Abstract
Aim:
To examine the anti-cancer effects of chamaejasmenin B and neochamaejasmin C, two biflavonones isolated from the root of Stellera chamaejasme L (known as the traditional Chinese herb Rui Xiang Lang Du) in vitro.
Methods:
Human liver carcinoma cell lines (HepG2 and SMMC-7721), a human non-small cell lung cancer cell line (A549), human osteosarcoma cell lines (MG63, U2OS, and KHOS), a human colon cancer cell line (HCT-116) and a human cervical cancer cell line (HeLa) were used. The anti-proliferative effects of the compounds were measured using SRB cytotoxicity assay. DNA damage was detected by immunofluorescence and Western blotting. Apoptosis and cell cycle distribution were assessed using flow cytometry analysis. The expression of the related proteins was examined with Western blotting analysis.
Results:
Both chamaejasmenin B and neochamaejasmin C exerted potent anti-proliferative effects in the 8 human solid tumor cell lines. Chamaejasmenin B (the IC50 values ranged from 1.08 to 10.8 μmol/L) was slightly more potent than neochamaejasmin C (the IC50 values ranged from 3.07 to 15.97 μmol/L). In the most sensitive A549 and KHOS cells, the mechanisms underlying the anti-proliferative effects were characterized. The two compounds induced prominent expression of the DNA damage marker γ-H2AX as well as apoptosis. Furthermore, treatment of the cells with the two compounds caused prominent G0/G1 phase arrest.
Conclusion:
Chamaejasmenin B and neochamaejasmin C are potential anti-proliferative agents in 8 human solid tumor cell lines in vitro via inducing cell cycle arrest, apoptosis and DNA damage.
Keywords: chamaejasmenin B, neochamaejasmin C, biflavones, Stellera chamaejasme L, anticancer agent, cell cycle arrest, apoptosis, DNA damage
Introduction
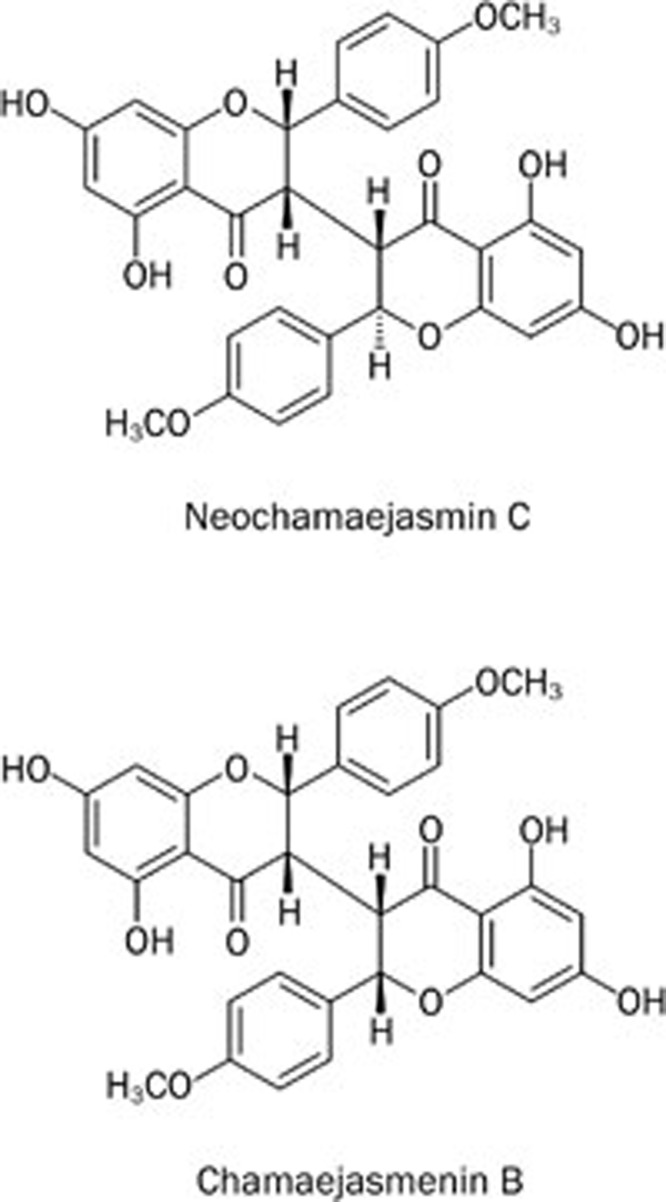
The plant Stellera chamaejasme L is a well-known traditional Chinese herbal medicine that is widely distributed in the north and southwest of China. This herb has been shown to possess both toxic and therapeutic effects1. The roots of this plant are commonly used for the treatment of scabies, tinea, stubborn skin ulcers, cancer and tuberculosis2,3. There have been continued efforts in isolating individual compounds from Stellera chamaejasme L, including biflavones, lignans and diterpenes4,5,6. Biflavones are broadly distributed in gymnosperm plants and have anti-bacterial, anti-fungal, anti-allergic, anti-viral, anti-hepatotoxic, anti-cancer and immune-suppressive effects7. Several natural biflavones have been found to possess cytotoxic and anti-tumor effects8,9. Recently, our group isolated a series of C3/C3″-biflavones, including the new compound neochamaejasmin C10. In this study, we examined two of these biflavones (chamaejasmenin B and neochamaejasmin C, shown in Figure 1) for their anti-tumor effects. The difference between chamaejasmenin B and neochamaejasmin C is the stereo configuration at C2″. The configuration in chamaejasmenin B is S/S/S/S at C2/C3/C2″/C3″, while the configuration of neochamaejasmin C is S/S/R/S at C2/C3/C2″/C3″.
