Abstract
In AT1 receptor (AT1R)-blocked rats, renal interstitial (RI) administration of des-aspartyl1-angiotensin II (Ang III), but not angiotensin II (Ang II), induces natriuresis via activation of angiotensin type-2 receptors (AT2R). In the present study, renal function was documented during systemic AT1R blockade with candesartan in Sprague-Dawley rats receiving unilateral RI infusion of Ang III. Ang III increased urine sodium excretion (UNaV), fractional excretion of sodium (FENa), and fractional excretion of lithium (FELi). RI co-infusion of specific AT2R antagonist PD-123319 (PD) abolished Ang III-induced natriuresis. The natriuretic response observed with RI Ang III was not reproducible with RI Ang (1–7) alone or together with angiotensin converting enzyme (ACE) inhibition. Similarly, neither RI Ang II alone nor in the presence of aminopeptidase A (APA) inhibitor to prevent degradation increased UNaV. In the absence of systemic AT1R blockade, Ang III alone did not increase UNaV, but natriuresis was enabled by the co-infusion of aminopeptidase N (APN) inhibitor and subsequently blocked by PD. In AT1R-blocked rats, RI administration of APN inhibitor alone also induced natriuresis that was abolished by PD. Ang III-induced natriuresis was accompanied by increased RI cyclic GMP levels and was abolished by inhibition of soluble guanylyl cyclase. RI and renal tissue Ang III levels increased in response to Ang III infusion and were augmented by APN inhibition. These data demonstrate that endogenous intrarenal Ang III, but not Ang II or Ang (1–7), induces natriuresis via activation of AT2Rs in the proximal tubule via a cyclic GMP-dependent mechanism and suggest APN inhibition as a potential therapeutic target in hypertension.
Keywords: angiotensin, sodium, natriuresis, kidney, receptor, cyclic GMP
INTRODUCTION
The basal state of renal sodium (Na+) excretion (UNaV) and its effect on blood pressure (BP) are regulated by the intrarenal renin-angiotensin system (RAS) (1,2). RAS actions are mediated largely by angiotensin II (Ang II) acting via two major receptors: angiotensin type-1 (AT1R) and angiotensin type-2 (AT2R). The activation of AT1Rs by Ang II induces vasoconstriction, increased renal tubule Na+ reabsorption, sympathetic nervous system activation and increased secretion of aldosterone, vasopressin and endothelin (1,2). Collectively, these actions lead to the elevation of systemic BP. Conversely, the activation of AT2Rs results in vasodilation and natriuresis (3,4). Angiotensin (1–7) [Ang (1–7)] is another product of the RAS whose role in the kidney has not fully been established. In vitro studies in rat renal proximal tubule cells (RPTC) demonstrate that Ang (1–7) inhibits Na+/K+-adenosine triphosphatase (ATPase) activity, suggesting that Ang (1–7) might induce natriuresis in vivo possibly mediated via AT2Rs, the Ang (1–7) mas receptor, or both (5). However, whether Ang (1–7) reduces tubular Na+ transport and induces natriuresis in vivo remains uncertain (5).
Ang II, the principal effector peptide of the RAS, is metabolized within the kidney to des-aspartyl1-Ang II (angiotensin III; Ang III) by aminopeptidase A (APA) (6). Intrarenal Ang III has recently been demonstrated to be a preferred AT2R agonist in the induction of natriuresis (7–9). The natriuretic effect of exogenous renal interstitial (RI) Ang III infusion is markedly augmented by co-infusion with the selective aminopeptidase N (APN) inhibitor PC-18, which blocks the degradation of Ang III (8). In contrast, RI Ang II infusion does not increase UNaV unless APN is concomitantly inhibited, and this natriuretic response is abolished when Ang II metabolism to Ang III is blocked with APA inhibitor EC-33 (9).
Previous studies have localized the precursors of angiotensin peptide synthesis, including angiotensinogen, renin and angiotensin converting enzyme (ACE) mRNA, to the renal proximal tubule (1). RPTCs, which are responsible for reabsorption of about 60% of filtered Na+ in the nephron, express a high level of AT2Rs as verified by Western blot analysis and immunohistochemistry (10,11).
A major cell signaling pathway of AT2Rs is the activation of the nitric oxide/soluble guanylyl cyclase/guanosine cyclic 3′,5′-monophosphate (cGMP) cascade (1,3), but whether this pathway is involved in the AT2R-natriuretic response is unknown. In the present study, we examined renal functional responses and RI fluid and tissue Ang II and Ang III levels to direct RI Ang III infusion to determine whether Ang III induces natriuresis by acting at AT2Rs in RPTCs by a soluble guanylyl cyclase-dependent mechanism. We also determined the natriuretic effects of (i) RI Ang III infusion in the presence or absence of specific AT2R antagonist PD-123319 (PD); (ii) RI Ang (1–7) infusion in the presence or absence of ACE inhibition with enalaprilate [to inhibit Ang (1–7) metabolism]; and (iii) RI Ang II infusion in the presence of APA inhibitor EC-33 used to block Ang II degradation and shunt the octapeptide to Ang (1–7) in rats with concurrent systemic AT1R blockade. Additionally, we studied the RI co-infusion of Ang III and APN inhibitor PC-18 in both the presence and absence of systemic AT1R blockade to determine the influence of AT1Rs on Ang III-induced natriuresis. Further, we explored RI infusion of PC-18 alone in the presence of systemic AT1R blockade to determine if endogenous Ang III can induce natriuresis. The results of these studies demonstrate that endogenous intrarenal Ang III, but not Ang II or Ang (1–7), induces natriuresis via activation of AT2Rs in a soluble guanylyl cyclase-dependent manner, likely in the renal proximal tubule.
METHODS
Animal Preparation
The experiments were conducted on 12-week-old female Sprague–Dawley rats except that the experiments in Protocol 8 were conducted on 10-week-old male Sprague-Dawley rats. Please see the Online Data Supplement at http://hyper.ahajournals.org for the following methods: renal cortical interstitial infusion, renal interstitial fluid (RIF) microdialysis, mean arterial pressure (MAP) measurements, pharmacologic agents, measurement of glomerular filtration rate (GFR) and fractional excretion of sodium (FENa) and lithium (FELi), measurement of Ang peptide levels in renal interstitial fluid and tissue and AT2R Western blot analysis. Except where described otherwise, all protocols were performed on rats subjected to 24 hour systemic AT1R blockade with candesartan (CAND).
