
Figure 2.
Effects of patient age and health status on therapeutic recommendations for anthracycline chemotherapy or non-anthracycline chemotherapy. Lines are model estimates for Good Health (solid lines), Average Health (dashed lines), and Poor Health (dotted lines). Symbols are observed percents for Good Health (square), Average Health (circle), and Poor Health (triangle).
The decision to employ endocrine therapy alone in the same hypothetical patients was influenced again by both age (P<0.0001) and health (P<0.0001). Oncologists were more likely to recommend endocrine therapy with increasing age and declining health status (Figure 1B). Selection of no therapy constituted an infrequent response, representing an average of 2% of responses overall (range, 0–9.3%). Similar to endocrine therapy, no therapy was increasingly recommended with advancing age (P=0.019) and declining health status (P=0.02) (Figure 1C)
Specific Endocrine Therapy
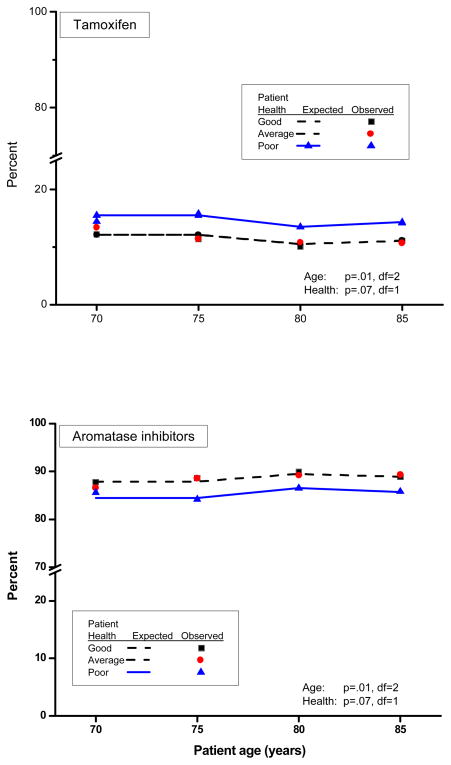
Oncologists who chose chemotherapy with endocrine therapy and endocrine therapy alone selected an AI more frequently than tamoxifen (87.5% versus 12.5%; P<0.0001). The preference for AI therapy increased with patient age (P<0.01), but was lower for patients with poor health compared to patients with good and average health (P=0.07). The percent recommending AI did not vary significantly between patients of good and average health (P=0.75) (Figure 3).
Figure 3.
Effects of patient age and health status on therapeutic recommendations for tamoxifen or aromatase inhibitors (anastrozole, letrozole or exemestane). Lines are estimates for Good Health (solid lines), Average Health (dashed lines), Poor Health (dotted lines). Symbols are observed percents for Good Health (square), Average Health (circle), and Poor Health (triangle).
Oncologists Characteristics Influencing Treatment Recommendations
By univariate GLMM multinomial analysis, oncologists’ treatment recommendations among three treatment choices (chemotherapy with endocrine therapy, endocrine therapy alone and no therapy) varied significantly according to number of patients treated for any disease in the prior month (P=0.0042). Oncologists who treated a larger number of patients were less likely to recommend hormone therapy alone (P=0.028) and even less likely to recommend no therapy (P=0.028), in favor of chemotherapy with endocrine therapy. However, treatment choice was not significantly influenced by their age (P=0.66), sex (P=0.85), geographic region (P=0.54), number of years in practice (P=0.45), number of breast cancer patients they were treating (P=0.66), number of post-menopausal breast cancer patients they were treating (P=0.57), percent of patients over 65 years of age in their practice (P=0.23), number of post-menopausal breast cancer patients treated on adjuvant hormonal therapy (P=0.17), number of post-menopausal breast cancer patients ever treated (P=0.18), or percentage of patient over 65 years (P=0.23). The effects of patient age and health status on treatment choice in multinomial analysis were not affected by number of patients treated.
Relative Impact of Age and Health Status
A significant interaction was observed between age and health status in the recommendation for chemotherapy with endocrine therapy and endocrine therapy alone (P<0.0001 for both). To compare the effect of age versus health status given their interaction, the relative frequency of treatment recommendations holding either variable constant was examined (Table 3). For example, compared to a 70-year old in good health (anchored at a frequency of 1.00), an 85-year old in good health was recommended to receive chemotherapy in addition to endocrine therapy 70% less often (frequency = 0.30, Table 3A). Alternatively, compared to 70-year old in good health (anchored at a frequency of 1.00), a 70-year old in poor health was recommended to receive chemotherapy in addition to endocrine therapy 35% less often (frequency = 0.65, Table 3B). Similar to the scenarios presented, age continued to have a greater effect on treatment recommendation as compared to health status across all strata of these two variables.
