Abstract
Gambogic acid (GA) is the main active ingredient of gamboge, a brownish to orange dry resin secreted from Garcinia hanburyi, a plant that is widely distributed in nature. Recent in vitro and in vivo studies have demonstrated that GA exerts potent antitumor effects against solid tumors of various derivations, and its antitumor mechanisms have been thoroughly investigated. On the other hand, normal cells remain relatively resistant to GA, indicating a therapeutic window. GA is currently in clinical trials in China. Over the last decade, our laboratory demonstrates that GA exhibits potent anticancer activities against hematological malignancies. This review focuses on the new mechanisms through which GA inhibits proliferation and induces apoptosis in malignant hematological cells. These include the regulation of expression and intracellular positioning of nucleoporin and nucleophosmin; downregulation of steroid receptor coactivator-3 (SRC-3) and its downstream proteins; upregulation of death inducer-obliterator (DIO-1); downregulation of HERG potassium channel; as well as induction of reactive oxygen species (ROS) accumulation.
Keywords: gambogic acid, hematological malignancies, death inducer-obliterator, HERG channel, nucleophosmin, nucleoporin, steroid receptor coactivator-3, reactive oxygen species
Introduction
It is extraordinarily important to maintain a balance between cell division and death to ensure the correct development and maintenance of multicellular organisms. Disruption of this dynamic balance has pathologic consequences and can lead to disturbed embryogenesis, neurodegenerative diseases, and tumor formation1. The multi-step process of tumor generation is typically linked to apoptosis resistance. For example, by interrupting the signals that can trigger mitochondrial perturbations, tumor cells gain the ability to abolish the mitochondrial pathway of apoptosis2. Additionally, the inability of most cancers to undergo apoptosis in response to chemotherapeutics is the key cause of treatment failure and presents one of the major unsolved bottlenecks in oncology3.
In recent decades, natural drugs have received much attention as cancer chemopreventive agents and therapeutics. Because of their capacity to bind multiple targets, natural drugs may have an advantage over rationally designed mono-targeted agents in the treatment of cancer with multigenic abnormalities4. The use of herbs in China can be dated back to the third century BCE, and more than 7000 species have been recorded in Chinese literature since then. One of these species, the gamboges, has been reported to cure tooth decay and edema in ancient Chinese books.
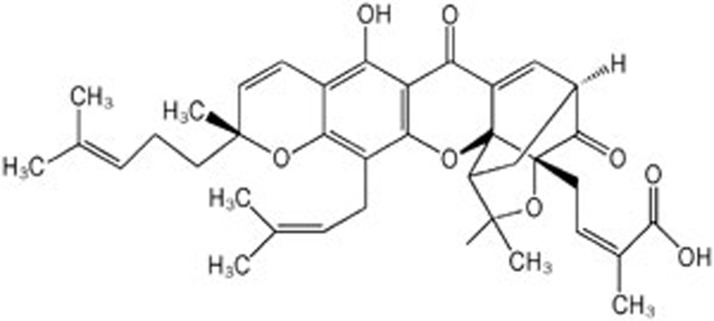
Gambogic acid (GA, C38H44O8, MW: 628.75, Figure 1) is the main active ingredient in gamboges and is a brownish to orange dry resin secreted from Garcinia hanburyi, a plant that mainly grows in South China, Cambodia, Vietnam, and Thailand5. Recent in vitro and in vivo studies demonstrated that GA had potent anti-tumor effects on solid tumors of various derivations, such as human lung carcinoma6 and hepatoma7. The mechanisms of GA anti-tumor activity include downregulating Bcl-2, activating p53, directly binding to c-myc and transferring receptors, and blocking vascular endothelial growth factor (VEGF) signaling4. Additionally, GA3, a new GA derivative, can produce potent cytotoxicity against a panel of cell lines8. In contrast, GA exhibits less toxicity to normal cells compared with cancer cells. For example, normal hepatocytes and breast cells are more resistant to GA than hepatoma and breast cancer cells4,9, indicating a therapeutic window. Over the last decade, our laboratory has demonstrated that GA exerts potent anticancer activity in hematological malignancies. This review will focus on the mechanisms through which GA kills malignant hematological tumor cells (Figure 2).
Figure 1.
Chemical structure of Gambogic acid (GA, C38H44O8, MW: 628.75).