Protocols
(1) Effects of Unilateral RI Infusion of Ang III on UNaV, MAP, GFR, FENa, and FELi in the Presence of Systemic AT1R Blockade
The left (experimental) kidney received RI infusion of Ang III (3.5, 7, 14, and 28 nmol/kg/min; each dose for 30-min) following a 30-min control period with RI vehicle (V; 5% dextrose in water) infusion. The right (control) kidney received RI V infusion for all periods. UNaV, MAP, GFR, FENa, and FELi were quantified separately for the experimental and control kidneys for each infusion period.
(2) Effects of Unilateral RI Infusion of Ang III ± AT2R Antagonist PD on UNaV and MAP in the Presence of Systemic AT1R Blockade
The left (experimental) kidney received RI infusion of Ang III (3.5, 7,14, and 28 nmol/kg/min; each dose for 30-min) ± PD (10 μg/kg/min) throughout the 4 experimental periods following a 30-min control period with RI V infusion. The right (control) kidney received RI V infusion for all periods. UNaV and MAP were quantified separately for the experimental and control kidneys for each infusion period.
(3) Effects of Unilateral RI Infusion of Ang (1–7) ± ACE Inhibitor Enalaprilate or Ang (1–7) antagonist A-779 on UNaV and MAP in the Presence of Systemic AT1R Blockade
The left (experimental) kidney received RI infusions of Ang (1–7) (3.5, 7, 14 and 28 nmol/kg/min; each dose for 30-min) ± enalaprilate (10 μg/kg/min) or A-779 (3.5, 7, 14 and 28 nmol/kg/min; each dose for 30-min) throughout the 4 experimental periods following a 30-min control period with RI V infusion. The right (control) kidney received RI V infusion for all periods. UNaV and MAP were quantified separately for the experimental and control kidneys for each infusion period.
(4) Effects of Unilateral RI Infusion of Ang II ± APA Inhibitor EC-33 on UNaV and MAP in the Presence of Systemic AT1R Blockade
The left (experimental) kidney received RI infusions of Ang II (3.5, 7, 14, and 28 nmol/kg/min; each dose for 30-min) ± EC-33 (25 μg/min) throughout the 4 experimental periods following a 30-min control period with RI V infusion. The right (control) kidney received RI V infusion for all periods. UNaV and MAP were quantified separately for the experimental and control kidneys for each infusion period.
(5) Effects of Unilateral RI Infusion of Ang III ± APN Inhibitor PC-18 or Ang III + PC-18 + AT2R Antagonist PD on UNaV and MAP in the Absence of Systemic AT1R Blockade
In this series, the rats were not subjected to 24-hr systemic AT1R blockade prior to study. The left (experimental) kidney received RI infusion of Ang III (3.5, 7, 14 and 28 nmol/kg/min) ± PC-18 (25 μg/min) or Ang III + PC-18 + PD (10 μg/kg/min) throughout the 4 experimental periods following a 30-min control period with RI V infusion. The right (control) kidney received RI V infusion for all periods. UNaV, and MAP were quantified separately for the experimental and control kidneys for each infusion period.
(6) Effects of Unilateral RI Infusion of APN Inhibitor PC-18 ± PD on UNaV and MAP in the Presence of Systemic AT1R Blockade
The left (experimental) kidney received RI infusion of PC-18 (25 μg/min) ± PD (10 μg/kg/min) throughout the 4 experimental periods following a 30-min control period with RI V infusion. The right (control) kidney received RI V infusion for all periods. UNaV and MAP were quantified separately for the experimental and control kidneys for each infusion period.
(7) Effects of Unilateral RI infusion of Ang III, Ang III + PC-18 ± PD or ± soluble guanylyl cyclase inhibitor 1-H-[1,2,4] oxadiazolo-[4,2-α] quinoxalin-1-one (ODQ) on RIF cGMP, UNaV and MAP in the Presence of Systemic AT1R Blockade
The left (experimental) kidney received RI infusion of Ang III (3.5, 7,14, and 28 nmol/kg/min; each dose for 30-min), Ang III + PC-18 (25 μg/min) or Ang III + PC-18 + PD or Ang III + PC-18 + ODQ throughout the 4 experimental periods following a 30-min control period with RI V infusion. The right (control) kidney received RI V infusion for all periods. RIF cGMP, UNaV and MAP were quantified separately for the experimental and control kidneys for each infusion period.
(8) Effects of Systemic AT2R Agonist Compound 21 (C21) alone and combined with RI PD infusion on UNaV and MAP in Uninephrectomized Male Sprague-Dawley Rats
Male rats (N=11) anesthetized with Inactin underwent acute unilateral nephrectomy. Following a one hour control period, the rats received a systemic infusion of C21 at 100, 200 and 300 ng/kg/min, each dose for 30 min either in the presence or absence of RI infusion of PD (10 μg/kg/min) or systemic AT1R blockade prior to the study. UNaV and MAP were quantified for the control and final infusion period.
(9) Effects of Systemic AT1R Blockade and Unilateral RI Infusion of Ang II or Ang III or Ang III + PC-18 on Renal Tissue and RIF Levels of Ang II and Ang III
The left (experimental) kidney received RI infusion of Ang II (3.5, 7, 14, and 28 nmol/kg/min; each dose for 30-min), Ang III or Ang III + PC-18 (25 μg/min following a 30-min control period with RI V infusion. Renal tissue and RIF Ang II and III levels were separated using HPLC and quantified separately by radioimmunoassay as previously described (12).
Statistical Analysis
Data are presented as mean ± 1 SE. Statistical significance was determined by using one-way analysis of variance (ANOVA) followed by multiple comparisons testing with the Student Newman-Keuls test with 95% confidence. The level of significance was set at P<0.05.
