Abstract
Objective:
To examine protocol adherence to structured intensive management in the Valsartan Intensified Primary carE Reduction of Blood Pressure (VIPER-BP) study involving 119 primary care clinics and 1562 randomized participants.
Methods:
Prospective criteria for assessing adherence to treatment prescription, uptitration, and visit attendance at 6, 10, 14, and 18 weeks postrandomization were applied to 1038 intervention participants. Protocol adherence scores of 1–5 (least to most adherent) were compared to blood pressure (BP) control during 26 weeks of follow-up.
Results:
Mean age was 59.3 ± 12.0 years, 963 (62%) were men, and 1045 (67%) had longstanding hypertension. Clinic attendance dropped from 91 (week 6) to 83% (week 26) and pharmacological instructions were followed for 93% (baseline) to 61% at week 14 (uptitration failures commonly representing protocol deviations). Overall, 26-week BP levels and BP target attainment ranged from 132 ± 14/79 ± 9 and 51% to 141 ± 15/83 ± 11 mmHg and 19% in those participants subject to the highest (n = 270, 26%) versus least (n = 148, 14%) per protocol adherence, respectively; adjusted relative risk (RR) 1.22 per unit protocol adherence score, 95% confidence interval (CI) 1.15–1.31; for achieving BP target (P < 0.001). Participants with a per protocol score of 4 or 5 (512/1038, 49.3%) were 1.54-fold (95% CI 1.31–1.81; P < 0.001) more likely to achieve their individual BP target compared with usual care. Clinics equipped with a practice nurse significantly influenced protocol adherence (adjusted RR 1.20, 95% CI 1.06–1.37; P = 0.004) and individual BP control (RR 1.21, 95% CI 1.04–1.41; P = 0.015).
Conclusion:
There is considerable potential for structured care management to improve BP control in primary care, especially when optimally applied.
Keywords: antihypertensive treatment, blood pressure, cardiovascular disease, hypertension, management, primary care, treatment targets
INTRODUCTION
Hypertension represents the single most important and preventable cause of cardiovascular disease (CVD) [1,2]. Despite evidence of reduced rates of CVD with progressively lower blood pressure (BP) [3] and a broad range of antihypertensive therapies [4] to achieve treatment targets, poorly controlled BP remains common [5,6]. Overcoming resistance to uptitrate therapy [7] to achieve earlier and greater BP lowering appears critical to reducing the risk of hypertension-related CVD events. In order to achieve this in the primary care setting in which hypertension is predominantly managed [8], there is evidence for the benefits of applying a framework, preferably multidisciplinary [9], of increased surveillance and guided stepped-care [10]. In addition, practice nurses are increasingly becoming an important component of primary healthcare in Australia [11] in terms of supporting family physicians to provide greater continuity of care for those with chronic conditions.
The Valsartan Intensified Primary carE Reduction of Blood Pressure (VIPER-BP) study [12], a large multicenter trial of a structured and intensive approach to BP management in primary care, reaffirmed the potential to achieve better BP control in a high-risk cohort of individuals with hypertension. The strategy was associated with an adjusted 28% increased likelihood of achieving individualized BP control at 26 weeks. However, despite a standardized protocol for incremental antihypertensive therapy, supported by a user-friendly, computer-based treatment algorithm tool, close examination of treatment patterns from the VIPER-BP study revealed that failure to uptitrate antihypertensive therapy was still common. Given the meticulous nature of data collected, this study provides a unique opportunity to better understand potential barriers to optimal BP control in primary care.
Study objective and hypothesis
We studied the extent and determinants of per protocol adherence, from both a participant and clinician perspective, during the VIPER-BP trial. A priori and as determined by prespecified criteria, we postulated that optimal adherence to the VIPER-BP protocol would significantly improve the primary endpoint of individualized BP control during 26-week follow-up and result in a greater decrease in BP levels compared to baseline, both within the intervention group and relative to usual care.
METHODS
Participants
As described in greater detail previously [13], individuals routinely managed by family physicians partaking in the VIPER-BP study were eligible to participate if they were aged at least 18 years; diagnosed with hypertension requiring active pharmacological treatment according to guidelines and; consented to participate. Exclusion criteria were as follows: mean initial sitting SBP at least 180 mmHg; prescription of at least three antihypertensive agents; severe renal disease (clinical diagnosis and/or estimated glomerular filtration rate <60 ml/min per 1.73 m2); and/or contraindications to an angiotensin receptor blocker, calcium channel blocker, or a thiazide diuretic.
Design
The VIPER-BP study was a multicenter randomized controlled trial that recruited 2337 hypertensive individuals via 119 primary care clinics and over 250 family physicians Australia-wide [13]. Participating clinics comprised a representative combination of small, independent (one to two physicians), and larger clinics with shared protocols and governance structures (an increasing feature of primary care in Australia). The primary endpoint reflected contemporary Australian guidelines [14] that recommended three different levels of BP control, comprising a lower BP target of 125/75 mmHg or less for those with proteinuria, an intermediate target of 130/80 mmHg or less for those with diabetes or other forms of end-organ damage, and the higher traditional target of 140/90 mmHg or less for all others. Participants were managed within the Australian universal health insurance scheme (Medicare) that provides reimbursed access (the majority of services without copayment) to primary care clinics and subsidized pharmacotherapy. The study was approved by relevant ethics committees (including the Royal Australian College of General Practitioners) and conducted in accordance with CONSORT guidelines for pragmatic trials [15]. Recruitment commenced in June 2010 with final study follow-up completed in July 2011.