Figure 1.
Chemical structure of neochamaejasmin C and chamaejasmenin B.
DNA is the main target of most cytotoxic anti-cancer drugs, and several cancer chemotherapeutic agents exert their cytotoxic effect by inducing DNA damage11. To maintain genomic stability after DNA damage, multicellular organisms activate checkpoints that induce cell cycle arrest or apoptosis12. Generally, mammalian cells respond to DNA-damaging agents by activating cell cycle checkpoints to delay cell cycle progression until the errors have been corrected13. The arrest at G0/G1, which prevents the cells from completing the cell cycle and proliferating, is regulated by the sequential activation and deactivation of complexes composed of CDK family proteins and cyclins, such as the CDK2/cyclin E complex14. Pausing the cell cycle is sometimes coupled to DNA damage repair. However, when DNA damage cannot be successfully repaired during the pause in cell division, the activation of the DNA damage checkpoint induces cell death by apoptosis15.
In our study, we showed for the first time that chamaejasmenin B had substantial anti-tumor efficacy against various human solid tumor cells. Interestingly, our results demonstrated that chamaejasmenin B and neochamaejasmin C led to increased γ-H2AX in both A549 and KHOS cells, indicating increased DNA damage. In addition, chamaejasmenin B and neochamaejasmin C induced apoptosis in A549 and KHOS cells. Furthermore, chamaejasmenin B and neochamaejasmin C triggered G0/G1 phase arrest in both A549 and KHOS cells. This article reports the in vitro anti-cancer effects of chamaejasmenin B and neochamaejasmin C in solid tumor cells to extend our knowledge about biflavones and provide support for their development as anti-tumor drug candidates.
Materials and methods
Extraction and isolation
Air-dried, powdered Stellera chamaejasme L roots (3.0 kg), collected from Yunnan Province, China, were extracted 4 times with 5 L 95% aqueous EtOH at room temperature. A crude extract (360 g) was obtained after concentration in vacuo and suspended in 1 L H2O. The suspension was extracted with petroleum ether (PE), AcOEt, and BuOH to yield 45, 160, and 86 g of product, respectively. The AcOEt extract was subjected to column chromatography with a PE/AcOEt gradient system of increasing polarity (9:1, 8:2, 7:3, 6:4, and 5:5) to yield six fractions (Fr 1–6). Fraction 3 was subjected to chromatography with a SiO2 column with MeOH/H2O (7:3, 8:2, and 9:1) to yield chamaejasmenin B (124 mg). Fraction 5 was subjected to chromatography with a SiO2 column with CHCl3/MeOH (99:1, 98:2, and 94:6) to yield neochamaejasmin C (238 mg). The chamaejasmenin B and neochamaejasmin C (>95% purity) structures were characterized by NMR and MS spectra10.
Materials
Primary antibodies against cyclin E (M-20), CDK-2 (D-12), Mcl-1 (S-19), p21CIP1 (F-5), PARP (H250), procaspase-3 (H-277), XIAP (A-7) and γ-H2AX (Ser139) and HRP-labeled secondary anti-mouse/anti-rabbit antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Primary antibodies against p-Rb (Ser-795) and cleaved-caspase-3 (D-175) were purchased from Cell Signaling Technology (Danvers, MA, USA), and antibodies against Rb, p53, and β-actin were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Boc-D-fmk was purchased from Calbiochem (Darmstadt, Germany).
Cell culture
Human liver carcinoma cell lines (HepG2 and SMMC-7721), a human non-small cell lung cancer cell line (A549), human osteosarcoma cell lines (MG63, U2OS, and KHOS), a human colon cancer cell line (HCT-116) and a human cervical cancer cell line HeLa16 were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China), and the genotypes were authenticated by DNA fingerprinting. The HepG2, HeLa, and HCT-116 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, and all other cell lines were cultured in RPMI-1640 supplemented with 10% fetal bovine serum. All the cells were maintained in a humidified atmosphere of 95% air plus 5% CO2 at 37 °C.
Cytotoxicity assay
The anti-proliferative effects of chamaejasmenin B and neochamaejasmin C were measured using the sulforhodamine blue (SRB) cytotoxicity assay. Briefly, cells were seeded in 96-well microtiter plates (4000 cells/well). After incubation for 24 h in the appropriate medium, the cells were incubated with the compounds for 72 h and fixed with 10% TCA solution for 1 h. The wells were rinsed 5 times with tap water and then stained with 0.4% SRB solution (100 μL per well) for at least 20 min at room temperature. The wells were rinsed with 1% acetic acid to remove unbound dye and left to air dry. The SRB dye was then solubilized by adding 100 μL unbuffered Tris-based solution to each well, and the absorbance at 515 nm was measured using a multi-scan spectrum. The inhibitory rate of cell proliferation was calculated for each well using the following formula: (A515 control cells - A515 treated cells)/A515 control cells×100%.