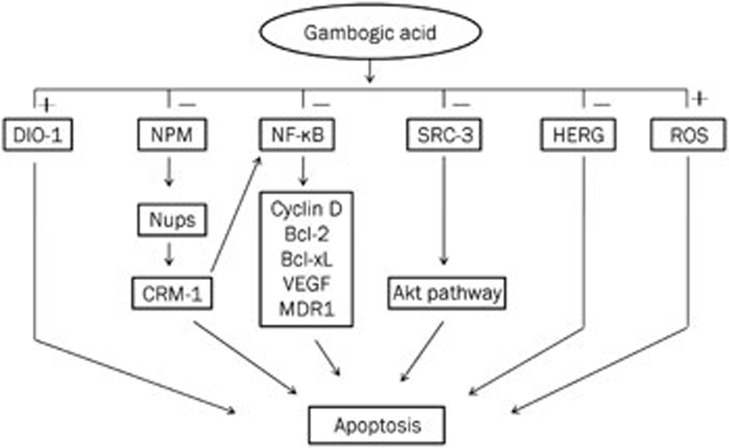
Figure 2.
The anti-hematologic malignancies mechanisms of GA. GA induces hematologic malignancies cells apoptosis via regulating the expression and intracellular positioning of nucleoporin and nucleophosmin (NPM); down-regulating steroid receptor coactivator-3 (SRC-3) as well as its downstream proteins; up-regulating death inducer-obliterator (DIO-1); down-regulating NF-κB; down-regulating HERG potassium channel; and induction of ROS accumulation.
Regulation of nucleoporin and nucleophosmin
Because various proteins are abnormally localized in the cytoplasm and nucleus of tumor cells, mechanisms relating to the nucleocytoplasmic transport of proteins may provide novel opportunities for anti-cancer drug research10. As the only nucleocytoplasmic pore, the nuclear pore complex (NPC) spans the two lipid bilayers of the nuclear envelope and is an essential mediator of all known transport events between the nucleus and the cytoplasm. In addition to mediating nucleocytoplasmic transport, the NPC seems to be either directly or indirectly involved in many other cellular processes, including chromosome segregation, gene expression and apoptosis. The NPC consists of more than 30 different proteins, named nucleoporins (Nups)11.
Nup88, an NPC protein, has received considerable attention as a potential marker for high-grade tumors. Nup88 is a non-phenylalanine-glycine (FG) nucleoporin located exclusively on the cytoplasmic side of the NPC12. It contains two repeating structural motifs: the N-terminal domain, which is predicted to form a β-propeller structure, and the C-terminal domain, which is predicted to contain coiled-coils13. Nup88 can form a sub-complex of the NPC together with the FG repeat nucleoporin Nup214 through the N-terminal β-propeller structure of Nup88 and a central coiled-coil domain of Nup21412. This Nup88/Nup214 sub-complex makes important contributions to nuclear export. It has been reported that both the FG repeat domain of Nup214 and the N-terminal β-propeller domain can bind directly to CRM-1/exportin-114,15, the receptor for the export of most proteins from the nucleus, such as nuclear factor kappaB (NF-κB)16, which is a ubiquitous transcription factor involved in the immune response, apoptosis, and cancer.
A monoclonal antibody raised against Candida albicans unexpectedly cross-reacted with human Nup8817, leading to the discovery of Nup88 overexpression in a broad spectrum of neoplasias, including carcinomas, sarcomas, lymphomas and mesotheliomas18. Immunoblotting several lung carcinoma samples demonstrated that Nup88 expression did not correlate with a concomitant overexpression of its interacting partner Nup214, suggesting selective nucleoporin dysregulation18. Moreover, the intensity of Nup88 staining generally correlates with tumor grade, and the highest expression is routinely detected in more advanced tumors and around the edges of tumors, suggesting a link to invasivity19.
Until now, the exact nature of the relationship between Nup88 overexpression and tumorigenesis has been uncertain. Perhaps, the overexpression of Nup88 leads to the assembly of the Nup88/Nup214 subcomplex, which traps CRM-1 at cytoplasmic foci and inhibits protein export15. For example, the depletion of Nup88 by small interfering RNA (siRNA) inhibited NF-κB-dependent reporter gene activation and the nuclear translocation of NF-κB without affecting the upstream activation pathway in mouse NIH3T3 fibroblast cells. In metastatic melanoma cells overexpressing Nup88, constitutive NF-κB activation was found in the nucleus and cytoplasm, and Nup88 depletion reduced the tumor necrosis factor (TNF)-induced nuclear accumulation of NF-κB subunits in these cells. These phenomena imply that Nup88 overexpression in tumor cells may contribute to constitutive NF-κB activation20.