Procedures
Figure 1 outlines the key features of the VIPER-BP study. Overall, 2185 consenting participants entered a standardized run-in period of valsartan 80 mg/day for 14–28 days and were subject to comprehensive clinical profiling. Of these, 416 (19%) achieved their individual BP target and 1562 were subsequently randomized (stratified according to three BP targets) into the study.
FIGURE 1.
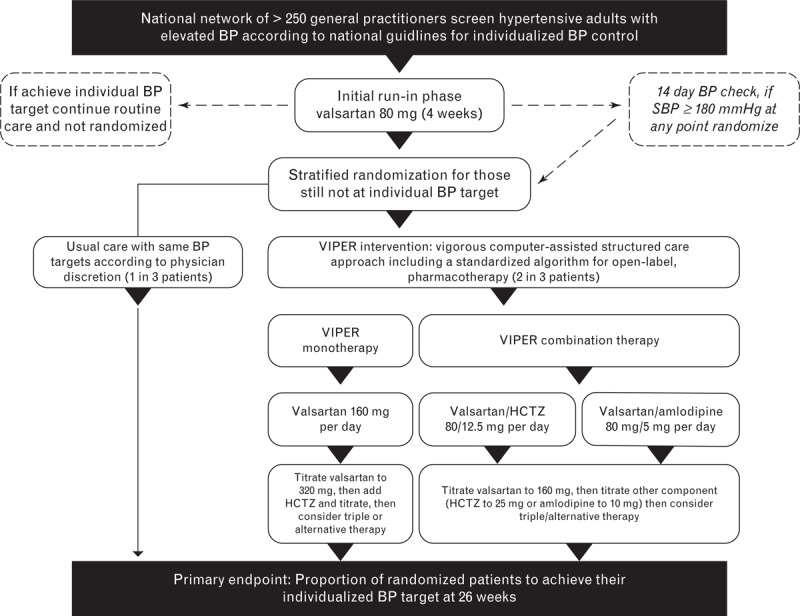
Overview of the Valsartan Intensified Primary carE Reduction of Blood Pressure (VIPER-BP) study design and treatment pathways. BP, blood pressure.
Those randomized to the usual care group (n = 524) were subject to an enhanced form of routine management with two mandatory visits at 6 and 26 weeks for BP comparisons. Participating physicians were asked to manage these participants as they normally would during 26-week follow-up according to national guidelines for BP management [14]. Notably, 367 participants (70%) in the usual care group continued to be prescribed the run-in dose of valsartan 80 mg/day at 6 weeks despite not achieving their individual BP target, with less than half (n = 228, 44%) subsequently prescribed combination or triple therapy by 26 weeks.
Those randomized to the study intervention group (n = 1038) were further randomized at a ratio of 1 : 2 to commence valsartan monotherapy (160 mg/day) or valsartan combination therapy as a single pill. Once randomized to combination therapy, physicians chose between valsartan and hydrochlorothiazide or amlodipine. Supported by a computerized treatment algorithm tool, the study protocol called for intervention participants to be reviewed at a series of mandatory visits at weeks 6, 10, 14, 18, and 26 postrandomization, with the instruction to uptitrate pharmacotherapy, as shown in Fig. 1, if a participant's BP remained above their individual target. According to a priori criteria, adherence to the study protocol within the treatment arms of the study was examined according to prescription of valsartan therapy as per group randomization and then uptitration of therapy if a participant remained above their individual BP target; and completion of prescheduled study BP monitoring and follow-up visits at weeks 6, 10, 14, and 18, with tolerance for ±7-day variance. Each visit was individually assessed on this basis with consideration of the reason for a clinical decision not to follow the study protocol (based on Study Investigator notes). For example, off-protocol treatment following an adverse event was still considered ‘per protocol’ if it was uptitrated in response to an elevated BP. A per protocol score of 0–5 was generated for each participant. One point was allocated for correct treatment at randomization and an additional point was given for each of the four visits up to 18 weeks according to correct treatment (e.g., uptitration if clinically indicated) and a visit within the allowed timeframe. A score of 4 or 5, allowing one deviation from the study protocol to account for potential treatment deviation due to an adverse event, was considered as ideal per protocol management.