RESULTS
Effects of Unilateral RI Infusion of Ang III on UNaV, MAP, GFR, FENa, and FELi in the Presence of Systemic AT1R Blockade
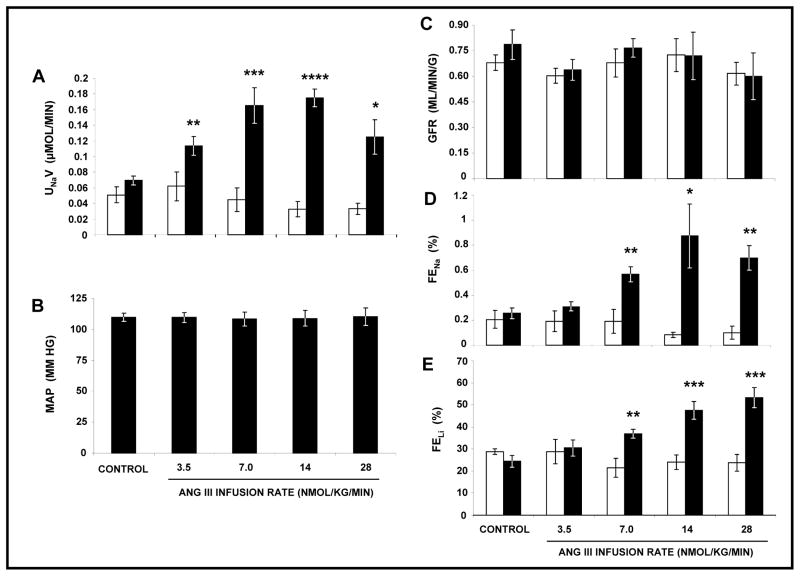
As shown in Figure 1, Panel A, cumulative RI delivery of Ang III in the presence of systemic AT1R blockade with CAND induced statistically significant natriuresis at all infusion rates (F=34.0, P<0.0001). UNaV increased from a control of 0.07 ± 0.01 μmol/min to 0.11 ± 0.01 (P<0.01), 0.17 ± 0.02 (P<0.01), 0.18 ± 0.01 (P<0.001), and 0.13 ± 0.02 μmol/min (P<0.05) at 3.5, 7, 14, and 28 nmol/kg/min of Ang III respectively. RI Ang III infusion had no significant effect on MAP (Panel B) as monitored by a direct carotid artery cannula or GFR (Panel C) as measured by inulin clearance. The rise in UNaV was accompanied by a significant increase in both FENa (F=14.6, P<0.0001) and FELi (F=14.9, P<0.0001). FENa (Panel D) increased from a control of 0.26 ± 0.04% to 0.57 ± 0.06 (P=0.01), 0.87 ± 0.26 (P<0.0001), and 0.70 ± 0.10% (P=0.0003) at Ang III infusion rates of 7, 14 and 28 nmol/kg/min, respectively. FELi (Panel E) increased in a dose-dependent fashion from a control of 24.4 ± 2.7% to 36.9 ± 1.9 (P=0.007), 47.4 ± 4.0 (P=0.0002), 53.3 ± 4.6% (P<0.001) at 7, 14, and 28 nmol/kg/min of Ang III, respectively. There were no changes in UNaV, MAP, GFR, FENa, or FELi in the V-infused control kidneys. Because most of the experiments employed systemically blocked AT1Rs for 24h, we measured total whole cell AT2R protein expression in cortical kidney samples by Western blot analysis and demonstrated that there was no change in AT2R expression under these conditions (data not shown).
FIGURE 1.
Panel A. Urine Na+ excretion (UNaV) in response to renal interstitial (RI) infusion of vehicle (□), (N=6) and Ang III (■), (N=6) in the presence of systemic AT1R blockade with candesartan. Results are reported as μmol/min. Panel B. Mean arterial pressure in response to conditions in Panel A. Results are reported as mm Hg. Panel C. Glomerular filtration rate (GFR) in response to conditions in Panel A. Results are reported as mL/min/gram kidney weight. Panel D. Fractional excretion of Na+ (FENa) in response to conditions in Panel A. Results are reported as a percentage. Panel E. Fractional excretion of Li+ (FELi) in response to conditions in Panel A. Results are reported as a percentage. Data represent mean ± 1 SE. *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001 from own control.
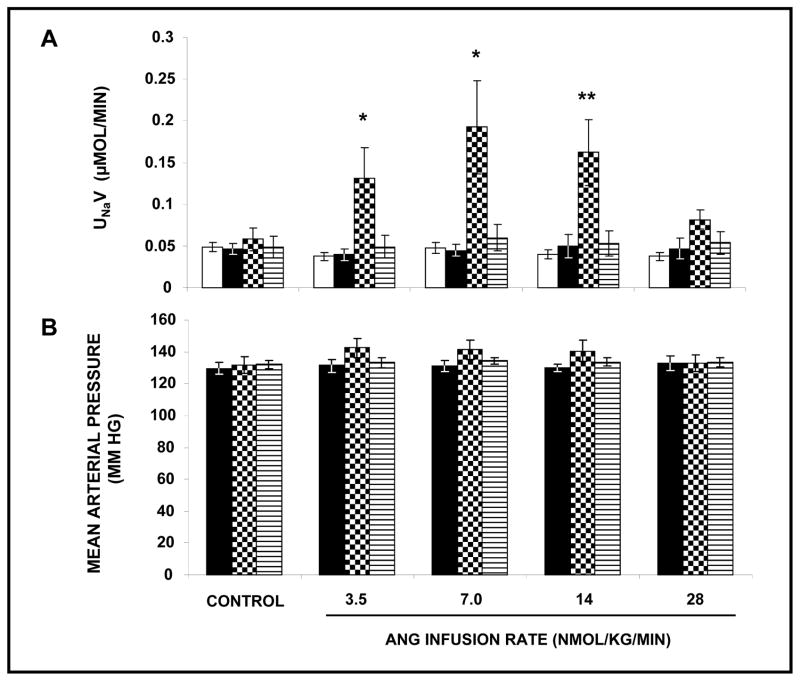
Effects of Unilateral RI Infusion of Ang III ± AT2R Antagonist PD on UNaV and MAP in the Presence of Systemic AT1R Blockade
As depicted in Figure S1 (Panel A) (Online Data Supplement at http://hyper.ahajournals.org), cumulative RI administration of Ang III in the presence of systemic CAND induced a rise in UNaV (F=23.5, P<0.001). UNaV increased from a control value of 0.05 ± 0.01 μmol/min to a peak of 0.14 ± 0.01 μmol/min (P<0.0001) at an Ang III infusion rate of 14 nmol/kg/min. The increase in UNaV in response to Ang III was abolished by co-infusion of the heptapeptide with AT2R antagonist PD. There was no significant change in MAP (Panel B) in any of these experiments nor in UNaV in the V-infused control kidneys (Panel A).
Effects of Unilateral RI Infusion of Ang (1–7) ± ACE Inhibitor Enalaprilate or Ang (1–7) antagonist A779 on UNaV and MAP in the Presence of Systemic AT1R Blockade
In contrast to the aforementioned results with Ang III, RI infusion of equimolar doses of Ang (1–7) (Figure S1, Panel A) did not increase UNaV at any of the administered infusion rates. Similarly, RI co-infusion of Ang (1–7) and ACE inhibitor enalaprilate [to reduce the catabolism of infused Ang (1–7)] failed to increase UNaV. There was also no significant change in MAP (Panel B) in any of these experiments nor in UNaV in the V-infused control kidneys (Panel A). We also demonstrated that Ang (1–7) does not engender natriuresis even in the absence of AT1R blockade with CAND and that Ang (1–7) antagonist A-779 alone has no effect UNaV (data not shown).