In our previous study, almost 96% of acute monocytic leukemia U937 cells expressed Nup88 by flow cytometry analysis. It has been reported that Nup88 is normally localized on the cytoplasmic side of NPCs12, but in U937 cells, immunofluorescence detection using confocal microscopy showed that it is extensively expressed in the nucleus and cytoplasm21. Compared with the control group, Nup88 in U937 cells treated with 1.0 mg/L GA for 24 h was re-distributed to the cytoplasmic side of NPCs. GA could correct the disrupted distribution of Nup88 in U937 cells and decrease the protein level by inhibiting gene transcription in a dose-dependent manner. As a result of the effect of GA on the regulation of Nup88, the function of nucleocytoplasmic traffic was affected, leading to the failure to meet the great demand for proteins in U937 cells. GA also downregulated the expression of Nup88 protein and mRNA levels in human HL-60 myeloid leukemia cells22 and Jurkat T cell leukemia cells23, which was accompanied by cell apoptosis.
Nucleophosmin is another key molecule in nucleocytoplasmic communication. It can shuttle continuously through the NPC between the nucleus and the cytoplasm during the cell cycle and sustains normal cell homeostasis24. The nucleophosmin gene (nucleophosmin1) belongs to a new category of genes that function as oncogenes or tumor-suppressor genes depending on its dosage, expression level, interacting partners and compartmentalization25. It has been reported that the nucleophosmin1 gene is one of the most frequent targets of genetic alterations in hematological malignancies, especially in de novo acute myeloid leukemia26. In pharmaceutical research on tumor treatments, the change in nucleophosmin expression is relevant to the destiny of cancer cells. For example, nucleophosmin expression is decreased in phorbol ester 12-otetradecanoylphorbol-13-acetate (TPA)-induced human K562 chronic myeloid leukemia cell differentiation27 and retinoic acid-induced differentiation and sodium butyrate-induced apoptosis of HL-60 cells28. In our previous research, GA decreased the expression of Nup88 and changed its location and decreased the expression of nucleophosmin in a dose-dependent manner23.
Altogether, these results demonstrated that GA has a potent ability to block the nucleocytoplasmic transport of proteins by regulating Nup88 and nucleophosmin, which results in apoptosis and the cell cycle arrest of Jurkat cells, HL60 cells and U937 cells.
Regulation of steroid receptor coactivator-3 (SRC-3)
SRC-3 is a member of the p160 family of SRCs, which also contains SRC-1 and SRC-2/TIF-2/GRIP-129. SRC-3 was first identified from an amplified region of the long arm of chromosome 20 (20q) in breast cancer in 1997 by Anzick SL and originally termed amplified in breast cancer 1 (AIB1)30. SRC-3 also has other names, such as nuclear receptor coactivator-3 (NCoA-3), receptor-associated coactivator-3 (RAC3), activator of thyroid hormone and retinoid receptor (ACTR), thyroid hormone receptor-activating molecule-1 (TRAMI), and p300/CBP-interacting protein (p/CIP)31. The SRC-3 protein contains three basic structural domains: the N-terminal basic helix-loop-helix-Per/ARNT/Sim domain (bHLH-PAS), through which SRC-3 interacts with other DNA-binding proteins; the receptor-interacting domain (RID), which includes three LXXLL motifs and through which SRC-3 binds to the ligand-activated nuclear receptors; and the C-terminal domain, which contains two intrinsic transcriptional activation domains named AD1 and AD2 and contributes to the interaction between SRC-3 and histone acetyltransferases or methyltransferases. The C-terminal of SRC-3 also has its own histone acetyltransferase activity32.
Since SRC-3 was first discovered to be amplified in breast tumors in 1997, the correlation between SRC-3 and tumors has been comprehensively discussed33. Recently, a growing body of evidence has demonstrated that SRC-3 plays a central role in tumor genesis, progression, invasion and metastasis. SRC-3 is often overexpressed in several hormone-dependent cancers, such as breast cancer33, ovarian cancer34, and endometrial cancer35. Moreover, SRC-3 dysregulation also leads to hormone-independent cancers and malignant disease, such as colorectal cancer36, hepatocellular cancer (HCC)37, pancreatic cancer38, and gastric cancer39. In animal models, overexpression of SRC-3 results in increasing cancer incidence in various organs including the lung, uterus, and breast40. With regard to hematological malignancies, SRC-3 has been found to be overexpressed in K562 chronic myelogenous leukemia cells and Raji Burkitt's lymphoma cells41. Additionally, SRC-3 is involved in leukemia-specific chromosome translocation in acute myelocytic leukemia (AML)-M4/M5, which leads to the expression of the MYST3/NCOA3 fusion gene42.