Apoptosis analysis by Annexin V and propidium iodide staining
Apoptosis was quantified using the Annexin V-FITC/PI Apoptosis Detection Kit (BD Biosciences) according to the manufacturer's instructions. Briefly, cells were incubated with 5 μL Annexin V at room temperature for 15 min in the dark. Before flow cytometric analysis, 5 μL of 50 mg/mL propidium iodide (PI) stock solution was added to the samples. For each sample, 1×104 cells were collected and analyzed using a FACSCalibur cytometer (Becton Dickinson), and apoptotic cells were identified as Annexin V- and FITC-positive cells.
Cell cycle analysis by propidium iodide staining
Cells were harvested and washed with PBS and fixed with pre-cooled 70% ethanol at 4 °C overnight. Fixed cells were then washed with PBS to remove residual ethanol, pelleted, resuspended in 500 μL PBS containing 50 μg RNase A at 37 °C and then stained with 5 μg PI in the dark at room temperature for 30 min. For each sample, 2×104 cells were collected and analyzed using a FACS-Calibur cytometer (Becton Dickinson, San Jose, CA, USA).
Cell lysate preparation and Western blot analysis
Proteins were extracted with lysis buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L EDTA, 0.1% SDS, 0.5% deoxycholic acid, 0.02% sodium azide, 1% NP-40, 2.0 μg/mL aprotinin, 1 mmol/L phenylmethylsulfonylfluoride). The lysates were centrifuged at 10 000×g for 30 min at 4 °C, the supernatants were transferred to a new tube, and the protein concentration was then determined. Proteins were fractionated on 8% to 15% Tris-glycine gels, and then were transferred to PVDF membrane (Millipore, Bedford, MA, USA) and probed with primary antibodies (dilution range 1:500–1:1000) followed by horseradish peroxidase-labeled secondary antibodies at a 1:5000 dilution. Antibody binding was then detected using a chemiluminescent substrate and visualized on autoradiography film17.
Immunofluorescence
Cells were fixed with 4% formaldehyde for 15 min. After washing with PBS, the cells were blocked with 10% serum in PBS for 10 min and incubated at 37 °C for 2 h with γ-H2AX-specific primary antibodies (1:200). The cells were then washed and incubated in the dark for 1 h at 37 °C with goat anti-rabbit (FITC)-conjugated antibodies (1:200, Earthox, San Francisco, CA, USA). After washing, the nuclei were counterstained with DAPI, and the cells were then washed in PBS and examined using a laser-scanning confocal microscope (Fluoview, Olympus, Tokyo, Japan)18.
Statistical analysis
Two-tailed Student's t-tests were used to determine the significance of the differences between the experimental conditions. Differences were considered significant at P<0.05.
Results
Cytotoxicity of chamaejasmenin B and neochamaejasmin C in human cancer cell lines
Chamaejasmenin B and neochamaejasmin C showed potent anti-tumor effects in various human cancer cells in vitroin cytotoxicity analyses using paclitaxel as a positive control. As shown in Table 1, chamaejasmenin B and neochamaejasmin C exhibited excellent anti-proliferative activity against the eight human cancer cell lines. Moreover, the IC50 of chamaejasmenin B and neochamaejasmin C ranged from 1.08 to 10.8 μmol/L and 3.07 to 15.97 μmol/L, respectively, indicating that chamaejasmenin B had a slightly higher cytotoxic effect on human cancer cells than neochamaejasmin C. Chamaejasmenin B showed a considerable anti-proliferative effect on the A549 non-small cell lung cancer cell line, with an IC50 of 1.08 μmol/L.
Table 1. IC50 values (mean±SD, n=3) in cell lines measured via SRB assay.
