SUMMARY
The renin-angiotensin system is a coordinated hormonal cascade critical for the regulation of blood pressure (BP) and kidney function. Angiotensin II (Ang II), the major angiotensin effector peptide, binds to two major receptors, type-1 (AT1Rs) and type-2 (AT2Rs). AT1Rs engender antinatriuresis and raise BP, whereas AT2Rs oppose these effects, inducing natriuresis and reducing BP
AT2Rs are highly expressed in the adult kidney, especially in the proximal tubule. In AT2R-null mice, long-term Ang II infusion results in pressor and antinatriuretic hypersensivivity compared to responses in wild-type animals.
The major endogenous receptor ligand for AT2R-mediated natriuretic responses appears to be des-aspartyl1-Ang II (Ang III) instead of Ang II. Recent studies have demonstrated that Ang II requires metabolism to Ang III by aminopeptidase A in order to induce natriuresis and that inhibition of aminopeptidase N increases intrarenal Ang III and augments Ang III-induced natriuresis.
The renal dopaminergic system is another important natriuretic pathway. Renal proximal tubule D1-like receptors (D1LIKERs) control approximately 50% of basal sodium (Na+) excretion. We have recently found that natriuresis induced by proximal tubule D1LIKERs requires AT2R activation and that D1LIKER stimulation induces recruitment of AT2Rs to the apical plasma membrane via a cyclic AMP-dependent mechanism.
Initial studies employing potent AT2R non-peptide agonist Compound 21 demonstrate natriuresis in both the presence and absence of AT1R blockade indicating the therapeutic potential of this compound in fluid retaining states and hypertension.
Keywords: Renin-angiotensin system, Angiotensin, Receptors, Natriuresis, Dopamine, Dopamine Receptors, Hypertension
INTRODUCTION
The renin-angiotensin system (RAS) is a coordinated hormonal cascade critical for the regulation of blood pressure (BP) and kidney function [1]. Angiotensin II (Ang II), the most important RAS effector peptide, classically acts by binding to two major Ang receptors, type-1 (AT1Rs) and type-2 (AT2Rs) [1–3]. Most of the physiological actions of Ang II are transduced via AT1R activation, including cellular dedifferentiation and proliferation; vasoconstriction; reduction of vascular compliance; cardiac contractility; increased renal tubule sodium (Na+) reabsorption; aldosterone, vasopressin and endothelin secretion; salt appetite; thirst; and stimulation of the sympathetic nervous system [1,3]. Knowledge of AT2R functions and their mechanisms is less well understood than those of AT1Rs. However, AT2Rs have been found to oppose Ang II actions, especially vasoconstriction and cellular proliferation, via AT1Rs under the majority of circumstances [1–4]. Indeed, the only instance to our knowledge in which AT1Rs and AT2Rs have been found to act in concert is in the negative feedback suppression of renin secretion directly at the renal juxtaglomerular cell [5].
AT2Rs are 7-transmembrane G protein-coupled receptors encoded by a single copy gene on the X chromosome and composed of 363 amino acids (molecular weight 41,220 Da) with only 34% amino acid sequence homology with AT1Rs [2]. The main sequence homology between AT1Rs and AT2Rs occurs in the transmembrane hydrophobic regions of the molecules which form their 7-transmembrane helical columns [2].
AT2R SIGNALING PATHWAYS
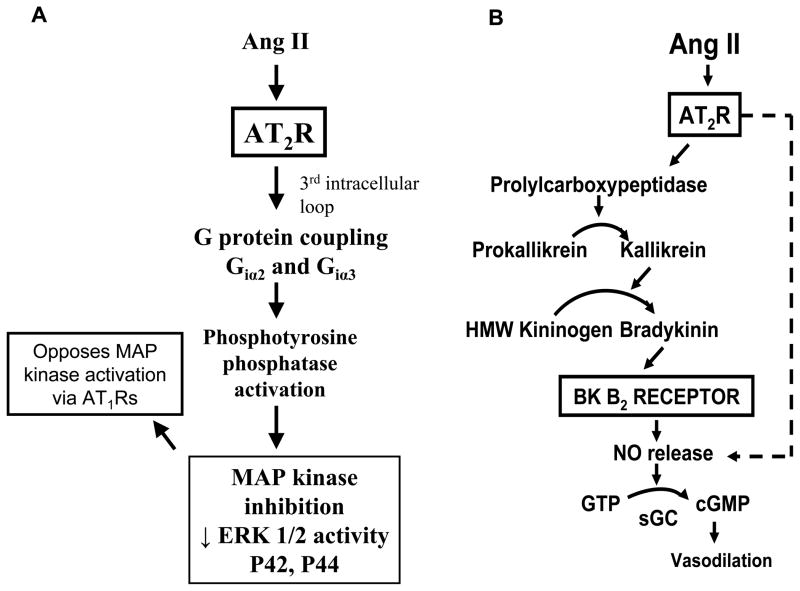
AT2R signaling mechanisms have been found to differ substantially from those of AT1Rs [1–4,6]. As summarized in Figure 1, Panel A, AT2R activation initiated by binding of Ang II to the receptors on the plasma membrane triggers G protein coupling through Giα2 and Giα3 via the third intracellular loop of the receptor. This signal activates phosphotyrosine phosphatases, the function of which is to inactivate mitogen-activated protein (MAP) kinases, such as extracellular signal-regulated kinase (ERK)-1 and ERK-2. Phosphotyrosine phosphatase activation can also occur through a G protein-independent mechanism. MAP kinase inhibition by AT2Rs is thought to be a major signaling mechanism by which AT2Rs oppose the actions of AT1Rs, which activate these kinases. AT2Rs can also activate lipid signaling pathways including increased phospholipase A2 activity and arachidonic acid release, and long-term AT2R activation can increase the biosynthesis of ceramides, which can activate stress kinases and caspases to induce apoptosis [6].