SRC-3 is related to the apoptosis resistance of cancer cells. The overexpression of SRC-3 partly blocked H2O2-mediated apoptosis in HEK293 cells in vitro43. Overexpression of SRC-3 in K562 cells has been implicated in cancer multidrug resistance, as the inhibition of SRC-3 by siRNA enhanced the sensitivity of K562 cells to TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis41. These results implied that SRC-3 had the potential to be a new target for antitumor drug research. Additionally, we confirmed that GA could downregulate the levels of both SRC-3 mRNA and protein in A549 cells and K562 cells44. It has been reported that SRC-3 can disturb apoptosis through Akt signaling, which is related to multiple apoptosis signaling pathways. Various studies have demonstrated that many components of the Akt signaling pathway are constitutively active in a wide range of human tumors, especially in leukemia45. Leukemia cells display increased expression of a phosphorylated form of Akt compared with granulocytes46. Additionally, the phosphorylation levels of GSK3β and S6k1, which stimulate protein synthesis and cell survival as downstream effectors of Akt, are also high in leukemia cells45.
It has been reported that the overexpression of SRC-3 will block apoptosis by enhancing Akt and NF-κB activity and increasing Bcl-2 expression43, while knockdown of SRC-3 by siRNA will decrease the expression of multiple genes associated with the Akt signal pathway and induce apoptosis in cancer cells47. Moreover, in the SRC-3 null mouse animal model, several components of the Akt signal pathway are also downregulated47. These phenomena imply that the Akt signal pathway is under the strict control of SRC-3. Consistent with previous findings, we found that Akt and its downstream targets GSK3β and S6k1 were constitutively activated in K562 cells, and GA inhibited their phosphorylation in a dose-dependent manner44, while their un-phosphorylated levels were not affected. In K562 cells, GA downregulated both the protein and mRNA levels of Bcl-2, which can act as an anti-apoptosis protein by maintaining mitochondrial membrane potential stability and is a crucial mediator downstream of the Akt signal pathway48.
Regulation of death inducer-obliterator (DIO-1) and NF-κB
DIO-1 was identified by differential display PCR in pre-B WOL-1 cells undergoing apoptosis by interleukin-7 (IL-7) starvation. The DIO-1 gene is located at 20q13.33 in Homo sapiens, and its amino acid sequence exhibits predicted transcriptional activation domains consisting of a canonical bipartite nuclear localization signal (NLS) in the N-terminal region, two Zn-finger motifs in the central region, and a C-terminal lysine-rich sequence49. The expression level of DIO-1 is low in WOL-1 cells in the logarithmic growth period. When apoptosis was induced in WOL-1 cells, the DIO-1 level increased. For example, DIO-1 was upregulated in MOL-1 cells treated with IFN-γ, dexamethasone or IL-7 deprivation but not in cells treated with etoposide or UV irradiation or cells undergoing p53-induced cell death. In MEF(10.1)Val5MycER cells, DIO-1 upregulation was observed in the absence of serum after addition of E2 but not before or at 32 °C. Transfection of a DIO-1 expression plasmid into BA/F3, A20, or FL5.12 cells resulted in cell apoptotic death, while the empty vector had no effect on cell survival; apoptotic cell death could be blocked by incubation with the pan caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk) or Bcl-2 overexpression49.
These data implied that DIO-1 upregulation could lead to cell apoptosis by activating caspase. Garcia-Domingo et al50 further confirmed that nuclear translocation was the main regulatory event in the DIO-1-induced apoptotic death pathway, as DIO-1 translocation upregulated protein levels of procaspase-3 and -9, which enhanced their own apoptosis-inducing activity. A nuclear localization signal deletion mutant of DIO-1 failed to translocate to the nuclear compartment in the absence of interleukin-3, upregulate procaspase levels or trigger cell death.