Table 3.
Rate ratios outlining the relative frequency of recommendation for chemotherapy with endocrine therapy or endocrine therapy alone (holding age or health status constant) in a patient with T2N2 hormone-receptor positive, HER2-negative breast cancer.
| A. Chemotherapy with endocrine therapy (Reference: Age = 70) | |||
|---|---|---|---|
| Age | Good | Average | Poor |
| 70 | 1.00 | 1.00 | 1.00 |
| 75 | 0.92 | 0.85 | 0.85 |
| 80 | 0.63 | 0.46 | 0.34 |
| 85 | 0.30 | 0.19 | 0.17 |
| B. Chemotherapy with endocrine therapy (Reference: Health status = Good) | |||
|---|---|---|---|
| Age | Good | Average | Poor |
| 70 | 1.00 | 0.94 | 0.65 |
| 75 | 1.00 | 0.87 | 0.60 |
| 80 | 1.00 | 0.69 | 0.35 |
| 85 | 1.00 | 0.60 | 0.37 |
DISCUSSION
In the current study, increasing age and decreasing health status of a hypothetical patient with T2N2 ER-positive, HER2-negative breast cancer led to less frequent recommendation for chemotherapy with endocrine therapy and increased utilization of endocrine therapy alone. An interaction between age and health status was observed for both of these treatment recommendations, indicating that oncologists use an amalgam of both factors in choosing therapies. Over the ranges of age and health status explored in the current study, an exploratory analysis suggests that variations in age may be a more substantial driver of treatment recommendations for either endocrine therapy alone or chemotherapy with endocrine therapy. The recommendation for no therapy was consistently low (<10%) across all strata of age and health status. Furthermore, given the choice of tamoxifen or AI therapy, oncologists more frequently chose the latter.
The trend towards declining use of chemotherapy with age observed in the current study is mirrored by two SEER database analyses of breast cancer patients age 65 and greater.10,11 In these analyses, older cohorts were up to four times less likely to receive adjuvant chemotherapy as compared to younger cohorts. A combination of factors may explain this practice pattern. The largest dataset exploring adjuvant therapy of breast cancer—the EBCTCG meta-analysis—suggested a decreasing benefit from chemotherapy with increasing age, albeit with limited representation of patients over the age of 70.1 Oncologists may further be discouraged from use of chemotherapy in older adults as a result of potential treatment related toxicities. A review of the International Breast Cancer Study Group (IBCSG) experience compiled data from 9 prospective trials utilizing cyclophosphamide, methotrexate, and fluorouracil (CMF)-based regimens.20 Results indicated a correlation between increasing age and treatment-related mortality. A similar correlation between age and chemotherapy-related toxicity was observed in a combined analysis of 3 prospective Cancer and Leukemia Group B (CALGB) trials utilizing anthracycline-based regimens for lymph node-positive breast cancer (CALGB 8541, 9344 and 9741).21 These data offer rationale for the declining recommendation of chemotherapy with increasing age in the current study. However, these meta-analyses are complicated by inclusion of relatively few patients over the age of 80; furthermore, eligibility criteria for participation in the associated cooperative group protocols may have resulted in a selection bias towards patients with fewer co-morbidities and a better overall health status.
Concerns regarding chemotherapy-related toxicity in older adults led to the development of a prospective trial (CALGB 49907) comparing adjuvant therapy regimens of varying intensity—specifically, capecitabine alone compared to doxorubicin-cyclophosphamide (AC) or CMF chemotherapy.13 Capecitabine therapy led to fewer hematologic adverse events; however, was associated with an increased risk of breast cancer mortality and poorer overall survival with 2 years median follow-up. Less than 5% of patients enrolled in the study were over the age of 80, while over 95% were characterized as demonstrating an Eastern Cooperative Oncology Group (ECOG) performance status (PS) between 0–1 on study entry. The FASG 08 trial represents the only other reported prospective trial to date in older adults with breast cancer; as previously noted, the results support the strategy of combined endocrine therapy and chemotherapy.12 Importantly, however, the published report lacks details related to patient distribution by age and performance status.