Effects of Unilateral RI Infusion of Ang II ± APA Antagonist EC-33 on UNaV and MAP in the Presence of Systemic AT1R Blockade
In contrast to Ang III, RI infusion of Ang II (Figure S1, Panel A) failed to increase UNaV. To determine whether an increase in endogenous Ang (1–7) is capable of increasing UNaV, we administered APA inhibitor EC-33 interstitially, to shunt endogenous Ang II to Ang (1–7). However, this maneuver also failed to increase UNaV (Figure S1, Panel A). Similarly, there were no significant changes in MAP (Panel B) in any of these experiments nor in UNaV in the V-infused control kidneys (Figure S1, Panel A).
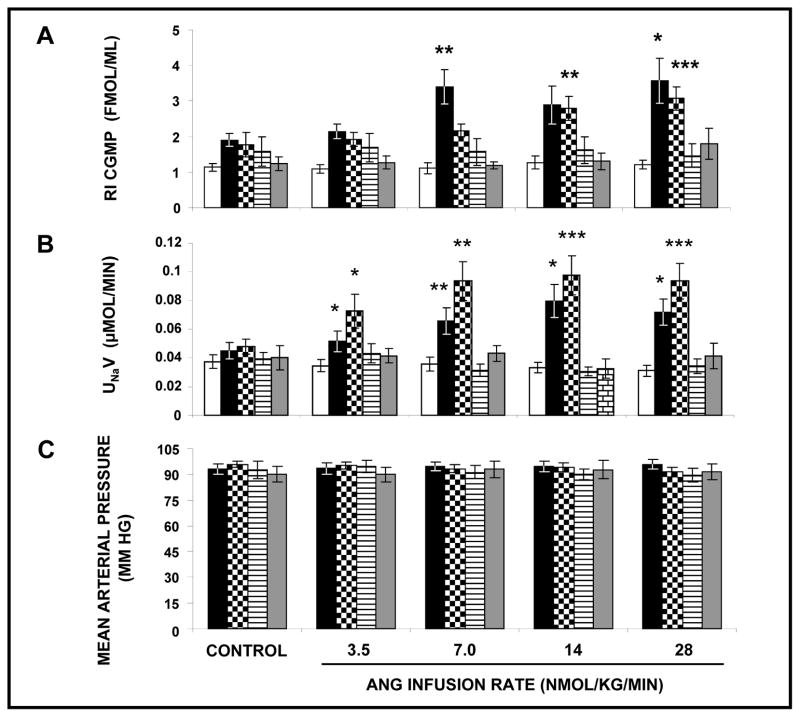
Effects of Unilateral RI Infusion of Ang III ± APN Inhibitor PC-18 or Ang III + PC-18 + AT2R Antagonist PD on UNaV and MAP in the Absence of Systemic AT1R Blockade
After demonstrating that RI Ang III infusion induces significant natriuresis in the presence of systemically blocked AT1Rs, we designed a set of experiments to determine whether Ang III-induced natriuresis is dependent upon reduced AT1R activation. As shown in Figure 2 (Panel A) in the absence of systemic CAND infusion, Ang III alone failed to engender a natriuretic response. However, co-infusion with APN inhibitor PC-18 enabled Ang III to induce a significant natriuresis (F=23.7, P<0.0001). Co-infusion of Ang III + PC-18 + PD abolished the natriuresis induced by Ang III at 3.5, 7 and 14 nmol/kg/min in the presence of APN inhibitor PC-18. During these experiments, no significant changes in MAP (Panel B) or UNaV (Panel A) in the V-infused control kidneys were observed. Because PD decreased UNaV to a value below V-infused control kidneys in these experiments, we also provide a control that PD alone does not reduce UNaV (data not shown).
FIGURE 2.
Panel A. Urine Na+ excretion (UNaV) in response to renal interstitial (RI) infusion of vehicle (□), (N=22), Ang III (■), (N=7), Ang III+APN inhibitor PC-18 (
 ), (N=10), and Ang III+PC-18+PD-123319 (▤), (N=7) in the absence of systemic AT1R blockade. Results are reported as μmol/min. Panel B. Mean arterial pressure in response to conditions in Panel A. Results are reported as mm Hg. Data represent mean ± 1 SE. *P<0.05 and **P<0.01 from own control.
), (N=10), and Ang III+PC-18+PD-123319 (▤), (N=7) in the absence of systemic AT1R blockade. Results are reported as μmol/min. Panel B. Mean arterial pressure in response to conditions in Panel A. Results are reported as mm Hg. Data represent mean ± 1 SE. *P<0.05 and **P<0.01 from own control.
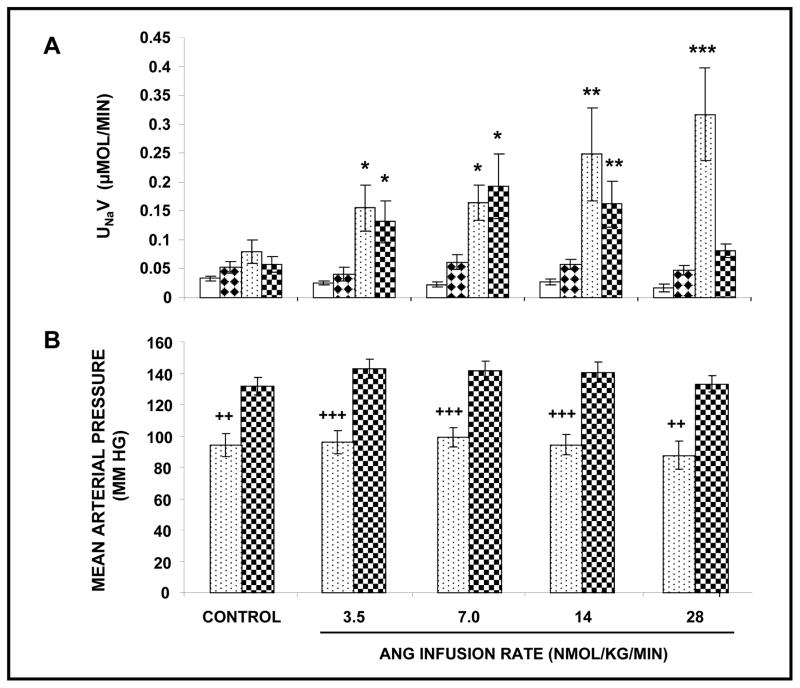
Figure 3 compares natriuretic and MAP responses to RI co-infusion of Ang III + PC-18 in the presence and absence of systemic AT1R blockade. As shown in Panel A, Ang III + PC-18 induced a robust, dose-dependent natriuresis in the presence of CAND (F=30.0; P<0.0001). UNaV increased from control values of 0.08 ± 0.02 μmol/min in a stepwise fashion to a peak of 0.32 ± 0.08 (P<0.0001) μmol/min at the highest infusion rate of Ang III (28 nmol/kg/min). In the absence of systemic CAND, RI infusion of Ang III + PC-18 induced a comparable natriuresis to that with CAND at 3.5 and 7 nmol/kg/min (P<0.05 from control values; P=NS from CAND). However, in the rats with uninhibited AT1Rs, Ang III + PC-18 was unable to sustain the natriuresis at the higher Ang III infusion rates and for the duration of the study. The overall natriuretic response to Ang III + PC-18 was greater in the presence than in the absence of systemic AT1R blockade (F=6.7; P<0.0001). As shown in Panel B, MAP was significantly lower in the CAND-treated rats than in those without CAND (F=28.4; P<0.0001), but MAP remained unaltered during the RI Ang III + PC-18 infusions (P=NS).