Statistical analyses
Continuous data are presented as a mean [±standard deviation and 95% confidence intervals (CIs), wherever appropriate] and categorical data as proportions. Consistent with the primary study outcome [12], between group comparisons of BP control at week 26 were performed using a log binomial generalized linear model with stratification status at randomization as a covariate. Stratification status was fit as a categorical variable with the three BP target groups (≤125/75, ≤130/80, and ≤140/90 mmHg) based on the participant's clinical profile. Change from baseline to 26 weeks in SBP and DBP was each subject to analysis of covariance with treatment group and stratification status as factors and centered baseline BP as a covariate. Study data were also analyzed using log binomial generalized linear models to determine the independent predictors of protocol adherence (score of 4/5) and achievement of the primary endpoint of individualized BP control during 26-week follow-up (n = 988 with an endpoint BP). In the latter model, per protocol adherence was included as a covariate along with a broad range of individual profiling data (including age, sex, and ethnicity) and key characteristics of the primary care clinic (e.g. location, size, and presence of a practice nurse). Where this would not converge, a robust Poisson regression model was used instead. Univariate models were first conducted, followed by a multivariate model. Variables entered into the multivariate analysis were those that were statistically significant (P ≤0.05) in the univariate analysis. Data were independently verified and analyzed by the study statistician (adverse event) using the Stata 11 statistical package (Stata Corp., College Station, Texas, USA).
RESULTS
Study cohort
Of the 1562 randomized study participants, 1038 (66.5%) were allocated to the intervention group with 360 (35%) and 678 participants allocated to the valsartan monotherapy and either of the combination therapy arms, respectively. Groups were well matched in respect to their baseline profile (Table 1). Overall, mean age was 59.3 ± 12.0 years, 963 (62%) were men, and 1045 (67%) had preexisting hypertension. Average BP at randomization was 149.7 ± 16.8/88.1 ± 11.0 mmHg, with 270 (17.3%), 843 (54.0%), and 449 (28.7%) participants, respectively, assigned a BP target of 125/75 mmHg or less, 130/80 or less, or 140/90 mmHg or less.
TABLE 1.
Baseline characteristics according to group randomization
| Usual care | VIPER-BP intervention | |||
| All (n = 524) | All (n = 1038) | Monotherapy (n = 360) | Combination therapy (n = 678) | |
| Sociodemographic profile | ||||
| Men | 323 (62%) | 640 (62%) | 222 (62%) | 418 (62%) |
| Age, years | 59 ± 12 | 59 ± 12 | 59 ± 12 | 59 ± 12 |
| Clinical profile | ||||
| Prior hypertension | 353 (67%) | 692(67%) | 253 (70%) | 439 (65%) |
| Heart disease | 38 (7%) | 93 (9%) | 35 (10%) | 58 (9%) |
| Type 2 diabetes | 106 (20%) | 195 (19%) | 69 (19%) | 126 (19%) |
| Proteinuria | 93 (17%) | 183 (18%) | 64 (18%) | 119 (18%) |
| Microalbuminuria | 127 (23%) | 242 (23%) | 73 (20%) | 169 (25%) |
| BP profile and BP targets | ||||
| SBP (mmHg) | 149 ± 17 | 150 ± 17 | 150 ± 17 | 150 ± 17 |
| DBP (mmHg) | 87 ± 11 | 88 ± 11 | 88 ± 11 | 89 ± 11 |
| BP target ≤140/90 (mmHg) | 145 (28%) | 304 (29%) | 106 (30%) | 198 (29%) |
| BP target ≤130/80 (mmHg) | 286 (55%) | 557 (54%) | 190 (53%) | 367 (54%) |
| BP target ≤125/75 (mmHg) | 93 (18%) | 177 (17%) | 64 (18%) | 113 (17%) |
BP, blood pressure; VIPER-BP, the Valsartan Intensified Primary carE Reduction of Blood Pressure.
Protocol adherence within the intervention group (n = 1038)
Pattern of clinic visits
Table 2 shows that although the timing of actual visits closely followed per protocol visits at days 42, 70, 98, 126 and 182, both the proportion of participants who attended a clinic visit (range 83–91%) and the timing of such a visit within 7 days either side of the scheduled date (range 59–86%), steadily declined during 26-week follow-up. Prior to the week 26 visit, participants managed by a clinic with a practice nurse were more likely to attend a scheduled visit (absolute difference of 2–5% for each time-point).
TABLE 2.
Summary of visits, clinical status, and per protocol treatment in the VIPER-BP intervention arm (n = 1038)
| Baseline | Week 6 day 42 | Week 10 day 70 | Week 14 day 98 | Week 18 day 126 | Week 26 day 182 | |
| Participants | 1085 (100%) | 945 (91.0%) | 872 (84.0%) | 831 (80.1%) | 829 (79.9%) | 857 (82.6%) |
| Mean days of visit | – | 42.5 ± 8.9 | 72.8 ± 12.0 | 102.5 ± 13.8 | 132.1 ± 15.4 | 186.1 ± 19.9 |
| Visit within 7 days of schedule | – | 812/945 (86%) | 678/872 (78%) | 595/831 (72%) | 586/829 (71%) | 502/857 (59%) |
| BP above individual target | 1085 (100%) | 722 (76.4%) | 625 (71.7%) | 536 (64.5%) | 499 (60.2%) | 514 (60.0%) |
| SBP within 1–5 mmHg of target | – | 97 (10.3%) | 110 (12.6%) | 104 (12.5%) | 112 (13.5%) | – |
| Treatment-related adverse event recorded | – | 135 (14.3%) | 148 (17.0%) | 154 (18.5%) | 154 (18.2%) | 141 (16.5%) |
| Per protocol treatment applied | 962/1085 (92.7%) | 701 (74.2%) | 568 (65.1%) | 509 (61.3%) | 541 (65.3%) | – |
VIPER-BP, the Valsartan Intensified Primary carE Reduction of Blood Pressure.