Figure 1.
A. Schematic representation of the major intracellular signaling pathway of AT2Rs. MAP = mitogen-activated protein; ERK = extracellular-regulated kinase; AT1R = angiotensin type-1 receptor. B. Schematic representation of the most important extracellular signaling pathway of AT2Rs: the autacrine/paracrine bradykinin (BK)- nitric oxide (NO)-cyclic GMP (cGMP) cascade. Recent studies also suggest that NO and cGMP may function as signaling molecules intracellularly in nuclei and mitochondria. Ang II = angiotensin II; HMW = high molecular weight; GTP = guanosine triphosphate; sGC = soluble guanylyl cyclase.
A major AT2R signaling pathway, involved in almost all of the reported physiological actions of AT2Rs is the bradykinin (BK) – nitric oxide (NO) – cyclic GMP (cGMP) signaling cascade shown in Figure 1, Panel B [1,3,7–12]. AT2Rs have consistently been found to increase the production of NO and cGMP either by stimulating an increase in BK production with consequent activation of BK B2 receptors or by direct activation of NO production independently of BK [12]. This pathway is critical, for example, for AT2R-induced vasodilation [1,3,4,6,13].
AT1R/AT2R INTERACTIONS IN NATRIURESIS
The RAS is a primary regulator of BP, fluid and electrolyte balance and is thought to be a key pathophysiological driving force for the development of hypertension. As most effectively articulated by Guyton and colleagues, the capacity of the kidney to excrete Na+ via the pressure-natriuresis mechanism is central in the regulation of BP [14,15]. Pressure-natriuresis is the protective mechanism by which an increase in BP and thereby renal perfusion pressure increases Na+ excretion bringing BP back towards control levels. The elegant servo-control studies by Hall and colleagues defined the importance of pressure-natriuresis as the mechanism of escape from the Na+-retaining consequences of a variety of hormonal influences, including those of norepinephrine, angiotensin II and aldosterone [16–18]. Under the Guyton hypothesis, there must be a defect in renal Na+ handling in order to sustain a chronic increase in BP and the development of hypertension [14,15]. Over the years, this hypothesis has been substantiated by kidney transplantation studies in genetically hypertensive rats showing that hypertension follows the transplanted kidney [19–21].
Recent work by the Coffman laboratory using cross-transplantation studies has confirmed the essential role of renal AT1Rs in the development of hypertension induced by Ang II [22–24]. Renal AT1Rs were required to sustain a hypertensive response to continuous Ang II infusion for two weeks in mice [23]. In particular, mice with AT1Rs specifically deleted from renal proximal tubule cells using Cre-lox technology under the control of a proximal tubule-specific Pepck promoter) had a 10 mmHg reduction in baseline BP accompanied by a 36% decrease in proximal tubule Na+/fluid reabsorption together with reduced expression of key proximal tubule apical Na+ transporter, Na+-hydrogen exchanger-3 (NHE-3) [24]. Importantly, these mice were protected from the antinatriuretic and pressor actions of infused Ang II [24]. Sigmund and colleagues also recently demonstrated that AT1R knockout specifically in the proximal tubule reduced baseline BP by approximately 14 mmHg and that AT1R over-expression in the proximal tubule increased BP by approximately 15 mm Hg [25,26]. These studies underscore the importance of renal proximal tubule AT1Rs in the control of BP by means of their effects on Na+ reabsorption.
While the effects of AT1Rs on Na+ reabsorption and BP are now relatively well understood, the potential role of AT2Rs in counteracting these actions is less appreciated. The purpose of this Mini-Review is to provide an up-to-date summary of the renal actions of AT2Rs with an emphasis of their role in the control of Na+ excretion and BP.
ANGIOTENSIN PEPTIDES AND AT2R-INDUCED NATRIURESIS
In the fetus, AT2Rs are expressed throughout the kidney, and their expression level decreases markedly during the post-natal period [27–29]. Nevertheless, in adulthood, AT2Rs continue to be expressed in glomeruli as well as vascular and tubule cells, albeit in smaller quantities [28,29]. However, AT2Rs are highly expressed in the adult proximal tubule [28,29].