| Cell type | Cell lines | Neochamaejasmin C IC50 (μmol/L) | Chamaejasmenin B IC50 (μmol/L) | Paclitaxel IC50 (μmol/L) |
|---|---|---|---|---|
| Human liver carcinoma | SMMC-7721 | 9.28±3.69 | 7.22±3.33 | 14.32±1.68 |
| Human liver carcinoma | HepG2 | 3.75±1.19 | 1.26±0.46 | 5.33±2.45 |
| Human non-small cell lung carcinoma | A549 | 5.72±2.37 | 1.08±0.40 | 7.33±2.46 |
| Human osteosarcoma | MG63 | 7.37±2.10 | 3.67±1.39 | 4.38±2.01 |
| Human osteosarcoma | U2OS | 8.76±1.84 | 7.82±2.06 | 10.41±2.31 |
| Human osteosarcoma | KHOS | 3.07±0.49 | 1.56±0.44 | 1.45±1.27 |
| Human colonic carcinoma | HCT-116 | 15.97±1.93 | 10.80±3.07 | 4.78±1.13 |
| Human cervical carcinoma | HeLa | 10.06±1.89 | 7.02±1.40 | 1.85±0.40 |
Chamaejasmenin B induced DNA damage
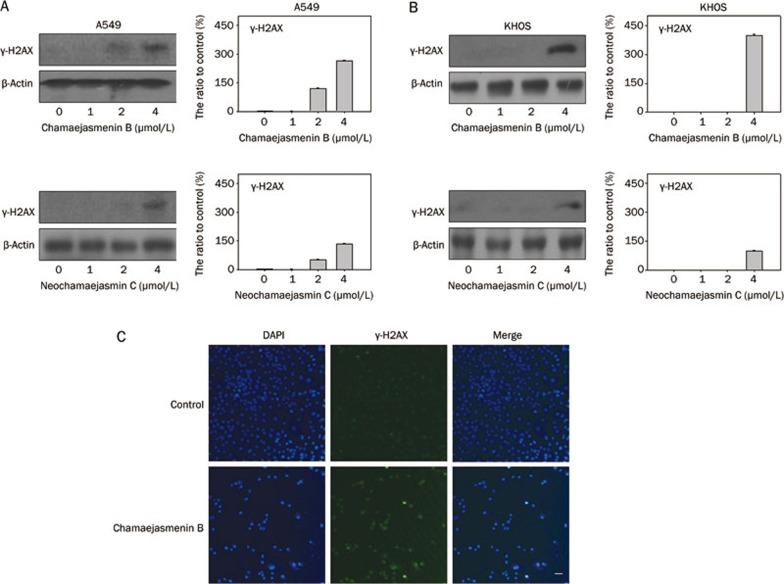
Next, we determined whether chamaejasmenin B and neochamaejasmin C could induce DNA damage in A549 and KHOS cells. The phosphorylation of H2AX (denoted as γ-H2AX) is a marker of DNA damage19. As shown in Figure 2A and 2B, the induction of γ-H2AX in both A549 and KHOS cells was observed by Western blot analysis, indicating that DNA damage might be involved in the anti-cancer effect of the 2 compounds. Furthermore, immunofluorescence showed that the number of γ-H2AX-positive A549 cells was significantly increased in the chamaejasmenin B treatment group (Figure 2C).
Figure 2.
Chamaejasmenin B and neochamaejasmin C induced DNA damage. (A) A549 cells were exposed to the compounds for 48 h, and protein extracts were immunoblotted for γ-H2AX detection. (B) KHOS cells were exposed to the compounds for 48 h, and protein extracts were immunoblotted for γ-H2AX detection. (C) A549 cells were treated with chamaejasmenin B (2 μmol/L) for 48 h and imaged by immunofluorescence for γ-H2AX (green) and nuclei (blue). γ-H2AX expression is indicated by a punctate appearance in the nucleus. Scale bar=40 μm.
Chamaejasmenin B and neochamaejasmin C induced apoptosis
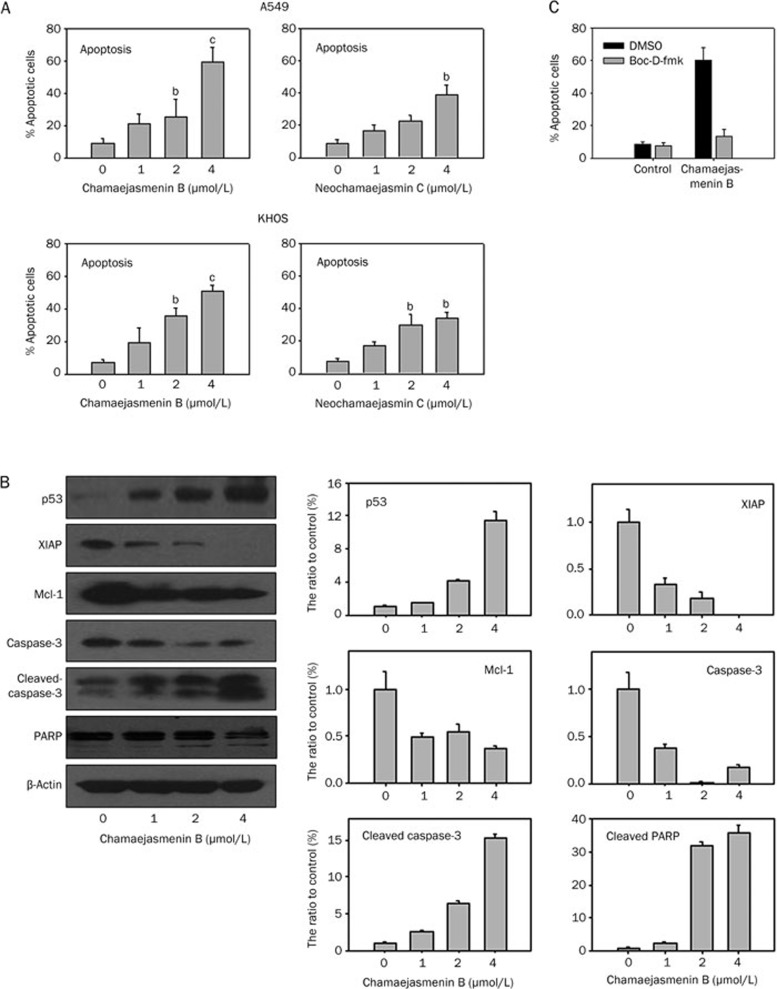
To determine whether chamaejasmenin B and neochamaejasmin C inhibited cancer cell proliferation by inducing apoptosis, we detected apoptosis in A549 and KHOS cells. As shown in Figure 3A, the percentage of apoptotic cells in the control group was 8.39%. Upon exposure to 1, 2, or 4 μmol/L chamaejasmenin B for 48 h, the percentage of apoptotic cells increased to 21.10%, 25.25% (P<0.05) and 59.50% (P<0.01), respectively. Treatment with chamaejasmenin B and neochamaejasmin C resulted in increased apoptosis in both A549 and