Adherence to treatment uptitration schedule
Table 2 also shows the number of participants at each scheduled clinic visit who had a BP above their individual target. The proportion of people with uncontrolled BP progressively fell from 76 to 60% between visits at week 6 and week 18, with a small increase in the proportion with a SBP within 1–5 mmHg (from 10.3 to 13.5% over the same timeframe) of their target. Simultaneously, 14.3–18.2% of participants had adverse events potentially related to study treatment. By week 26, 49 (4.7%) were prescribed alternative pharmacotherapy. Overall, adherence to the treatment protocol declined from 74.2% at week 6 to 65.3% at week 18.
Overall per protocol adherence
A total of 54 participants (5.2%) in the intervention group had a protocol adherence score of 0, the majority of whom (n = 50) were withdrawn early from the study. A further 146 (14%) participants recorded a minimum score of 1 and 162 (16%), 164 (16%), 242 (23%), and 270 (26%) had progressively higher adherence scores of 2, 3, 4, and 5, respectively. Overall, 49% (512/1038) of those randomized to the VIPER-BP intervention recorded a protocol adherence score of 4 or 5.
Univariate predictors of study protocol adherence (score of 4 or 5) were treatment modality, BP target, ethnicity of the participant, BMI, presence of a practice nurse, and nature of the clinic. Of these, a less stringent BP target [BP target of 140/90 versus 125/75 mmHg – relative risk (RR) 1.36, 95% confidence interval (CI) 1.13–1.65], ethnicity (white versus rest – RR 1.36, 95% CI 1.12–1.65), and a practice nurse (versus rest – RR 1.23, 95% CI 1.09–1.40) were associated with an increased likelihood of protocol adherence. On an adjusted basis, randomization to monotherapy was associated with a reduced probability of protocol adherence (RR 0.75, 95% CI 0.65–0.87; P < 0.001). Alternatively, the independent predictors of protocol adherence were the patient's ethnicity (white being the predominant group versus rest – RR 1.36, 95% CI 1.13–1.65; P = 0.004) and the practice nurse attended clinics (RR 1.20, 95% CI 1.06–1.37; P = 0.004).
Impact of protocol adherence on blood pressure control
Figure 2 summarizes the BP measurements recorded at the structured clinic visits as part of the study intervention according to three key parameters observed at the preceding visit (e.g. lack of uptitration of treatment at week 6 impacting on the BP recorded at week 10). These were adherence to the treatment prescription and uptitration protocol; whether the participant achieved their BP target; and whether the participant had an adverse event potentially related to study