Definitive evidence now exists that renal AT2Rs mediate natriuresis [30–35]. This hypothesis was initially strongly suggested by AT2R knockout studies in mice wherein receptor-null mice had markedly exaggerated antinatriuretic responses to systemically infused Ang II compared to wild-type mice [36]. AT2R-null mice also had marked pressor hypersensitivity to Ang II, and their pressure-natriuresis mechanism was markedly impaired. The mechanism of the antinatriuresis in AT2R-null mice was identified as reduction in intrarenal generation of BK, NO and cGMP in these studies [36].
Further investigation of the role of endogenous renal AT2Rs in natriuresis incorporated the novel technique of direct renal interstitial microinfusion of pharmacological and molecular probes in mice and rats, enabling the direct evaluation of renal function without systemic hemodynamic or hormonal influences. With this technique, selective intrarenal AT1R blockade with candesartan in rats induced a highly significant natriuresis that was abolished with concurrent intrarenal administration of the AT2R-specific antagonist PD-123319 (PD) [30]. These results suggested that the natriuretic response to intrarenal angiotensin receptor blockade is dependent upon Ang activation of unblocked AT2Rs.
ANGGIOTENSIN III (Ang III) AND AT2R-INDUCED NATRIURESIS
We were surprised to find, however, that in the presence of systemic AT1R blockade, direct intrarenal administration of Ang II, even at very high infusion rates, was unable to induce a natriuretic response [30]. Because the heptapeptide des-aspartyl1-Ang II (Ang III), an Ang II metabolite, is highly active at AT2Rs in the brain, we investigated whether Ang III might be an agonist for AT2R-mediated responses in the kidney [30]. When systemic AT1Rs were blocked, direct intrarenal Ang III infusion induced a highly significant increase in renal Na+ excretion that was abolished by concurrent AT2R blockade with PD. However, intrarenal Ang III infusion alone demonstrated no effect on renal Na+ excretion in the absence of systemic AT1R blockade [30].
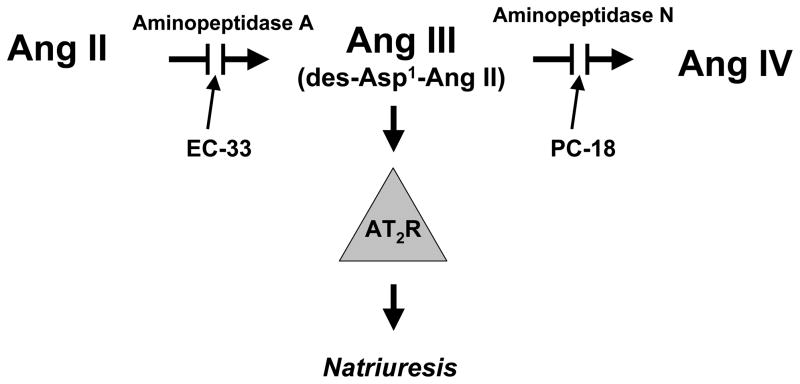
To explain the effectiveness of Ang III, but not Ang II, to induce natriuresis, we hypothesized that Ang II must be converted to Ang III in order to interact with AT2Rs within the kidney [31]. Ang II is converted to Ang III by aminopeptidase A (APA), and Ang III is converted to the hexapeptide angiotensin IV (Ang IV) by aminopeptidase N (APN). In the presence of systemic AT1R blockade, intrarenal infusion of Ang III again resulted in a natriuretic response, which was markedly augmented by intrarenal co-administration of the APN inhibitor 2-amino-4-methylsulfonyl-butane-thiol, methane-thiol (PC-18) [31]. The augmentation in Ang III-induced natriuresis due to APN inhibition was also abolished by intrarenal AT2R blockade with PD [31]. Intrarenal infusion of the canonical Ang receptor ligand Ang II induced a natriuretic response only in the presence of APN inhibition and this response was blocked completely by concomitant intrarenal APA inhibition with 3-amino-4-thio-butyl-sulfonate (EC-33) [32]. These results strongly suggest that Ang III, not Ang II, is the canonical AT2R ligand in the regulation of renal Na+ excretion and that Ang II must be converted to Ang III to mount an AT2R-dependent natriuretic response (Figure 2).
Figure 2.
Schematic diagram depicting the necessity of conversion of Ang II to Ang III for the induction of a natriuretic response via AT2R activation. PC-18, a selective aminopeptidase N inhibitor; EC-33, a selective aminopeptidase A inhibitor.
These studies are potentially important for disease states such as type-2 diabetes mellitus and obesity, wherein AT2Rs are upregulated in renal proximal tubule cells, inhibit Na+/K+ ATPase (NKA) and reduce Na+ transport [35–37]. The inhibition of NKA appears to be mediated via a NO/cGMP-dependent pathway, and inhibition of NAD(P)H oxidase potentiates AT2R-mediated natriuresis [37].