In a clinical study, 100% of human myelodysplastic syndrome (MDS)/myeloproliferative disorder (MPD) patients who were analyzed showed DIO misexpression, which was also found in other myeloid neoplasm patients but not in lymphoid neoplasm patients or healthy donors51. In our previous study, we showed that GA could not only upregulate the DIO-1 protein expression level in a dose-dependent manner, but could also change the location of DIO-1 in adult acute lymphoblastic leukemia Jurkat T cells. In the control group, DIO-1 was mainly located in the cytoplasm, although the nucleus showed slightly scattered green fluorescence of DIO-1, and the nucleus per se was intact. After Jurkat T cells were treated with GA, DIO-1 was mainly located in the nucleus and became aggregated in each early apoptotic cell, which exhibited relatively intact but condensed chromatins52.
NF-κB, as a collective term for a small family of dimeric transcription factors containing p65, RelB, c-Rel, p50/p105, and p52/p100, plays a pivotal role in lymphocyte development, proliferation, and survival and is pivotal in lymphoid malignancies, such as Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL) and multiple myeloma (MM). When activated, NF-κB proteins trans-activate target genes encoding regulators of the cell cycle (eg, cyclin D1 and cyclin D2), anti-apoptotic genes (eg, Bcl-2 and Bcl-xL), angiogenesis regulators (eg, VEGF) and drug efflux pumps (MDR1)53. In Jurkat cells, NF-κB is highly expressed and may contribute to the overexpression of Bcl-2, which inhibits cell apoptosis. Overexpression of Bcl-2 could also block DIO-1-induced apoptosis50.
In normal cells, the balance between NF-κB-regulated cell proliferation and DIO-1-mediated apoptosis may be a dynamic equilibrium. However, in some tumor cells, overexpression of NF-κB will block DIO-1-mediated apoptosis by upregulating Bcl-2 protein expression. In our experiment, GA downregulated Bcl-2 expression by inhibiting NF-κB and caused DIO-1 upregulation and a change in localization. These results contributed to pro-caspase3 activation and induced Jurkat T cell apoptosis52.
Consistent with the results above, we confirmed that GA could induce apoptosis in Burkitt's lymphoma Raji cells by upregulation of DIO-1 and downregulation of NF-κB and Bcl-xL. Together, these effects contributed to the activation of caspase-3, leading to Raji cell apoptosis54. Recently, LU L et al55 reported that GA could inhibit the TNF-α-induced invasion of human prostate cancer PC3 cells by inhibiting the NF-κB signaling pathway.
Regulation of HERG
Ion channels are membrane proteins that balance the transport of ions through the hydrophobic lipid bilayer of the cell membrane. Generally speaking, they play an important role in maintaining normal organism functions, such as regulation of blood pressure, nerve/muscle excitation, and sperm motility or capacitation. K+ channels are the main determinants of the resting membrane potential of cells. Studies suggest that K+ channels are important regulators of cell proliferation. The activation of K+ channels is essential for the progression of cells through the G1 phase to the S phase of the cell cycle56.
The human eag-related gene (herg), which encodes a channel contributing to the cardiac repolarizing current Ikr, belongs to an evolutionary conserved multigenic family of voltage-activated K+ channels, the eag (ether a-go-go) family. Although a tiny current, Iherg has profound physiological importance in cardiac rhythm57. Mutations of this channel cause long Q-T syndrome 2, leading to cardiac arrhythmias and sudden death. The acquisition of functional mutations in this channel contributes to short Q-T syndrome and sudden infant death58. Moreover, recent studies demonstrated that HERG was abundantly expressed in a variety of tumor cell lines of different histogenesis but absent in the healthy cells from which the respective tumor cells were derived59. HERG was not expressed in resting peripheral mononuclear cells (PBMNCs) obtained from normal donors or CD34+ cells collected from peripheral blood (PBCD34+). However, HERG was rapidly upregulated in the latter upon induction of proliferation of PBMNCs or PBCD34+ by cytokines/growth factors. This activation is necessary for CFU-GM proliferation in vitro57.
Various myeloid leukemia cell lines, including K562 and HL60, express herg and HERG channels. It has been reported that HERG is expressed in 36/46 (78%) of AML patients examined, with the highest incidence in the M1 (AML without maturation), M2 (AML with maturation), M3 (acute promyelocytic leukemia) and M4 (acute myelomonocytic leukemia) groups. In addition, M2 and M4 accounted for the majority of the AML patients examined57. Moreover, HERG channel inhibitors (antiarrhythmic drugs, such as E4031 and Way 123, 398, or CsCl) had the potential to inhibit the proliferation of FLG 29.1 and K562 cells and primary AML cells derived from AML patients and arrest them at the G1 phase of the cell cycle, while the same inhibitors were not able to block the proliferation of cell lines not expressing HERG57.