To broaden the applicability of data to the population of older adults, future prospective efforts should include a wider range of patients based on health status with no upper age limit. In addition to extending inclusion criteria, incorporation of novel geriatric assessment tools for prospective stratification may enhance the applicability of clinical trial data in older adults. Risk estimators such as Adjuvant! Online are strongly sensitive to input related to co-morbid illnesses; however, the classification of co-morbid illnesses in this model lacks stringent criteria and is thus subject to interpreter variability.22,23 In contrast, the geriatric assessment includes a detailed analysis of functional status, medications, social support, nutritional status, psychological state and cognition.24 Output from the assessment provides a more precise estimation of an individual’s health status and life expectancy, allowing the oncologist to balance these considerations against the anticipated benefit of therapeutic interventions. Future studies may aid in optimizing use of these prognostic tools; as one example, the ongoing Ibandronate With or Without Capecitabine in Elderly Patients with Early Breast Cancer (ICE) trial will compare the Charlson co-morbidity score and the Vulnerable Elder Survey (VES)-13, two validated geriatric assessments.25–27
Results of the ATAC and BIG 1–98 trials have shown similar benefits in DFS in older adults as compared to younger postmenopausal cohorts.4,8 These results have extrapolated to treatment practice recommendations, as reflected in our survey study. As survey respondents were only queried regarding their first-line choice for endocrine therapy, the study did not assess preference for a ‘switching’ approach—presumably, respondents may have recommended initial therapy with tamoxifen with the intention of changing treatment to an AI within several years. Several randomized trials have validated this approach.2,3,6
The design of the current study renders several limitations. Firstly, it is challenging to elucidate the rationale for clinical decision-making. For example, with respect to the inverse relationship of patient age and recommendation for chemotherapy, it remains unclear whether concerns related to therapeutic efficacy or chemotherapy-related toxicity motivated this trend. Secondly, the survey presented the health status of the hypothetical patient as discrete variables. As previously noted, health status is more precisely represented using output from geriatric assessment tools, placing co-morbidities and other relevant patient characteristics on a continuum. Finally, the survey does not include an assessment of the respondents’ medical knowledge. To this end, experiential information derived from practice composition and volume serves as a surrogate for this data.
Despite these limitations, the current study provides valuable insight into practice patterns of chemotherapy and endocrine therapy use in older adults with hormone receptor-positive breast cancer. These data underscore the need for further randomized trials in older adults, with extension of inclusion criteria to encompass a broader range of health conditions and patients over the age of 80. Prospective evaluation of validated geriatric or frailty assessment screeners in this setting may allow the oncologist to individualize the patient’s therapy based on better estimations of health status and life expectancy. Although this study demonstrated that oncologist are influence by both age and health status in treatment selections, it is not clear whether the follow a systematic approach to the determination of approach treatment.. These decision are now even more complex given the unique biologic characteristics of breast cancer in the older adult (i.e., higher ER- and PR-expression and lower HER2 expression)28. Molecular decision tools such as the 21-gene recurrence score (OncotypeDx; Genomic Health, San Francisco, CA) may need to be combined with assesment tools to aid in decision-making.29 Ultimately, a personalized approach with integration of biologic data and comprehensive clinical information may represent the most effective strategy in the older adult with breast cancer.
References
- 1.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Boccardo F, Rubagotti A, Guglielmini P, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol. 2006;17(Suppl 7):vii10–4. doi: 10.1093/annonc/mdl941. [DOI] [PubMed] [Google Scholar]
- 3.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–70. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 4.Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 5.Goss PE, Ingle JN, Martino S, et al. Efficacy of letrozole extended adjuvant therapy according to estrogen receptor and progesterone receptor status of the primary tumor: National Cancer Institute of Canada Clinical Trials Group MA.17. J Clin Oncol. 2007;25:2006–11. doi: 10.1200/JCO.2006.09.4482. [DOI] [PubMed] [Google Scholar]