The exact mechanism whereby the AT2R responds to Ang III and not to Ang II in the nephron is currently unknown. However, it is likely to be due to the role of the N-terminal arginine in stabilizing the binding pocket of the AT2R allowing optimal binding of the remaining c-terminal amino acid residues. In addition to the kidney, two other cardiovascular hormonal systems have been linked to Ang III as the preferred agonist for AT2Rs, the coronary vascular bed and the adrenal zona glomerulosa. In the coronary microcirculation, Ang III, rather than Ang II, is the preferred ligand to induce AT2R-mediated vasodilation [38]. Ang III is also the preferred agonist for AT2R-mediated aldosterone secretion from the adrenal cortex [39]
Recent in vivo studies using endogenous Na+/lithium clearance techniques, indicate that renal AT2Rs engender natriuresis mainly by reducing Na+ reabsorption in the renal proximal tubule without systemic or intrarenal hemodynamic changes [40]. Endogenous intrarenal Ang III was shown to be the major agonist for the natriuretic response, which could not be induced either by Ang II or Ang (1–7) [40], Ang III-induced natriuresis was accompanied by an increase in renal cGMP levels and was abolished by soluble gualylyl cyclase inhibitor 1-H-(1,2,4) oxadiazolo-(4,2-α) quinoxalin-1-one (ODQ), strongly suggesting that Ang III-induced natriuresis is mediated by renal production of cGMP [40].
Recent studies also have shown that AT2Rs may induce natriuresis in part through an action in the thick ascending limb of Henle (TAL) [41,42]. Ang II increases NO production in TALs by activation of AT2Rs, and NO inhibits the Na+/K+/2Cl− co-transporter, thereby reducing Na+ reabsorption in this nephron segment. However, no effort was made to determine if Ang II conversion to Ang III is required to inhibit Na+ reabsorption in these studies. Interestingly, the natriuretic effect of TAL AT2R activation is decreased in Dahl salt-sensitive rats, a model of salt-sensitive hypertension in humans in which Na+ retention occurs predominantly in TAL.
In vitro studies also have recently shown that renal AT2Rs decrease AT1R function in the proximal tubule by the common NO/cGMP pathway and also reduce AT1R expression at the transcriptional level via the ubiquitous transcription factor Sp1 [43]. AT2Rs also have been shown following ligand activation to dimerize with AT1Rs, reducing their expression level via a direct protein-protein interaction at the cell surface. Therefore, renal AT2Rs may oppose AT1R actions by multiple signaling pathways [43].
In most studies so far reported, Ang III-induced natriuresis has required the concurrent blockade of systemic AT1Rs. However, we recently demonstrated that the heptapeptide is capable of inducing natriuresis in the absence of systemic AT1R blockade if intrarenal Ang III levels are increased by APN inhibition [40]. Therefore, in a pure physiological sense, intrarenal Ang III probably does not play a significant role in the regulation of Na+ excretion when AT1Rs are intact because the antinatriuresis by Ang II acting at AT1Rs with substantially greater renal expression levels would overwhelm the ability of AT2Rs to induce natriuresis. Thus, AT2Rs would be expected to induce natriuresis when AT1Rs are blocked or when APN is blocked in the absence of AT1R inhibition.
We also conducted a series of experiments to clarify whether endogenous Ang III is capable of inducing natriuresis in the normal rat [40]. In the AT1R-blocked rat, we found that intrarenal administration of APN antagonist PC-18 alone was able to increase Na+ excretion in a sustained fashion and that the natriuresis was abolished by concurrent AT2R blockade [40]. Therefore, endogenous intrarenal Ang III induces natriuresis via AT2R activation under certain circumstances.
AT2R-DOPAMINE RECEPTOR INTERACTIONS IN NATRIURESIS
The renal dopaminergic system is an important regulator of renal Na+ excretion and blood pressure. Dopamine (DA) is synthesized from filtered L-dihydroxyphenylalanine (L-DOPA) transported from the nephron lumen into proximal tubule cells [44]. The synthesized DA is secreted from the proximal tubule cell predominantly across the apical plasma membrane into the tubule lumen, where it binds to D1-like receptors (D1LIKER, D1 and D5 subtypes). D1LIKER activation inhibits Na+ reabsorption into the proximal tubule cell through an adenylate cyclase - cAMP - signaling mechanism [44]. Systemic administration of fenoldopam, a highly selective D1LIKER agonist, in animals and humans elicits a robust natriuretic response that is based almost exclusively upon inhibition of proximal tubule Na+ reabsorption [45–48]. Renal dopaminergic tone accounts for approximately 50–60% of basal Na+ excretion, as selective intrarenal administration of SCH-23390 (SCH), a highly selective D1LIKER antagonist, induces antinatriuresis of this magnitude in dose-dependent fashion under conditions of moderate Na+ balance [49]. Conversely, intrarenal fenoldopam administration induces a highly significant natriuresis that is blocked completely by SCH in the sodium-loaded rat [48]. Surprisingly, however, we discovered that the natriuretic response to fenoldopam was also abolished by concomitant intrarenal administration of AT2R antagonist PD [48,50].