KHOS cell lines. To confirm the effect of chamaejasmenin B on the induction of the apoptotic pathway, we examined the effect of chamaejasmenin B on the activation of caspase-3, cleavage of PARP, and the expression of XIAP, p53, and Mcl-1. The results showed that chamaejasmenin B treatment efficiently induced caspase-3 cleavage and cleavage of the 85 kDa inactive PARP intermediate in a dose-dependent manner (shown in Figure 3B). These results confirmed that chamaejasmenin B-induced apoptosis was mediated by PARP cleavage and caspase-3 activation. Furthermore, our data showed that chamaejasmenin B resulted in a dose-dependent decrease in XIAP and Mcl-1 protein levels in A549 cells, and similar treatment also caused a significant accumulation of p53 protein expression. Only 13.54% of the cells were apoptotic when pretreated with Boc-D-fmk for 1 h prior to incubation with chamaejasmenin B compared with 59.86% in the chamaejasmenin B treatment group, suggesting that the cytotoxicity induced by combination treatment in A549 cells was caspase dependent (Figure 3C).
Figure 3.
Chamaejasmenin B and neochamaejasmin C induced apoptosis. (A) Cells were treated with the compounds for 48 h and analyzed for apoptosis. The experiments were repeated three times, and error bars represent the standard deviation. bP<0.05, cP<0.01 (two-sided Student's t-test). (B) A549 cells were exposed to the compounds for 48 h, and protein extracts were immunoblotted with specific antibodies against p53, XIAP, Mcl-1, caspase-3, cleaved-caspase-3, and PARP. (C) A549 cells were pretreated with the pan-caspase inhibitor Boc-D-fmk (10 μmol/L) for 1 h and then treated with 4 μmol/L chamaejasmenin B for 48 h. The cells were analyzed for apoptosis by flow cytometry.
Chamaejasmenin B and neochamaejasmin C treatment caused G0/G1 phase arrest
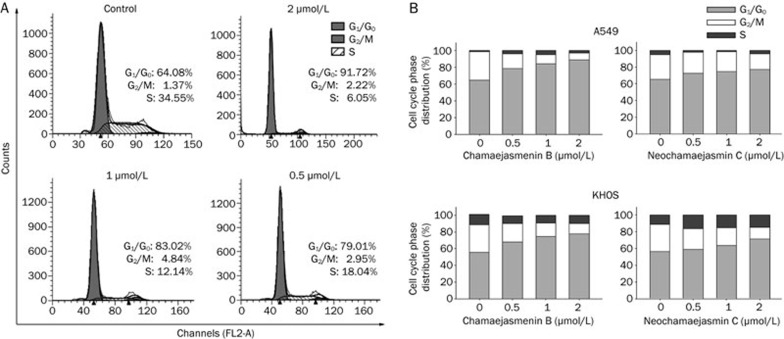
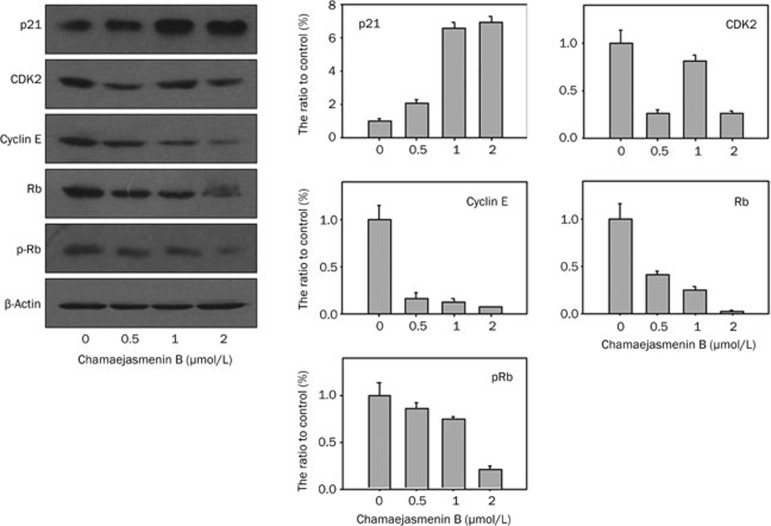
Next, we investigated the cell cycle in cancer cells treated with chamaejasmenin B and neochamaejasmin C. Our study showed that chamaejasmenin B and neochamaejasmin C treatment led to prominent G0/G1 arrest in both A549 and KHOS cells (Figure 4A and 4B). We analyzed the possible alterations of cell cycle regulators reported to play important roles in the regulation of the G0/G1 phase20. Figure 5 shows that the level of p21CIP1 changed with increases in the concentration of chamaejasmenin B. In addition, chamaejasmenin B induced the down-regulation of CDK2, cyclin E, Rb, and pRb proteins in A549 cells.