treatment. We observed a widening and positive impact on BP control as the 26-week follow-up period progressed in response to per protocol treatment during the preceding visit. Those who achieved their BP goal at the preceding visit were more likely to once again achieve this goal at the next visit. Alternatively, a prior adverse event was associated with a higher BP at the subsequent visit, although no difference was observed on this basis at week 26.
FIGURE 2.
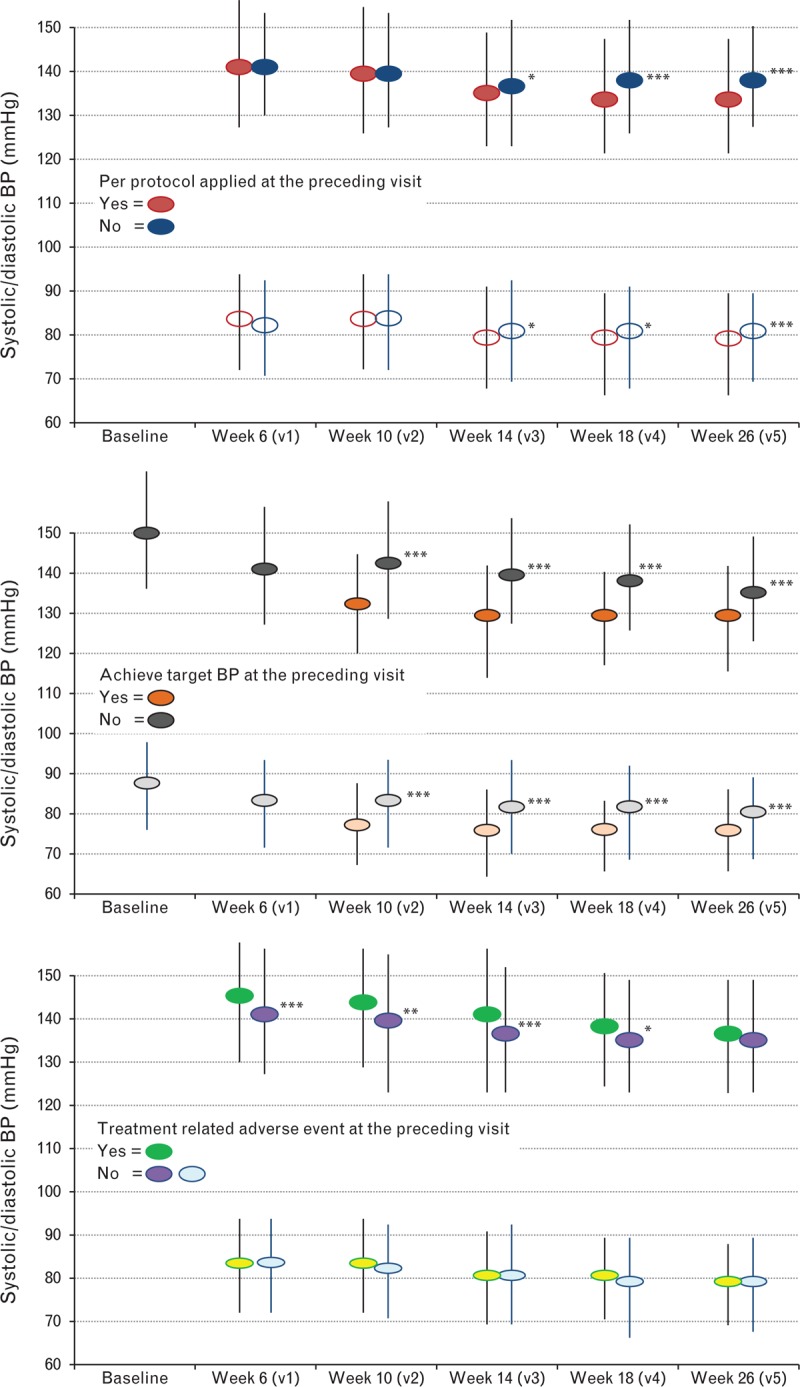
Pattern of blood pressure (BP) levels according to per protocol treatment (a), achievement of BP target (b), and any adverse events (c). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. All three figures show the impact of events/decisions occurring at a preceding visit (e.g., visit 1 at week 6) on BP levels recorded at the next study visit (e.g., visit 2 at week 10).
Figure 3 shows the relationship between per protocol adherence and BP control in those who completed 26-week follow-up (n = 857).
FIGURE 3.
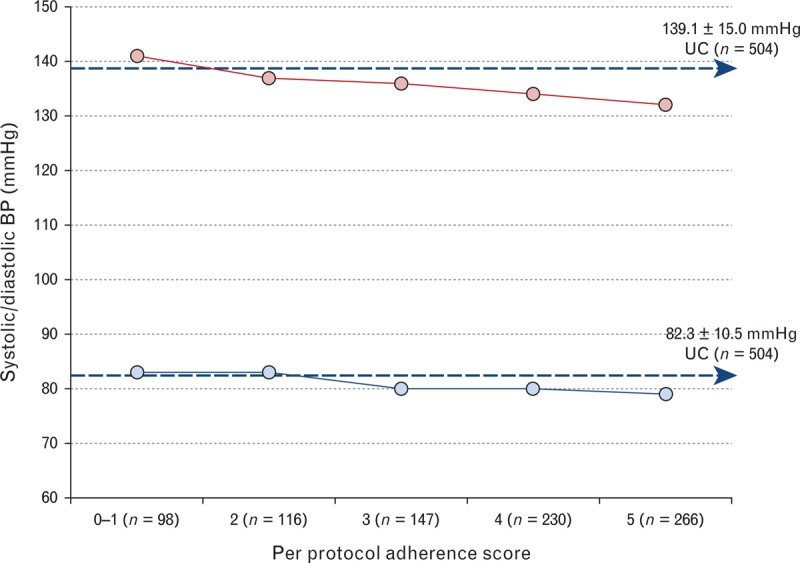
Impact of increasing protocol adherence on mean SBP and DBP at 26 weeks (n = 857). Blue dashed arrows indicate the mean endpoint blood pressure (BP; systolic and diastolic) achieved by the usual care (UC) group as a comparator. Mean (95% confidence interval, CI) differences in SBP and DBP relative to a per protocol score of 0–1 are provided with significant differences (P < 0.05) observed for scores of 3 (P < 0.05) or more (4 or 5; P < 0.001). Standard error bars are contained within symbols.
Figure 4[16–18] plots the baseline predictors of the primary endpoint (individual BP control) for participants in the VIPER-BP intervention group with available endpoint BP data (n = 988). Men were one third more likely to achieve their individual BP target compared with women (adjusted RR 1.31, 95% CI 1.13–1.53; P < 0.001) and those with a higher BMI less likely (RR 0.97, 95% CI 0.96–0.98 per kg/m2; P < 0.001). The less stringent the BP target (RR 2.02, 95% CI 1.38– 2.95 and 3.36, 95% CI 2.31–4.89 for a BP target of 130/80 and 140/90 mmHg, respectively, versus 125/75 mmHg), the more likely this was achieved. Those attending a clinic staffed with a practice nurse were also more likely to achieve the primary endpoint (RR 1.21, 95% CI 1.04–1.41; P = 0.015). Alternatively, attendance at a clinic part of a larger network was a negative predictor for achieving BP target (ranging from 26.5 to 36.5% in these clinics versus 50.4% for the rest). Increasing per protocol adherence was a strong predictor of achieving individual BP control (RR 1.22, 95% CI 1.15–1.31; P < 0.001 per unit protocol score).