To learn the possible mechanism of AT2R participation in D1LIKER - induced natriuresis, we investigated cellular AT2R trafficking in renal proximal tubule cells [48,50]. Fenoldopam administration in vivo was accompanied by translocation of AT2Rs from intracellular sites to the apical plasma membranes of renal proximal tubule cells [48,50]. Neither total nor basolateral proximal tubule cell AT2R expression was changed in response to fenoldopam, suggesting that apically distributed AT2Rs are involved in the natriuretic response [48]. D1LIKERs translocate to the cell surface in response to D1LIKER activation in cultured kidney cells, kidney section preparations, and isolated proximal tubules and this response requires an intact microtubule network [51]. Recently, we extended these findings to the natriuretic mechanism of renal AT2Rs in vivo [50]. Fenoldopam-induced natriuresis and AT2R translocation were completely abolished during intrarenal co-infusion of nocodazole (NOC), which disrupts the microtubule network but preserves the actin microfilament cytoskeleton of renal proximal tubule cells [50]. Thus, microtubules are not only necessary for D1LIKER recruitment but also for AT2R recruitment in response to fenoldopam, a common trafficking pathway for the natriuretic function of these receptors.
Since D1LIKERs signal through cAMP/protein kinase A to mediate natriuresis, the role of renal interstitial cAMP generation in AT2R-mediated natriuresis was investigated in vivo [50]. Renal interstitial accumulation of cAMP [via direct activation of adenylyl cyclase with forskolin and selective inhibition of cAMP degradation with 3-isobutyl-1-methylxanthine (IBMX)] caused a significant and sustained increase in AT2R-mediated natriuresis [50]. Direct agonist stimulation of D1LIKERs was not necessary for AT2R-mediated natriuresis since forskolin+IBMX-induced natriuresis persisted in the presence of D1LIKER blockade with SCH [50]. Thus, one mechanism by which AT2Rs and D1LIKERs interact during high Na+ balance states to mediate natriuresis is D1LIKER-cAMP signaling, which provides the stimulus necessary for AT2R translocation and natriuresis. Because the effect is independent of specific D1LIKER-induced activation of adenylyl cyclase, stimulation of other receptors that signal through cAMP/protein kinase A pathway may be advantageous in promoting AT2R-mediated natriuresis in vivo. Indeed, administration of parathyroid hormone, through its cAMP/protein kinase A-dependent but not phospholipase C/protein kinase C–dependent signaling, has been shown to redistribute Na+ transporters such as Na+-hydrogen exchanger-3 and inhibit Na+-K+-ATPase activity in a direction favoring natriuresis and diuresis [52]. Renal AT2Rs are known to inhibit Na+-K+-ATPase activity, and increased renal cAMP may regulate this process [35,53].
The above-mentioned in vivo results in experimental animals have been confirmed by recent in vitro studies in a human renal proximal tubule cell line showing that D1R, but not D5R, stimulation of AT2R plasma membrane recruitment is cAMP- and protein phosphatase 2A (PP2A)-dependent [54]. These studies also demonstrated that D1R stimulation recruits plasma membrane AT2Rs from very low quantities to levels comparable to those of AT1Rs and that AT2R recruitment to the cell surface is required for its various functions [54].
THERAPEUTIC IMPLICATIONS OF RENAL AT2R-INDUCED NATRIURESIS
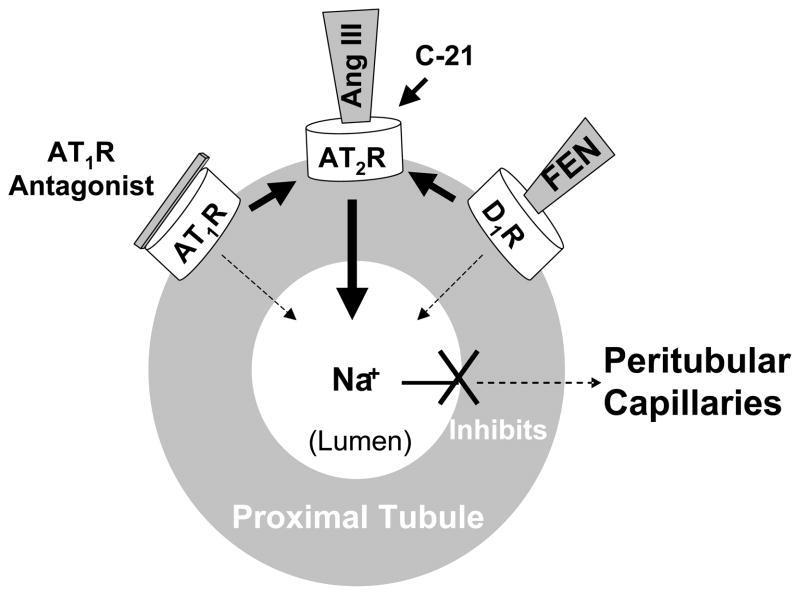
From the foregoing discussion, it is apparent that renal AT2Rs are natriuretic receptors, counter-balancing the Na+ retaining actions of Ang II via AT1Rs. Whereas the primary AT1R agonist in the kidney is Ang II, the preferred AT2R agonist governing Na+ excretion is the Ang II metabolite Ang III. Either blocking renal AT1Rs, indirectly activating renal AT2Rs, or directly activating AT2Rs with their endogenous ligand Ang III induces natriuresis (Figure 3). Evidence also has been provided that AT2R recruitment to the plasma membrane and activation are required for renal D1R-induced natriuresis. Thus, AT2R activation appears to be common to the natriuretic capacity of two of the most powerful hormonal systems, the intrarenal RAS and the renal dopaminergic system, governing Na+ homeostasis in the body. Consequently, targeting AT2Rs would be predicted to offer a new, potentially complimentary therapeutic approach to AT1R blockade in disorders of fluid retention and hypertension.