Figure 4.
Chamaejasmenin B and neochamaejasmin C induced G0/G1 arrest. (A) and (B) Cells treated with the compounds for 48 h were collected and processed for cell cycle distribution analysis.
Figure 5.
Effect of chamaejasmenin B and neochamaejasmin C on the expression of cell cycle regulators. A549 cells were treated with the compounds for 48 h, and the expression of p21CIP1, CDK2, cyclin E, and Rb was evaluated using Western blotting.
Discussion
Because biflavonoids from various natural plants have been found to possess cytotoxic and anti-tumor effects, we isolated chamaejasmenin B and neochamaejasmin C from Stellera chamaejasme L to test their anti-cancer efficacy. Our results showed that chamaejasmenin B caused a slightly higher cytotoxic effect than neochamaejasmin C in eight human cancer cells in vitro. Moreover, the IC50 values of chamaejasmenin B and neochamaejasmin C against normal Chang liver cells and H9C2 cardiomyoblast cells (both >50 μmol/L) were much higher than their IC50 values against cancer cell lines, suggesting that chamaejasmenin B might have a selective cytotoxic effect on rapidly proliferating cells compared with quiescent or slowly proliferating normal cells. The evidence showing the anti-proliferative effect of chamaejasmenin B in a wide variety of tumor cell lines supports further investigation into the anti-cancer efficacy of chamaejasmenin B in vivo.
Anti-cancer agents that induce DNA damage are some of the most effective agents in clinical use and have resulted in significantly improved cancer patient survival21. Among the different forms of complex DNA damage, double-strand breaks (DSBs) are considered to be among the most lethal forms of DNA damage, severely compromising genomic stability22. A number of anti-cancer drugs exert their effects by causing DSBs that induce subsequent apoptosis, including DNA replication inhibitors, crosslinking agents and topoisomerase inhibitors23. H2AX has received increased attention in analyzing cellular responses to DNA damage after the discovery that it is locally phosphorylated on Ser139 (denoted as γ-H2AX) in the vicinity of DSBs24, which is one of the earliest events in DNA damage signaling and repair25. In our study, a clear increase in γ-H2AX expression was observed in both A549 and KHOS cells incubated with chamaejasmenin B and neochamaejasmin C. Several studies have characterized ROS-induced DNA damage, which includes single- and double-strand breaks, abasic sites, and base damages26,27. Our data showed that chamaejasmenin B and neochamaejasmin C could not induce ROS in A549 cells, indicating that ROS might not be involved in the DNA damage induced by the two biflavones. Further investigation will be required to determine whether chamaejasmenin B and neochamaejasmin C cause DNA damage by binding to DNA directly or by indirectly inducing other factors. To maintain genomic stability after DNA damage, multicellular organisms activate checkpoints that induce apoptosis12. Our results indicated that chamaejasmenin B-induced apoptosis was mediated through PARP cleavage and caspase-3 activation. The p53 tumor-suppressor gene is important in the cellular response to DNA damage, with the activation of p53 increasing apoptosis induced by DNA damage28,29. Western blot analysis showed a marked increase in p53 expression in A549 cells after chamaejasmenin B treatment, indicating that chamaejasmenin B might cause DNA damage-induced apoptosis via a p53-dependent pathway. XIAP, a member of a novel family of inhibitor of apoptosis (IAP) proteins, regulates DNA damage-induced apoptosis through downstream caspase-9 cleavage30. In our study, decreased XIAP expression was detected in A549 cells treated with chamaejasmenin B, indicating that apoptosis suppression was impaired. Mcl-1 is an essential modulator of survival during development and maintenance in a variety of cell lineages and is a key regulator of apoptosis after DNA damage31,32. Our data showed that chamaejasmenin B-induced apoptosis was accompanied by a large decrease in Mcl-1 protein. These results indicated that chamaejasmenin B might induce DSBs in A549 cells and activate apoptotic pathways, resulting in the observed anti-proliferative effect.
In response to DNA damage, p53 stimulates the transcription of several genes that inhibit the cell cycle progression to G133. Many of the important genes associated with G0/G1 regulation have been shown to play a key role in proliferation, differentiation and apoptosis34. Cell cycle regulation of the G0/G1 phase has attracted a great deal of attention as a promising target for the research and treatment of cancer35. The cell cycle is regulated by the temporal activation of different cyclin-dependent kinase (CDK)/cyclin complexes in eukaryotic cells36. The cell cycle progression from G1 phase to S phase is initiated by phosphorylated Rb, which is regulated by CDK/cyclin complexes and multiple suppressor proteins37. The cyclin E-CDK2 complex is required for the G1-S transition in the cell cycle, and the activity of this complex is thought to be required for the G1-S transition35,38. In our study, we found that chamaejasmenin B potently inhibited the expression of CDK2, cyclin E, Rb and pRb, resulting in G0/G1 cell cycle arrest and anti-proliferation. p21CIP1 is a potent tight-binding inhibitor of CDKs and can inhibit the phosphorylation of Rb by cyclin A-CDK2, cyclin E-CDK2, cyclin D1-CDK4, and cyclin D2-CDK4 complexes39. When A549 cells were treated with chamaejasmenin B, an increase in the amount of p21CIP1was detected. These results suggested that chamaejasmenin B could arrest A549 cells at the G0/G1 phase via modulating p21CIP1, CDK2, and cyclin E, and the findings provided additional evidence that regulation of the cell cycle might play a crucial role in chamaejasmenin B-induced anti-tumor effects.