FIGURE 4.
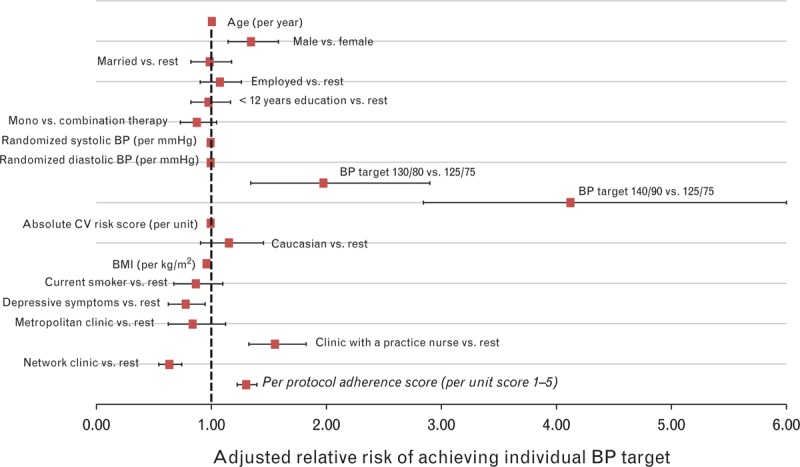
Predictors of individual blood pressure (BP) control in the intervention arm (n = 988). Monotherapy versus combination therapy is based on intention-to-treat randomization. Absolute cardiovascular risk is based on Framingham criteria [16,17] for 5-year risk of a cardiovascular event. BMI was calculated by standardized anthropometric profiling. Depressive symptoms were defined as a positive response to the two-item Arroll screening tool [18]. CV, cardiovascular.
Comparison of per protocol treatment versus usual care
In comparison to usual care, intervention participants managed as per study protocol were 1.5-fold more likely to achieve their individual BP target [RR 1.54, 95% CI 1.31–1.81; 138/504 (27.4%) versus 245/512 (47.8%), P < 0.001]. They were also 1.3-fold more likely to achieve a BP of less than 140/90 mmHg [RR 1.33, 95% CI 1.21–1.47; 272/504 (54.0%) versus 363/512 (70.9%), P < 0.001]. Consequently, compared with usual care, the numbers needed to treat in the intervention group to achieve this BP target was 5.9 (95% CI 4.4–9.1). Mean change in BP from baseline to 26 weeks was markedly higher in the per protocol treatment group (−15.6 ± 16.1/−8.9 ± 9.4 mmHg) compared with the usual care group (−10.2 ± 17.5 /−5.2 ± 10.2 mmHg); the adjusted mean differences in BP in favor of the VIPER-BP group being −5.8 (95% CI −7.4 to −4.1)/−3.5 (95% CI −4.6 to −2.5) mmHg (P < 0.001 for both comparisons).
DISCUSSION
Although there is strong evidence to support the application of structured programs in primary care to optimize BP management [10], there is a paucity of detail to describe how they best work and can be effectively applied. Using comprehensive data from a large multicenter randomized controlled trial of intensive BP management [12], we prospectively examined the predictors and consequences of greater adherence to the study protocol on subsequent BP control. As expected [7], we found some resistance to uptitrating antihypertensive therapy when clinically indicated, but not typically when participants were close to their BP target. As also expected [19], although adverse events appeared to initially influence prescribing patterns and BP control, they did not unduly affect BP goal attainment at 6 months. Ultimately, it was the level of BP control being sought and level of adherence to the study protocol that most influenced individual BP control. Compared with those who were least managed according to the study protocol, participants in the intervention group subject to optimal protocol adherence recorded a 26-week BP that was around 9/5 mmHg less. Moreover, they were two-fold more likely to attain their individual BP target (even if at the lower target of 125/75 mmHg).
Significantly, the presence of a practice nurse independently predicted the successful application of the study intervention (including timing and attendance at clinic visits and likelihood of treatment uptitration) and subsequent attainment of BP control. In Australia, as in many other healthcare systems, practice nurses typically assume a combination of clinical and administrative duties [20]. Despite data supporting a more proactive role for practice nurses to optimize BP management [9], this represents an underdeveloped component of primary care, particularly when considering the evidence in favor of other nurse-coordinated models of care in chronic disease [21,22]. Successful, algorithm-based interventions [9,10] to improve BP management have increased potential if there is someone like a practice nurse to implement them. Our observations that larger clinics with shared protocols/governance were less likely to achieve individualized BP control reinforce the need to maintain high standards of care across larger organizations with typically higher volume caseloads per physician [20].