Figure 3.
Schematic diagram of the renal proximal tubule summarizing the Interrelationships among AT1Rs, AT2Rs and D1Rs in the control of renal Na+ reabsorption. AT1R antagonists inhibit Na+ reabsorption leading to natriuresis via activation of unblocked AT2Rs. D1LIKER activation with selective agonist fenoldopam (FEN) induces natriuresis via AT2R activation. Ang III, the preferred endogenous AT2R agonist, activates AT2Rs engendering natriuresis. Compound 21 (C-21), a highly selective non-peptide AT2R agonist, activates AT2Rs inducing natriuresis. For diagrammatic purposes, the receptors are shown on the basolateral membranes of tubule cells; in fact, these receptors are distributed on both the apical and basolateral membranes of these cells.
Is there any existing evidence that AT2R activation might hold therapeutic potential? Compound 21 (C21) is a new, potent, highly selective non-peptide AT2R agonist [55,56]. Three papers, published in 2012, reported the natriuretic effects of C21 in experimental animals [40,57,58]. Kemp et al. published that systemic C21 administration (0.3 μg/kg/min) increased urinary Na+ excretion (UNaV) by 12-fold in male uninephrectomized Sprague-Dawley rats [40]. The natriuretic response was significantly augmented by AT1R blockade and was abolished with concomitant renal interstitial administration of AT2R antagonist PD [40]. Hilliard et al. reported that intravenous C21 infusion (0.3 μg/kg/min) induced an approximately 3-fold increase UNaV and fractional excretion of Na+(FENa) that was abolished by intravenous co-infusion of AT2R antagonist PD in normotensive male and female rats [57]. Ali and Hussain also reported that intravenous C21 (5.0 μg/kg/min; a 16-fold higher dose) increased UNaV, FENa and fractional excretion of lithium (FELi) and that this response was neutralized with systemic PD coinfusion in obese Zucker rats [58]. These three studies encourage further research on the therapeutic potential of pharmacologic AT2R activation, possibly in combination with AT1R blockade, in the treatment of disorders associated with fluid retention and hypertension.
ABBREVIATIONS
- Ang II
angiotensin II
- Ang III
des-aspartyl1-angiotensin II
- APA
aminopeptidase A
- APN
aminopeptidase N
- AT1R
angiotensin type-1 receptor
- AT2R
angiotensin type-2 receptor
- BK
bradykinin
- BP
blood pressure
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- DA
dopamine
- D1LIKER
dopamine D1-like receptor
- ERK
extracellular signal-regulated kinase
- FELi
fractional excretion of lithium
- FENa
fractional excretion of sodium
- IBMX
3-isobutyl-1-methylxanthine
- MAP
mitogen-activated protein
- L-DOPA
L-dihydroxyphenylalanine
- Na+
sodium
- NHE-3
sodium-hydrogen exchanger-3
- NKA
sodium/potassium ATPase
- NO
nitric oxide
- ODQ
soluble guanylyl cyclase inhibitor
- PD
PD-123319
- PP2A
protein phosphatase 2A
- RAS
renin-angiotensin system
- SCH
Schering-23390, D1-like receptor antagonist
- TAL
thick ascending limb of Henle
- UNaV
urinary sodium excretion
References
- 1.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal disease. Endocr Rev. 2003;24:261–71. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 2.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72. [PubMed] [Google Scholar]
- 3.Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor: the AT2 receptor comes of age. Hypertension. 2005;45:840–4. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 4.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siragy HM, Xue C, Abadir P, Carey RM. Angiotensin subtype-2 receptors inhibit renin biosynthesis and angiotensin II formation. Hypertension. 2005;45:133–7. doi: 10.1161/01.HYP.0000149105.75125.2a. [DOI] [PubMed] [Google Scholar]
- 6.Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol. 2001;281:H2337–65. doi: 10.1152/ajpheart.2001.281.6.H2337. [DOI] [PubMed] [Google Scholar]
- 7.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97:1978–82. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siragy HM, Jaffa AA, Margolius HS, Carey RM. Renin-angiotensin system modulates renal bradykinin production. Am J Physiol Reg Int Comp Physiol. 1996;271(Pt 2):R1090–5. doi: 10.1152/ajpregu.1996.271.4.R1090. [DOI] [PubMed] [Google Scholar]
- 9.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–9. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siragy HM, Jaffa AA, Margolius HS. Bradykinin B2 receptor modulates renal prostaglandin E2 and nitric oxide. Hypertension. 1997;29:757–62. doi: 10.1161/01.hyp.29.3.757. [DOI] [PubMed] [Google Scholar]