FIGURE 3.
Panel A. Urine Na+ excretion (UNaV) in response to renal interstitial (RI) infusion of vehicle (□), (N=7) and Ang III+APN inhibitor PC-18 (
 ), (N=7) in the presence of systemic AT1R blockade with candesartan and RI infusion of vehicle (
), (N=7) in the presence of systemic AT1R blockade with candesartan and RI infusion of vehicle (
 ), (N=9) and Ang III+PC-18 (
), (N=9) and Ang III+PC-18 (
 ), (N=10) in the absence of systemic AT1R blockade. Results are reported as μmol/min. Panel B. Mean arterial pressure in response to conditions in Panel A. Results are reported as mm Hg. Data represent mean ± 1 SE. *P<0.05, **P<0.01, and ***P<0.001 from own control. ++P<0.01 and +++P<0.001 within groups.
), (N=10) in the absence of systemic AT1R blockade. Results are reported as μmol/min. Panel B. Mean arterial pressure in response to conditions in Panel A. Results are reported as mm Hg. Data represent mean ± 1 SE. *P<0.05, **P<0.01, and ***P<0.001 from own control. ++P<0.01 and +++P<0.001 within groups.
Effects of Unilateral RI Infusion of APN Inhibitor PC-18 ± PD on UNaV and MAP in the Presence of Systemic AT1R Blockade
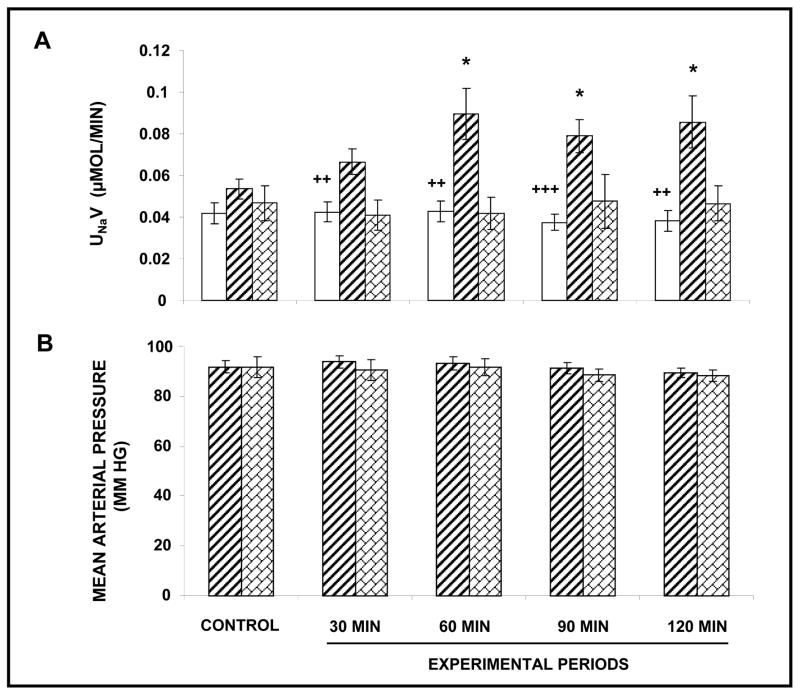
In order to determine whether endogenous Ang III is capable of inducing natriuresis, we infused PC-18 alone interstitially in AT1R-blocked rats. As shown in Figure 4 (Panel A), PC-18 induced a sustained natriuresis (F=14.7; P<0.0001) with UNaV rising from 0.05 ± 0.004 μmol/min to a peak of 0.09 ± 0.008 μmol/min (P<0.05) after 60 min of infusion. The natriuretic response to RI PC-18 infusion was abolished by the co-administration of AT2R antagonist PD. During these experiments, no significant change in MAP (Panel B) or UNaV (Panel A) in the V-infused control kidneys was observed.
FIGURE 4.
Panel A. Urine Na+ excretion (UNaV) in response to renal interstitial (RI) infusion of vehicle (□), (N=22) APN inhibitor PC-18 (▨), (N=17), and PC-18+ PD-123319 (
 ), (N=7) in the presence of systemic AT1R blockade with candesartan. Results are reported as μmol/min. Panel B. Mean arterial pressure in response to conditions in Panel A. Results are reported as mm Hg. Data represent mean ± 1 SE. *P<0.05 from own control. ++P<0.01 and +++P<0.001 within groups.
), (N=7) in the presence of systemic AT1R blockade with candesartan. Results are reported as μmol/min. Panel B. Mean arterial pressure in response to conditions in Panel A. Results are reported as mm Hg. Data represent mean ± 1 SE. *P<0.05 from own control. ++P<0.01 and +++P<0.001 within groups.
Effects of Unilateral RI infusion of Ang III, Ang III + PC-18 ± PD or ± ODQ on RIF cGMP, UNaV and MAP in the Presence of Systemic AT1R Blockade
As shown in Figure 5, Panel A, RI administration of Ang III increased RIF cGMP concentrations (F=8.49, P<0.0001). PC-18 did not significantly augment the cGMP response to Ang III. The increased cGMP response to Ang III + PC-18 was abolished with both PD and ODQ. Ang III alone and in the presence of PC-18 increased natriuresis (Figure 5, Panel B; F=6.5, P<0.0001; F=12.55, P<0.0001, respectively). Natriuretic responses to Ang III + PC-18 were numerically greater than with Ang III alone, although this did not achieve statistical significance (F=1.90, P=0.11). Ang III-induced natriuresis was abolished by both AT2R blocker PD and by guanlylyl cyclase inhibitor ODQ. There was no significant difference in MAP in response to any of the interstitially infused agents (Figure 5, Panel C).