At the individual level, three factors independently influenced successful BP control. First, men were approximately one-third more likely than women to achieve the primary endpoint. Although this finding appears to contrast with earlier study findings [23], it probably reflects the randomization of female participants with more persistent forms of hypertension and well described, sex-based differences in treatment response [24]. Reinforcing the links between a high population prevalence of both obesity and hypertension [25], increasing BMI was also negatively correlated with BP response to treatment. It is known that more obese individuals have increased sympathetic drive [26] that would not necessarily respond to the antihypertensive agents used in this study. We also found that participants with depressive symptoms were also less likely to attain their individual BP target. Given that this condition is also not uncommon in hypertensive individuals, these data reinforce the need to proactively routinely screen for depression in order to optimize adherence and treatment response to antihypertensive therapy. Overall, these data reinforce the therapeutic benefits of individualizing treatment pathways within a structured care approach.
There are a number of limitations that influence interpretation of these data and their applicability to other healthcare systems and wider clinical practice. As originally described [12], study physicians are likely to represent those most interested in hypertension management and they recruited participants by invitation. The application of the study protocol was facilitated by the Australian healthcare system with health subsidies and copayments for participants. As highlighted by potential variances in the size and nature of practices, there is a degree of heterogeneity in Australian primary care that is difficult to describe and control for; this is one of the reasons for not applying a cluster design. It is also possible that lower socioeconomic or some ethnic groups may be underrepresented in the study cohort. Similar results from an equivalent trial undertaken in Canada [27] and a systematic review of the overall impact of structured BP management programs [10] support the validity of our findings beyond Australia. Finally, given the significant findings in relation to practice nurses, it is important to note we did not document their specific role in study management and their precise impact remains unknown.
In summary, one in three participants randomized to the VIPER-BP intervention was exposed to the optimal level of protocol adherence and this was associated with markedly better BP control. Predictors of greater per protocol adherence and achievement of individualized BP control depended on a range of clinical, treatment, practice, and individual patient factors. These data reinforce the potential to better tailor management to individual needs in order to optimize BP management in primary care. A potentially key role for practice nurses was identified in this regard and further research is required to best translate the evidence from trials such as this into clinical practice.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge all primary care investigators and Study Nurse Coordinators for participating in the VIPER-BP study.
S.S., G.L.J., and M.C. are supported by the National Health and Medical Research Council of Australia. The VIPER-BP study was sponsored by Novartis Pharmaceuticals Australia Ltd. and was supported in part by the Victorian Government's Operational Infrastructure Support Program.
Conflicts of interest
VIPER-BP was designed by Baker IDI Heart and Diabetes Institute (S.S., M.L.C. and G.L.J.) in consultation with a Scientific Advisory Board [Craig Anderson, John Amerena, Alex Brown, L.M.B., F.J.deL., M.H. (no honorarium), Joseph Hung, Henry Krum, Mark Nelson, Markus Schlaich, N.P.S.] who received honorarium from Novartis Pharmaceuticals Australia Ltd as study consultants. The sponsors participated in discussions regarding design and conduct of the study and provided logistical support during the trial. Analyses were independently generated by the study statistician (Adrian Esterman, University of South Australia) on behalf of the other members of the Clinical Safety and Efficacy Committee (Colin Johnston, Mark Nelson, Richard Gerraty) who received remuneration from Novartis Pharmaceuticals as study consultants. Study data were assessed jointly by the study investigators and the sponsor. All authors have disclosed any conflict(s) of interest and declared that they had a form of support and specific relationships (as described above) with Novartis Pharmaceuticals for the submitted work.
Reviewers’ Summary Evaluations
Reviewer 1
Modern antihypertensive agents are designed to reduce blood pressure. Unfortunately, for reasons depending on the patients and/or the treating physicians these drugs are often not used optimally or not at all. Better adherence to a vigorous treatment protocol must be expected to improve blood pressure control of hypertensive patients, and this is exactly what the authors report. Any other finding would have been difficult to accept. The structured approach of the investigation and the number of patients enrolled gives this observation some weight.
Reviewer 2
The aim of the study was to analyze the determinants of per protocol adherence and to compare usual care and VIPER-BP intervention in achieving BP control. The study looked at the proportion of hypertensive patients achieving three different levels of BP control (≤ 125/75 mmHg for proteinuric patients; < 130/80 mmHg for patients with diabetes or/and organ damage; and < 140/90 mmHg for all other patients) reflecting the current Australian guidelines, which are different from the 2013 ESH/ESC guidelines.
A specific per protocol score was generated for each participant to assess adherence and treatment. A score of 4–5 (allowing one deviation from the study protocol) was considered ideal per protocol management, and was more likely to achieve individual BP targets compared with usual care. The study identified a potentially key role for practice nurses to be translated into clinical practice.