- 11.Tsutsumi Y, Matsubara H, Masaki H, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–35. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600–4. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 13.Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol. 2003;140:809–24. doi: 10.1038/sj.bjp.0705448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyton AC, Coleman TG, Cowley AW, Jr, et al. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–94. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 15.Guyton AC. The surprising kidney-fluid mechanism for pressure control: its infinite gain. Hypertension. 1990;16:725–730. doi: 10.1161/01.hyp.16.6.725. [DOI] [PubMed] [Google Scholar]
- 16.Hall JE, Granger JP, Smith MJ, Jr, Premen AJ. Role of renal hemodynamics and arterial pressure in aldosterone “escape”. Hypertension. 1984;6:1183–92. doi: 10.1161/01.hyp.6.2_pt_2.i183. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE, Granger JP, Hester RL, Coleman TG, Smith MJ, Jr, Cross RB. Mechanisms of escape from sodium retention during angiotensin II hypertension. Am J Physiol Renal Physiol. 1984;246:F627–34. doi: 10.1152/ajprenal.1984.246.5.F627. [DOI] [PubMed] [Google Scholar]
- 18.Mizelle HL, Montani JP, Hester RL, Didlake RH, Hall JE. Role of pressure natriuresis in long-term control of renal electrolyte excretion. Hypertension. 1993;22:102–10. doi: 10.1161/01.hyp.22.1.102. [DOI] [PubMed] [Google Scholar]
- 19.Graf C, Maser-Gluth C, de Muinck Keizer W, et al. Sodium retention and hypertension after kidney transplantation in rats. Hypertension. 1993;21:724–30. doi: 10.1161/01.hyp.21.5.724. [DOI] [PubMed] [Google Scholar]
- 20.Kopf D, Waldherr R, Rettig R. Source of kidney determines blood pressure in young renal transplanted rats. Am J Physiol Renal Physiol. 1993;265:F104–11. doi: 10.1152/ajprenal.1993.265.1.F104. [DOI] [PubMed] [Google Scholar]
- 21.Rettig R, Folberth CG, Stauss H, et al. Hypertension in rats induced by renal grafts from renovascular hypertensive donors. Hypertension. 1990;15:429–35. doi: 10.1161/01.hyp.15.4.429. [DOI] [PubMed] [Google Scholar]
- 22.Crowley SD, Gurley SB, Oliverio MI, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–9. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowley SD, Gurley SB, Oliverio MI, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Nat Acad Sci USA. 2006;103:17985–90. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurley SB, Riquier-Brison AD, Schnermann J, et al. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–75. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Weatherford ET, Davis DR, et al. Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1067–77. doi: 10.1152/ajpregu.00124.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding Y, Davisson RL, Hardy DO, et al. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem. 1977;272:28142–8. doi: 10.1074/jbc.272.44.28142. [DOI] [PubMed] [Google Scholar]
- 27.Zhuo JAA, Alcorn D, Aldred GP, MacGregor DP, Mendelsohn FA. The distribution of angiotensin II receptors. Hypertension. 1995;35:155–63. [Google Scholar]
- 28.Ozono R, Wang Z-Q, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–46. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 29.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol. 1999;277:F437–46. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 30.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–44. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 31.Padia SH, Kemp BA, Howell NL, Siragy HM, Fournie-Zaluski MC, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type 1 receptor-blocked rats. Hypertension. 2007;49:625–30. doi: 10.1161/01.HYP.0000254833.85106.4d. [DOI] [PubMed] [Google Scholar]
- 32.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski M-C, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-induced natriuresis in rats. Hypertension. 2008;51:460–5. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 33.Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension. 2005;45:270–5. doi: 10.1161/01.HYP.0000151622.47814.6f. [DOI] [PubMed] [Google Scholar]
- 34.Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2006;290:F503–8. doi: 10.1152/ajprenal.00092.2005. [DOI] [PubMed] [Google Scholar]
- 35.Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol. 2006;290:F1430–6. doi: 10.1152/ajprenal.00218.2005. [DOI] [PubMed] [Google Scholar]