- 6.Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–62. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 7.Thurlimann BJ, Keshaviah A, Mouridsen H, et al. BIG 1–98: Randomized double-blind phase III study to evaluate letrozole (L) vs. tamoxifen (T) as adjuvant endocrine therapy for postmenopausal women with receptor-positive breast cancer. Journal of Clinical Oncology, 2005 ASCO Annual Meeting Proceedings. 2005;23(16S):511. Part I of II (June 1 Supplement) [Google Scholar]
- 8.Crivellari D, Sun Z, Coates AS, et al. Letrozole compared with tamoxifen for elderly patients with endocrine-responsive early breast cancer: the BIG 1–98 trial. J Clin Oncol. 2008;26:1972–9. doi: 10.1200/JCO.2007.14.0459. [DOI] [PubMed] [Google Scholar]
- 9.Buzdar A, Howell A, Cuzick J, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7:633–43. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 10.Elkin EB, Hurria A, Mitra N, et al. Adjuvant Chemotherapy and Survival in Older Women With Hormone Receptor-Negative Breast Cancer: Assessing Outcome in a Population-Based, Observational Cohort. J Clin Oncol. 2006;24:2757–2764. doi: 10.1200/JCO.2005.03.6053. [DOI] [PubMed] [Google Scholar]
- 11.Giordano SH, Duan Z, Kuo Y-F, et al. Use and Outcomes of Adjuvant Chemotherapy in Older Women With Breast Cancer. J Clin Oncol. 2006;24:2750–2756. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- 12.Fargeot P, Bonneterre J, Roche H, et al. Disease-Free Survival Advantage of Weekly Epirubicin Plus Tamoxifen Versus Tamoxifen Alone As Adjuvant Treatment of Operable, Node-Positive, Elderly Breast Cancer Patients: 6-Year Follow-Up Results of the French Adjuvant Study Group 08 Trial. J Clin Oncol. 2004;22:4674–4682. doi: 10.1200/JCO.2004.02.145. [DOI] [PubMed] [Google Scholar]
- 13.Muss HB, Berry DL, Cirrincione C, et al. Standard chemotherapy (CMF or AC) versus capecitabine in early-stage breast cancer (BC) patients aged 65 and older: Results of CALGB/CTSU 49907. J Clin Oncol. 2008;26 (May 20 suppl; abstr 507) [Google Scholar]
- 14.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of Patients 65 Years of Age or Older in Cancer-Treatment Trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 15.Muss HB, Woolf S, Berry D, et al. Adjuvant Chemotherapy in Older and Younger Women With Lymph Node-Positive Breast Cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 16.Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8:1101–15. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 17.Naeim A, Sawhney R, MacLean C, et al. Quality indicators for the care of breast cancer in vulnerable elders. J Am Geriatr Soc. 2007;55:S258–69. doi: 10.1111/j.1532-5415.2007.01331.x. [DOI] [PubMed] [Google Scholar]
- 18.Hurria A, Wong FL, Villaluna D, et al. Role of age and health in treatment recommendations for older adults with breast cancer: the perspective of oncologists and primary care providers. J Clin Oncol. 2008;26:5386–92. doi: 10.1200/JCO.2008.17.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurria A, Wong FL, Pal S, et al. Perspectives and Attitudes on the Use of Adjuvant Chemotherapy and Trastuzumab in Older Adults with HER2-Positive Breast Cancer. A Survey of Oncologists. doi: 10.1634/theoncologist.2009-0056. (submitted) [DOI] [PubMed] [Google Scholar]
- 20.Colleoni M, Price KN, Castiglione-Gertsch M, et al. Mortality during adjuvant treatment of early breast cancer with cyclophosphamide, methotrexate, and fluorouracil. The Lancet - Vol. 1999 Jul 10;354(9173):130–131. doi: 10.1016/s0140-6736(99)02015-2. [DOI] [PubMed] [Google Scholar]
- 21.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of Older and Younger Patients Treated With Adjuvant Chemotherapy for Node-Positive Breast Cancer: The Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 22. [Last accessed December 1, 2008];Adjuvant! Online. (Available at http://www.adjuvantonline.com)
- 23.Ozanne EM, Braithwaite D, Sepucha K, et al. Sensitivity to Input Variability of the Adjuvant! Online Breast Cancer Prognostic Model. J Clin Oncol. 2009;27:214–219. doi: 10.1200/JCO.2008.17.3914. [DOI] [PubMed] [Google Scholar]
- 24.Extermann M, Hurria A. Comprehensive Geriatric Assessment for Older Patients With Cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 25.National Institutes of Health. [Last accessed April 15, 2008];Clinical Trials.gov. (Available at http://www.clinicaltrials.gov)
- 26.Extermann M, Overcash J, Lyman GH, et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 27.Min LC, Reuben DB, MacLean CH, et al. Predictors of Overall Quality of Care Provided to Vulnerable Older People. Journal of the American Geriatrics Society. 2005;53:1705–1711. doi: 10.1111/j.1532-5415.2005.53520.x. [DOI] [PubMed] [Google Scholar]
- 28.Diab SG, Elledge RM, Clark GM. Tumor Characteristics and Clinical Outcome of Elderly Women With Breast Cancer. J Natl Cancer Inst. 2000;92:550–556. doi: 10.1093/jnci/92.7.550. [DOI] [PubMed] [Google Scholar]
- 29.Paik S, Tang G, Shak S, et al. Gene Expression and Benefit of Chemotherapy in Women With Node-Negative, Estrogen Receptor-Positive Breast Cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 30.Paik S, Shak S, Tang G, et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]