Footnotes
Abbreviations: BP, blood pressure; CI, confidence interval; CVD, cardiovascular disease; RR, relative risk; VIPER-BP, Valsartan Intensified Primary carE Reduction of Blood Pressure
REFERENCES
- 1.He FJ, MacGregor GA. Blood pressure is the most important cause of death and disability in the world. Eur Heart J Suppl 2007; 9 Supp B:B23–B28 [Google Scholar]
- 2.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 2012; 307:1273–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219 [DOI] [PubMed] [Google Scholar]
- 5.Carrington MJ, Jennings GL, Stewart S. Pattern of blood pressure in Australian adults: results from a national blood pressure screening day of 13 825 adults. Int J Cardiol 2010; 145:461–467 [DOI] [PubMed] [Google Scholar]
- 6.Carrington MJ, Jennings GL, Stewart S. Pressure points in primary care: blood pressure and management of hypertension in 532 050 patients from 2005 to 2010. J Hypertens 2013; 31:1265–1271 [DOI] [PubMed] [Google Scholar]
- 7.Ernst ME. Resistant hypertension or resistant prescribing? Hypertension 2011; 58:987–988 [DOI] [PubMed] [Google Scholar]
- 8.Britt H, Miller G, Henderson J, Charles J, Valenti L, Harrison C, et al. General practice activity in Australia 2011–2012. Sydney:Sydney University Press; 2012 [Google Scholar]
- 9.Clark CE, Smith LF, Taylor RS, Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ 2010; 341:c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev 2010. CD005182. [DOI] [PubMed] [Google Scholar]
- 11.Australian Medicare local Alliance. 2012 General Practice Nurse National Survey Report; 2012. http://amlalliance.com.au/medicare-local-support/nigp [Accessed 20 August 2013] [Google Scholar]
- 12.Stewart S, Carrington MJ, Swemmer CH, Anderson C, Kurstjens NP, Amerena J, et al. Effect of intensive structured care on individual blood pressure targets in primary care: multicentre randomised controlled trial. BMJ 2012; 345:e7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart S, Carrington MJ, Swemmer C, Kurstjens N, Jennings GL. Optimising management of hypertension in primary care: the Valsartan Intensified Primary Care Reduction of Blood Pressure (VIPER-BP) study. Int J Cardiol 2011; 153:317–322 [DOI] [PubMed] [Google Scholar]
- 14.National Heart Foundation of Australia (National Blood Pressure and Vascular Disease Advisory Committee). Guide to Management of Hypertension 2008. Updated December 2010. http://www.heartfoundation.org.au/information-for-professionals/Clinical-Information/Pages/hypertension.aspx [Accessed 20 August 2013] [Google Scholar]
- 15.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008; 337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Vascular Disease Prevention Alliance. Guidelines for the assessment of absolute cardiovascular disease risk: National Heart Foundation of Australia; 2009. http://www.heartfoundation.org.au/information-for-professionals/Clinical-Information/Pages/absolute-risk.aspx [Accessed 20 August 2013] [Google Scholar]
- 17.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991; 121:293–298 [DOI] [PubMed] [Google Scholar]
- 18.Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ 2003; 327:1144–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrao G, Parodi A, Zambon A, Heiman F, Filippi A, Cricelli C, et al. Reduced discontinuation of antihypertensive treatment by two-drug combination as first step. Evidence from daily life practice. J Hypertens 2010; 28:1584–1590 [DOI] [PubMed] [Google Scholar]
- 20.Phillips CB, Pearce C, Hall S, Kljakovic M, Sibbald B, Dwan K, et al. Enhancing care, improving quality: the six roles of the general practice nurse. Med J Aust 2009; 191:92–97 [DOI] [PubMed] [Google Scholar]
- 21.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44:810–819 [DOI] [PubMed] [Google Scholar]
- 22.Stewart S, Carrington MJ, Marwick TH, Davidson PM, Macdonald P, Horowitz JD, et al. Impact of home versus clinic-based management of chronic heart failure: the WHICH? (Which Heart Failure Intervention Is Most Cost-Effective & Consumer Friendly in Reducing Hospital Care) multicenter, randomized trial. J Am Coll Cardiol 2012; 60:1239–1248 [DOI] [PubMed] [Google Scholar]
- 23.Stewart S, Carrington MJ, Swemmer CH, Kurstjens NP, Brown A, Burrell LM, et al. Determinants of achieving early blood pressure control with monotherapy in a primary care setting. J Clin Hypertens (Greenwich) 2013; 15:674–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt E, Joyce C, Dornelles A, Morisky D, Webber LS, Muntner P, et al. Sex differences in barriers to antihypertensive medication adherence: findings from the cohort study of medication adherence among older adults. J Am Geriatr Soc 2013; 61:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsson AC, Wandell P, Osby U, Zarrinkoub R, Wettermark B, Ljunggren G. High prevalence of diagnosis of diabetes, depression, anxiety, hypertension, asthma and COPD in the total population of Stockholm, Sweden: a challenge for public health. BMC Public Health 2013; 13:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert E, Sari CI, Dawood T, Nguyen J, McGrane M, Eikelis N, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension 2010; 56:351–358 [DOI] [PubMed] [Google Scholar]
- 27.Feldman RD, Zou GY, Vandervoort MK, Wong CJ, Nelson SA, Feagan BG. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension 2009; 53:646–653 [DOI] [PubMed] [Google Scholar]