FIGURE 5.
Panel A. Renal interstitial (RI) cGMP levels in response to RI infusion of vehicle (□), (N=13), Ang III (■), (N=11), Ang III + APN inhibitor PC-18 (
 ), (N=8), Ang III + PC-18 + PD-123319 (▤), (N=11), and Ang III + PC-18 + soluble guanylyl cyclase inhibitor ODQ (
), (N=8), Ang III + PC-18 + PD-123319 (▤), (N=11), and Ang III + PC-18 + soluble guanylyl cyclase inhibitor ODQ (
 ), (N=7). Results are reported as fmol/mL. Panel B. Urine Na+ excretion (UNaV) in response to conditions in Panel A. Results are reported as μmol/min. Panel C. Mean arterial pressure in response to conditions in Panel A. Results are reported as mm Hg. Data represent mean ± 1 SE. *P<0.05, **P<0.01, and ***P<0.001 from own control.
), (N=7). Results are reported as fmol/mL. Panel B. Urine Na+ excretion (UNaV) in response to conditions in Panel A. Results are reported as μmol/min. Panel C. Mean arterial pressure in response to conditions in Panel A. Results are reported as mm Hg. Data represent mean ± 1 SE. *P<0.05, **P<0.01, and ***P<0.001 from own control.
Effects of Systemic AT2R Agonist C21 Alone with or without Systemic AT1R Blockade and Combined with RI Infusion of PD on UNaV and MAP in Uninephrectomized Male Rats
C21 increased UNaV from 0.46 ± 0.08 to 6.22 ± 1/33 μmol/min (P<0.01). This response was augmented by systemic AT1R blockade (P<0.05) and blocked by intrarenal PD (P<0.05) (Figure S3; Online Data Supplement at http://hyper.ahajournals.org). MAP did not change significantly after C21 or PD (data not shown).
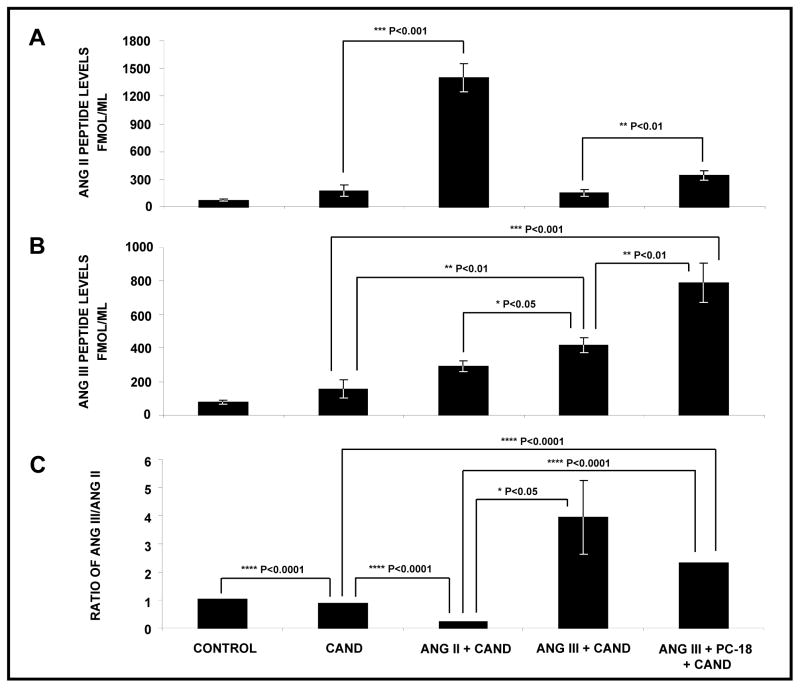
Measurement of RIF and Renal Tissue Ang II and III Levels in Response to Systemic AT1R Blockade and RI Infusion of Ang II or Ang III alone and + PC-18
As demonstrated in Figure 6, RIF Ang II (Panel A) and Ang III (Panel B) levels in the basal state were 76±12.0 and 77.4±12.1 fmol/ml, respectively (ratio 1.0; Panel C). Systemic AT1R blockade with CAND did not significantly change RIF Ang II or Ang III levels. RI Ang II infusion in the presence of systemic CAND increased RI Ang II levels by 8-fold (P<0.001) compared with those during CAND alone. Although numerically increased, Ang III levels were not significantly different from those with systemic CAND alone. As expected, RI administration of Ang III, while not increasing RIF Ang II levels, increased Ang III levels from those with CAND alone (P<0.01). RI co-administration of Ang III + PC-18 further increased RIF Ang III levels to 789.7±115.9 fmol/ml (P<0.01) but unexpectedly increased Ang II levels significantly.
FIGURE 6.
Angiotensin peptide levels in renal cortical interstitial fluid. Panel A. Ang II peptide levels following the renal interstitial (RI) infusion of vehicle in the absence of systemic AT1R blockade (N=6) and the RI infusion of vehicle (N=5), Ang II (N=6), Ang III (N=7), and Ang III + APN inhibitor PC-18 (N=11) in the presence of systemic AT1R blockade with candesartan. Results are reported as fmol/gram kidney weight. Panel B. Ang III peptide levels in response to conditions in Panel A. Panel C. Ratios of Ang III to Ang II peptide levels in response to conditions in Panel A. Data represent mean ± 1 SE.
Baseline renal tissue levels of Ang II and Ang III (Figure S3, Panels A and B; Online Data Supplement at http://hyper.ahajournals.org) were 313.0±60.9 and 77.8±18.1 fmol/gram kidney weight, respectively. Systemic AT1R blockade did not alter the levels of either peptide but significantly increased the ratio of Ang III to Ang II (Panel C). Ang II infusion increased both Ang II (P<0.01) and Ang III (P<0.05) and decreased the Ang III:II ratio (P<0.05). Ang III infusion increased levels of Ang III (P<0.0001) and also unexpectedly Ang II (P<0.01) but increased the ratio of Ang III:II. Ang III infusion in the presence of PC-18 also unexpectedly increased the levels of both peptides (each P<0.01) to approximately the same extent. Because Ang III increased the tissue concentrations of both peptides, we performed additional experiments in vitro in which Ang III was added to the assay system and Ang II was quantified. There was no significant cross-reactivity of Ang II with Ang III that could account for the increased renal Ang II in response to Ang III or Ang III + PC-18 administration (data not shown).