In conclusion, chamaejasmenin B and neochamaejasmin C were potential anti-proliferative agents and had marked anti-tumor efficacy in various cancer cells in vitro. The mechanism of their anti-tumor effects might be associated with cell cycle control, apoptosis and DNA damage. Considering their potent anti-tumor molecular mechanism and effects, chamaejasmenin B and neochamaejasmin C might serve as efficient therapeutic anti-cancer candidates and warrant further evaluation as therapeutic agents against solid tumors.
Author contribution
Chong ZHANG, Li-huang ZHANG, Jian-ping PAN, and Shuang-shuang ZHOU organized the study. Chong ZHANG and Shuang-shuang ZHOU carried out the cell culture and molecular studies and analyzed the data. Chong ZHANG, Shuang-shuang ZHOU, Jun-bo WANG, Lin-yi FENG, and Da-yong ZHANG performed the Western blot analyses. Neng-ming LIN, Jie LI, and Jian-ping PAN designed and coordinated the study. Chong ZHANG, Shuang-shuang ZHOU, and Li-huang ZHANG interpreted the results and helped write the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors gratefully acknowledge financial support from the Teachers Research Fund of Zhejiang University City College (J-12019, J-12021), the Zhejiang Provincial Foundation of National Science (Y2100682, LQ12H31001 and LQ12H30003), the Science Research Foundation of Zhejiang Health Bureau (2012KYA068 and 2012KYB066), the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (2010-190-4), the Scientific Research Fund of Zhejiang Provincial Education Department (Y201120633) and the Student Research Fund of Zhejiang University City College (XZ2012562091 and X2012562098).
References
- Blenn C, Wyrsch P, Althaus FR. The ups and downs of tannins as inhibitors of poly(ADP-ribose)glycohydrolase. Molecules. 2011;16:1854–77. doi: 10.3390/molecules16021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZH, Qin GW, Li XY, Xu RS. New biflavanones and bioactive compounds from Stellera chamaejasme L. Yao Xue Xue Bao. 2001;36:669–71. [PubMed] [Google Scholar]
- Wang L, Duan H, Wang Y, Liu K, Jiang P, Qu Z, et al. Inhibitory effects of Lang-du extract on the in vitro and in vivo growth of melanoma cells and its molecular mechanisms of action. Cytotechnology. 2010;62:357–66. doi: 10.1007/s10616-010-9283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZH, Tanaka T, Sakamoto T, Kouno I, Duan JA, Zhou RH. Biflavanones, diterpenes, and coumarins from the roots of Stellera chamaejasme L. Chem Pharm Bull (Tokyo) 2002;50:137–9. doi: 10.1248/cpb.50.137. [DOI] [PubMed] [Google Scholar]
- Yang G, Liao Z, Xu Z, Zhang H, Chen D. Antimitotic and antifungal C-3/C-3″-biflavanones from Stellera chamaejasme. Chem Pharm Bull (Tokyo) 2005;53:776–9. doi: 10.1248/cpb.53.776. [DOI] [PubMed] [Google Scholar]
- Liu X, Li Y, Yang Q, Liu A, Zhu X. Anti-tumor effect of alcohol extract of Stellera chamaejasme in vitro. Zhongguo Zhong Yao Za Zhi. 2010;35:3048–51. [PubMed] [Google Scholar]
- Kim HP, Park H, Son KH, Chang HW, Kang SS. Biochemical pharmacology of biflavonoids: implications for anti-inflammatory action. Arch Pharm Res. 2008;31:265–73. doi: 10.1007/s12272-001-1151-3. [DOI] [PubMed] [Google Scholar]
- Cao Y, Tan NH, Chen JJ, Zeng GZ, Ma YB, Wu YP, et al. Bioactive flavones and biflavones from Selaginella moellendorffii Hieron. Fitoterapia. 2010;81:253–8. doi: 10.1016/j.fitote.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Silva GL, Chai H, Gupta MP, Farnsworth NR, Cordell GA, Pezzuto JM, et al. Cytotoxic biflavonoids from Selaginella willdenowii. Phytochemistry. 1995;40:129–34. doi: 10.1016/0031-9422(95)00212-p. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao W, Hu JL, Cao X, Yang J, Li XR. A new C-3/C-3 “-biflavanone from the roots of Stellera chamaejasme L. Molecules. 2011;16:6465–9. doi: 10.3390/molecules16086465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr, Levrero M, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–9. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C elegans. Mol Cell. 2000;5:435–43. doi: 10.1016/s1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhu H, Yang X, Lou J, Zhu D, Lu W, et al. P53 and p38 MAPK pathways are involved in MONCPT-induced cell cycle G2/M arrest in human non-small cell lung cancer A549. J Cancer Res Clin Oncol. 2010;136:437–45. doi: 10.1007/s00432-009-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Hayashida M, Ito T, Kawano H, Nakano T, Miura M, et al. Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16(INK4a) and Cdk2/cyclin E complex activation. Oncogene. 2000;19:3225–34. doi: 10.1038/sj.onc.1203665. [DOI] [PubMed] [Google Scholar]