- 36.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Nat Acad Sci USA. 1999;96:6506–10. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabuhi R, Asghar M, Hussain T. Inhibition of NAD(P)H oxidase potentiates AT2 receptor agonist-induced natriuresis in Sprague-Dawley rats. Am J Physiol Renal Physiol. 299:F815–20. doi: 10.1152/ajprenal.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batenburg WW, Garrelds IM, Bunasconi CL, et al. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- 39.Yatabe J, Yoneda M, Yatabe MS, et al. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinol. 2011;152:1582–8. doi: 10.1210/en.2010-1070. [DOI] [PubMed] [Google Scholar]
- 40.Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Carey RM. Intrarenal angiotensin II is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension. 2012;60:387–95. doi: 10.1161/HYPERTENSIONAHA.112.191403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrera M, Garvin JL. Angiotensin II stimulates thick ascending limb NO production via AT(2) receptors and Akt1-dependent nitric oxide synthase 3 (NOS3) activation. J Biol Chem. 2010;285:14932–40. doi: 10.1074/jbc.M110.109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong NJ, Garvin JL. Angiotensin II type 2 receptor-mediated inhibition of NaCl absorption is blunted in thick ascending limbs from Dahl salt-sensitive rats. Hypertension. 2012 doi: 10.1161/HYPERTENSIONAHA.112.199216. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, Hopfer U, Jose PA, Zeng C. Angiotensin II AT2 receptor decreases AT1 receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens. 2012;30:1176–84. doi: 10.1097/HJH.0b013e3283532099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carey RM. Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38:297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- 45.Hughes JM, Beck TR, Rose CE, Jr, Carey RM. Selective dopamine-1 receptor stimulation produces natriruesis by a direct tubular action. J Hypertens Suppl. 1986;4:S106–8. [PubMed] [Google Scholar]
- 46.Hughes JM, Ragsdale NV, Felder RA, Chevalier RL, Keng B, Carey RM. Diuresis and natriuresis during continuous dopamine-1 receptor stimulation. Hypertension. 1988;11:169–74. doi: 10.1161/01.hyp.11.2_pt_2.i69. [DOI] [PubMed] [Google Scholar]
- 47.Ragsdale NV, Lynd M, Chevalier RL, Felder RA, Peach MJ, Carey RM. Selective peripheral dopamine-1 receptor stimulation: defferential responses to sodium loading and depletion in humans. Hypertension. 1990;15:914–21. doi: 10.1161/01.hyp.15.6.914. [DOI] [PubMed] [Google Scholar]
- 48.Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, Felder RA, Carey RM. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49:155–61. doi: 10.1161/01.HYP.0000251881.89610.ee. [DOI] [PubMed] [Google Scholar]
- 49.Siragy HM, Felder RA, Howell NL, Chevalier RL, Peach MJ, Carey RM. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol Renal Physiol. 1989;257:F469–77. doi: 10.1152/ajprenal.1989.257.3.F469. [DOI] [PubMed] [Google Scholar]
- 50.Padia SH, Kemp BA, Howell NL, Keller SR, Gildea JJ, Carey RM. Mechanisms of dopamine D(1) and angiotensin type 2 receptor interaction in natriuresis. Hypertension. 2012;59:437–45. doi: 10.1161/HYPERTENSIONAHA.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci U S A. 1998;95:5573–8. doi: 10.1073/pnas.95.10.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou NH, Mitcheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol Renal Physiol. 1999;276:F711–19. doi: 10.1152/ajprenal.1999.276.5.F711. [DOI] [PubMed] [Google Scholar]
- 53.Hakam AC, Hussein T. Angiotensin type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not lean Zucker rats. Hypertension. 2006;47:1117–24. doi: 10.1161/01.HYP.0000220112.91724.fc. [DOI] [PubMed] [Google Scholar]
- 54.Gildea JJ, Wang X, Shah N, Tran H, Spinosa M, Van Sciver R, Sasaki M, Yatabe J, Carey RM, Jose PA, Felder RA. Dopamine and angiotensin type 2 receptors cooperatively inhibit sodium transport in human renal proximal tubule cells. Hypertension. 2012;60:396–403. doi: 10.1161/HYPERTENSIONAHA.112.194175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 2011;121:297–303. doi: 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- 56.Steckelings UM, Ludovit P, Namsolleck P, Unger T. AT2 receptor agonists: hypertension and beyond. Curr Opin Nephrol Hypertens. 2012;21:142–6. doi: 10.1097/MNH.0b013e328350261b. [DOI] [PubMed] [Google Scholar]
- 57.Hilliard LM, Jones ES, Steckelings UM, Unger TM, Widdop RE, Denton KM. Sex-specific inflence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension. 2012;59:409–14. doi: 10.1161/HYPERTENSIONAHA.111.184986. [DOI] [PubMed] [Google Scholar]
- 58.Ali Q, Hussein T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens Res. 2012;35:654–60. doi: 10.1038/hr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]