DISCUSSION
The present study demonstrates the ability of Ang III to induce natriuresis via activation of soluble guanylyl cyclase by AT2Rs specifically localized in the RPTC. RI Ang III-induced natriuresis was abolished by co-infusion with the highly selective AT2R antagonist PD, specific for AT2Rs in the low doses employed in this study (13). In the absence of changes in GFR, FENa increased during Ang III infusions, indicating that the observed natriuretic effects of Ang III are attributable to reduced tubular transport and not to systemic or intrarenal hemodynamic changes. In Na+-replete experimental animals, FELi localizes changes in renal Na+ reabsorption to the proximal tubule with a maximum error rate of 4% (14). FELi increased in a dose-dependent fashion, strongly suggesting - but not unequivocally proving - that Ang III acts predominantly in the proximal tubule, where AT2Rs are highly expressed (10,11). Ang III-induced natriuresis was accompanied by increased RIF cGMP levels and was abolished by the soluble guanylyl cyclase inhibitor ODQ, strongly suggesting that Ang III-induced natriuresis is mediated by renal production of cGMP.
Our study also provides data showing that, at equimolar concentrations, neither intrarenal Ang II nor Ang (1–7) engender natriuresis in the AT1R blocked rat. We also demonstrated no effect of Ang (1–7) antagonist A-779, excluding an effect of endogenous Ang (1–7) on Na+ excretion. Previous studies from our laboratory demonstrated that Ang II was only capable of inducing natriuresis in the presence of an APN inhibitor to decrease Ang III metabolism (7,9). In the present study, Ang II, at an equivalent molar infusion rate as Ang III, either in the presence or absence of APA inhibitor to retard Ang II degradation was ineffective in increasing UNaV. In these studies, Ang (1–7), also at the equivalent molar concentration, failed to induce natriuresis either when infused alone or in the presence of an ACE inhibitor to reduce its degradation. It is certainly possible that alternative pathways, such as neprilysin (15), can metabolize Ang (1–7), but ACE has been identified as a major degrading enzyme for this peptide (15,16). Also, APA inhibition, which would be expected to increase Ang II which would then be available for conversion to Ang (1–7) via ACE-2 and/or alternative pathways, did not induce natriuresis. Taken together, these results support the concept that Ang III is the predominant agonist of AT2R-mediated natriuresis.
Previous studies from our laboratory have indicated that Ang III-induced natriuresis requires the concurrent blockade of systemic AT1Rs (7). Results from the present study confirm this finding. However, in the present study we demonstrated that, when APN is inhibited to reduce Ang III metabolism, the heptapeptide is capable of inducing natriuresis in the presence of unblocked systemic AT1Rs. Thus, in a pure physiological sense, intrarenal Ang III would not be expected to play a significant role in the control of Na+ excretion when AT1Rs are intact, as the antinatriuretic actions of Ang II via AT1Rs, which are widely expressed in the kidney, would overwhelm the ability of AT2Rs to engender natriuresis. AT2R-induced natriuresis would only apply when AT1Rs are blocked or, as shown by our data, when APN is blocked in the absence of AT1R inhibition. We also demonstrated that the aforementioned natriuretic response is specifically related to renal AT2R activation. In this regard, it is interesting to compare the natriuretic responses to Ang III with and without systemic AT1R blockade. In rats with AT1R blockade, natriuretic responses to Ang III and the APN inhibitor were sustained in a dose-dependent manner, whereas in those without AT1R blockade an initial natriuretic response was followed by return to baseline levels in spite of increasing peptide infusion rates. These data suggest that AT1R blockade enhances both the magnitude and duration of the natriuretic response to Ang III, albeit in the short-term.
In light of these results, we hypothesized that endogenous Ang III is capable of inducing natriuresis in the normal rat. In AT1R-blocked rats, we found that intrarenal administration of APN inhibitor PC-18 alone induced a significant and sustained natriuresis that was abolished by AT2R antagonist PD. These results indicate that, in addition to exogenous Ang III, endogenous renal Ang III induces natriuresis via AT2R activation. The magnitude of the natriuretic response to PC-18 was relatively small compared with that due to exogenous Ang III in the presence of PC-18, indicating that renal heptapeptide biosynthesis may be a rate-limiting step in Ang III-induced natriuresis.
The present study is limited by the exclusive use of pharmacologic agents as heuristic tools to dissect the relative contributions of Ang peptides to the natriuretic response. In the future, it will be important to employ approaches targeting, for example, APN at the molecular level. However, our study does demonstrate that renal Ang II and III levels (i) are approximately equal in the basal state and (ii) are appropriate for their respective peptide infusions, and (iii) that PC-18 effectively inhibits renal APN. The increase in renal Ang II during Ang III infusion may be due to product inhibition of APA, but this will require further study.
We employed female rats for these studies. Hilliard et al (17,18) have recently shown that AT2R activation caused a similar increase in Na+ excretion via an action at the renal tubule in both male and female Sprague-Dawley rats. Identified sex differences were limited to renal hemodynamic (vasodilatory) function that did not influence Na+ excretion in their studies. In addition, Hakam and Hussain have shown that AT2R activation directly inhibits renal tubule sodium-potassium ATPase activity both in male obese Zucker rats (19) and in male Sprague-Dawley rats (20). Furthermore, Sabuhi et al. (21) demonstrated that AT2R activation induces natriuresis via a renal tubule mechanism in male Sprague-Dawley rats. Thus, there is a large body of evidence in the literature that renal tubule AT2R activation induces natriuresis in both male and female rats. However, we do show here that Compound 21, a highly selective AT2R agonist (13), induces natriuresis in male rats and that this response is augmented by concomitant AT1R blockade and blocked with intrarenal PD.
In addition to the kidney, two other cardiovascular/hormonal systems have been linked to Ang III as a preferred agonist for AT2Rs, the coronary vascular bed and adrenal zona glomerulosa. In the coronary microcirculation, Ang III, rather than Ang II, is the preferred ligand to induce AT2R-mediated vasodilation (22). Ang III also is the preferred agonist for AT2R-mediated aldosterone secretion from the adrenal cortex (23). Together with the results of the present study, the evidence suggests that at least in certain tissues Ang III may be a better molecular fit within the AT2R binding pocket than Ang II. The precise molecular conformation that renders Ang III a better fit awaits future investigation.