- Norbury CJ, Zhivotovsky B. DNA damage-induced apoptosis. Oncogene. 2004;23:2797–808. doi: 10.1038/sj.onc.1207532. [DOI] [PubMed] [Google Scholar]
- Ibrahim AM, Mansour IM, Wilson MM, Mokhtar DA, Helal AM, Al Wakeel HM. Study of survivin and X-linked inhibitor of apoptosis protein (XIAP) genes in acute myeloid leukemia (AML) Lab Hematol. 2012;18:1–10. doi: 10.1532/LH96.11005. [DOI] [PubMed] [Google Scholar]
- Liu XW, Su Y, Zhu H, Cao J, Ding WJ, Zhao YC, et al. HIF-1alpha-dependent autophagy protects HeLa cells from fenretinide (4-HPR)-induced apoptosis in hypoxia. Pharmacol Res. 2010;62:416–25. doi: 10.1016/j.phrs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Cao J, Xu D, Wang D, Wu R, Zhang L, Zhu H, et al. ROS-driven Akt dephosphorylation at Ser-473 is involved in 4-HPR-mediated apoptosis in NB4 cells. Free Radic Biol Med. 2009;47:536–47. doi: 10.1016/j.freeradbiomed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Dinis J, Silva V, Gromicho M, Martins C, Laires A, Tavares P, et al. DNA damage response in imatinib resistant chronic myeloid leukemia K562 cells. Leuk Lymphoma. 2012;53:2004–14. doi: 10.3109/10428194.2012.681654. [DOI] [PubMed] [Google Scholar]
- Luo P, He Q, He X, Hu Y, Lu W, Cheng Y, et al. Potent antitumor activity of 10-methoxy-9-nitrocamptothecin. Mol Cancer Ther. 2006;5:962–8. doi: 10.1158/1535-7163.MCT-05-0385. [DOI] [PubMed] [Google Scholar]
- Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002;2:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–94. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi S, Hiraku Y. Amplification of anticancer drug-induced DNA damage and apoptosis by DNA-binding compounds. Curr Med Chem Anticancer Agents. 2004;4:415–9. doi: 10.2174/1568011043352867. [DOI] [PubMed] [Google Scholar]
- Mah LJ, El-Osta A, Karagiannis TC. GammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–86. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- Smart DJ, Ahmedi KP, Harvey JS, Lynch AM. Genotoxicity screening via the gammaH2AX by flow assay. Mutat Res. 2011;715:25–31. doi: 10.1016/j.mrfmmm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Hemnani T, Parihar MS. Reactive oxygen species and oxidative DNA damage. Indian J Physiol Pharmacol. 1998;42:440–52. [PubMed] [Google Scholar]
- Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–4. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- Yuan ZM, Huang Y, Ishiko T, Kharbanda S, Weichselbaum R, Kufe D. Regulation of DNA damage-induced apoptosis by the c-Abl tyrosine kinase. Proc Natl Acad Sci U S A. 1997;94:1437–40. doi: 10.1073/pnas.94.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R, Oki E, Endo K, Biedermann V, Ren J, Kufe D. XIAP regulates DNA damage-induced apoptosis downstream of caspase-9 cleavage. J Biol Chem. 2000;275:31733–8. doi: 10.1074/jbc.M910231199. [DOI] [PubMed] [Google Scholar]
- Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC, et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28:6068–78. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H, Dong XW, et al. Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther. 2011;10:1264–75. doi: 10.1158/1535-7163.MCT-10-1091. [DOI] [PubMed] [Google Scholar]
- DiPaola RS. To arrest or not to G2-M cell-cycle arrest: commentary re: A K Tyagi, et al. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G(2)-M arrest, and apoptosis. Clin Cancer Res. Clin Cancer Res. 2002;2002;88:3512–3519. 3311–4. [PubMed] [Google Scholar]
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–8. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Owa T, Yoshino H, Yoshimatsu K, Nagasu T. Cell cycle regulation in the G1 phase: a promising target for the development of new chemotherapeutic anticancer agents. Curr Med Chem. 2001;8:1487–503. doi: 10.2174/0929867013371996. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–80. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- Huang WW, Yang JS, Pai SJ, Wu PP, Chang SJ, Chueh FS, et al. Bufalin induces G0/G1 phase arrest through inhibiting the levels of cyclin D, cyclin E, CDK2 and CDK4, and triggers apoptosis via mitochondrial signaling pathway in T24 human bladder cancer cells. Mutat Res. 2012;732:26–33. doi: 10.1016/j.mrfmmm.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Zurlo D, Leone C, Assante G, Salzano S, Renzone G, Scaloni A, et al. Cladosporol a stimulates G1-phase arrest of the cell cycle by up-regulation of p21waf1/cip1 expression in human colon carcinoma HT-29 cells Mol Carcinog 2011. doi: 10.1002/mc.20872 [DOI] [PubMed]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]