PERSPECTIVES
In the present study in the normal Na+-replete rat, we demonstrated that endogenous intrarenal Ang III increases UNaV and that this response is mediated by AT2Rs by a cGMP-dependent mechanism in the proximal tubule. We showed that Ang III is the predominant angiotensin peptide agonist at renal tubule AT2Rs as neither Ang II nor Ang (1–7) altered UNaV in our experimental model. Our recent published studies have shown that spontaneously hypertensive rats have defective AT2R-mediated natriuresis which can be restored by intrarenal APN inhibition (24). On the basis of these findings, we hypothesize that in hypertension renal APN activity is increased leading to accelerated Ang III metabolism reducing AT2R-mediated Na+ excretion. We further hypothesize that restoration of APN activity could be beneficial in the treatment of hypertension. These hypotheses will require validation in future studies.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New
Angiotensin III, but not angiotensin II or angiotensin (1–7), induces natriuresis during AT1 receptor blockade.
This effect is mediated by activation of AT2 receptors in the renal proximal tubule.
In the absence of AT1 receptor blockade, natriuretic responses to angiotensin III require concurrent inhibition of aminopeptidase N.
Aminopeptidase N inhibition alone can induce natriuresis.
Renal AT2 receptor activation induces natriuresis by a cyclic GMP dependent mechanism.
What is Relevant?
What is new?
Endogenous intrarenal angiotensin III, but not angiotensin II or angiotensin (1–7), induces natriuresis by activation of AT2 receptors in the renal proximal tubule.
This action of AT2 receptors is mediated by renal formation of cyclic GMP.
What is relevant?
A major initiating event in hypertension is thought to be increased renal proximal tubule reabsorption of sodium.
Renal AT1 receptor activation increases proximal tubule sodium reabsorption, and this response is counteracted by renal AT2 receptor activation triggered by endogenous intrarenal angiotensin III.
Angiotensin III activation of AT2 receptors is markedly augmented by blocking aminopeptidase N.
These studies suggest that aminopeptiase N inhibition would be a novel therapeutic target in hypertension.
Summary
This study demonstrates that endogenous intrarenal angiotensin III, but not angiotensins II or (1–7), induces natriuresis via activation of AT2Rs in the proximal tubule via a cyclic GMP-dependent mechanism and suggest APN inhibition as a potential therapeutic target in hypertension.
Acknowledgments
Analytical studies performed at Tulane University were also supported by CoBRE grant (P20RR017659) from the Institutional Development Award Program of the National Center for Research Resources.
References
- 1.Carey RM, Siragy HM. Newly recognized components of the renin- angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 2.Navar LG, Kobori H, Prieto MC, Gonzales-Villalobos RA. Intratubular renin- angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey RM, Padia SH. Angiotensin AT2 receptors: control of renal sodium excretion and blood pressure. Trends Endocrinol Metab. 2008;19:84–87. doi: 10.1016/j.tem.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dilauro M, Burns KD. Angiotensin (1-7) and its effects in the kidney. The Scientific World Journal. 2009;9:522–535. doi: 10.1100/tsw.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardaillou R, Chansel D. Synthesis and effects of active fragments of angiotensin II. Hypertension. 1999;33:746–752. doi: 10.1038/ki.1997.476. [DOI] [PubMed] [Google Scholar]
- 7.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin type-1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 8.Padia SH, Kemp BA, Howell NL, Siragy HM, Fournie-Zaluski MC, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type-1 receptor-blocked rats. Hypertension. 2007;49:625–630. doi: 10.1161/01.HYP.0000254833.85106.4d. [DOI] [PubMed] [Google Scholar]
- 9.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski MC, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor mediated natriuresis in rats. Hypertension. 2008;51:460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 10.Ozono R, Wang Z-Q, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype-2 angiotensin (AT2) receptor protein in the rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 11.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 12.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-Ang II-infused rats. Am J Physiol Renal Physiol. 2009;296:F1067–F1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosnyak S, Jones ES, Christopoulos A, Aguilar M-I, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci. 2011;121:297–303. doi: 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen K, Shirley DG. The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron. 1997;77(2):125–38. doi: 10.1159/000190264. [DOI] [PubMed] [Google Scholar]
- 15.Allred AJ, Diz DI, Ferrario CM, Chappel MC. Pathways for angiotensin (1-7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol. 2000;279:F841–F850.0. doi: 10.1152/ajprenal.2000.279.5.F841. [DOI] [PubMed] [Google Scholar]
- 16.Ferrario CM, Varagic J. The Ang-(1-7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Renal Physiol. 2010;298:F1297–F1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM. Gender differences in pressure-natriuresis and renal autoregulation: role of the angiotensin type 2 receptor. Hypertension. 2011;57:275–282. doi: 10.1161/HYPERTENSIONAHA.110.166827. [DOI] [PubMed] [Google Scholar]
- 18.Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension. 2012;59(part 2):409–414. doi: 10.1161/HYPERTENSIONAHA.111.184986. [DOI] [PubMed] [Google Scholar]
- 19.Hakam AC, Hussein T. Angiotensin II type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not lean Zucker rats. Hypertension. 2006;47:1117–1124. doi: 10.1161/01.HYP.0000220112.91724.fc. [DOI] [PubMed] [Google Scholar]
- 20.Hakam AC, Hussein T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP pathway. Am J Physiol Renal Physiol. 2006;290:F1430–F1436. doi: 10.1152/ajprenal.00218.2005. [DOI] [PubMed] [Google Scholar]
- 21.Sabuhi R, Asghar M, Hussein T. Inhibition of NAD(P)H oxidase potentiates AT2 receptor agonist-induced natriuresis in Sprague-Dawley rats. Am J Physiol Renal Physiol. 2010;299:F815–F820. doi: 10.1152/ajprenal.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Esch JH, Oosterveer CR, Batenburg WW, van Veghel R, Danser AH. Effects of angiotensin and its metabolites in the rat coronary vascular bed: is angiotensin III the preferred ligand of the angiotensin AT2 receptor? Eur J Pharmacol. 2008;588:286–293. doi: 10.1016/j.ejphar.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Yatabe J, Yoneda M, Yatabi MS, Watanabe T, Felder RA, Jose PA, Sanada H. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinol. 2011;152 doi: 10.1210/en.2010-1070. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Padia SH, Howell NL, Kemp BA, Fournie-Zaluski M-C, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition restores defective AT2R- mediated natriuresis in SHR. Hypertension. 2010;55:474–480. doi: 10.1161/HYPERTENSIONAHA.109.144956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.