These studies demonstrated that the HERG channel is an important regulator of proliferation and a prerequisite for the G1/S progression of the leukemia cell lines, primary AML, and PBCD34+ cells. HERG was also upregulated in lymphoid leukemia. The HERG transcript level was not correlated with the B-cell subset, as it was elevated in both immature neoplastic B-CLL cells (CD5+) and a CD5- Burkitt's lymphoma Raji cell line. However, in Sjögren's syndrome cells (enriched in CD5+ B-cells) or Epstein-Barr virus-transformed B-cells (CD5- cells), the HERG expression level was not elevated60. Pillozzi et al61 further revealed that a HERG K+ channel, FLT-1 and β1 integrin could form a macromolecular signaling complex, which mostly recruited the HERG1B isoform of the HERG channel, and its assembly was necessary for AML cell migration. The complex was also found in primary AML blasts obtained from AML patients. The co-expression of FLT-1 and HERG conferred a pro-migratory phenotype to AML blasts. The HERG-positive blasts were more likely to invade the peripheral circulation and extramedullary sites after engraftment into NOD-SCID mice. Furthermore, HERG expression was associated with a higher probability of relapse and shorter survival periods in leukemia patients. These data demonstrated that the HERG channel not only promoted the proliferation of leukemia cells but also conferred a greater abilityto migrate and invade61.
In addition to leukemia cells, the HERG gene and HERG protein are also expressed in many colon cancer cells, such as the DLD1, HCT8, HCT116, and H630 cell lines. In particular, HCT116 and H630 cells expressed HERG at the highest level, whereas HCT8 cells expressed it at the lowest level. The activity of the HERG channel regulated the cell invasiveness of these colon cancer cell lines, and the amount of HERG protein was correlated with a more invasive phenotype of colon cancer cells. Although no expression of the HERG channel has been detected in normal human colonic mucosa or adenomas, a high percentage of primary colorectal cancers express the HERG channel, with a higher incidence in metastatic cancers62, which is consistent with the hypothesis above that high expression of the HERG channel contributed to the invasion and migration of leukemia cells.
From the above studies, it is clear that blocking the HERG channel will inhibit tumor cell proliferation, invasion and migration and thus block tumor disease progression and prolong the survival time of cancer patients. For example, knocking down HERG gene expression using short hairpin RNA (shRNA) for the HERG1 and HERG1b isoforms reduced the growth rate and cell viability of neuroblastoma cells, inhibited their colony formation, and restricted them to the G0/G1 phase of the cell cycle. Moreover, treatment with shRNA for HERG also inhibited tumor cell growth when injected into nude mice63.
In our previous study, we first revealed that GA had the potential ability to downregulate HERG mRNA and protein expression in chronic myeloid leukemia K562 cells in a dose-dependent manner. Consistent with the viewpoint that the HERG channel is an important modulator of the progression of cells through G1 phase to S phase of the cell cycle56, K562 cells treated with GA were arrested at G0/G1 phase, while the rate of S phase cells decreased, which was accompanied by the downregulation of the HERG channel. As the concentration of GA increased, the expression of HERG protein in K562 cells decreased, while the rate of cells in G0/G1 phase increases. These studies implied that GA could arrest K562 cells at the G0/G1 phase of the cell cycle, perhaps by blocking HERG channel conformation in the tumor cells64.
Induction of reactive oxygen species (ROS) and their accumulation
ROS, a term that describes the superoxide anion, hydroxyl, peroxyl, alkoxyl and O2-derived non-radical species, such as hydrogen peroxide, are generally highly reactive and short-lived65. ROS are mainly produced by mitochondria through electron leakage form complexes I and III. ROS are also produced by NAD(P)H oxidases.
Lastly, some metabolic enzymes often create ROS through nonspecific reactions66. As products or by-products of the process of cell metabolism, ROS can act as either signaling molecules or cell toxicants depending on their generated site, spatial distribution, pulse concentration and temporal duration67. ROS levels are mainly regulated by non-enzymatic antioxidants (eg, glutathione) and antioxidant enzymes (eg, superoxide dismutase)68. If the balance between ROS levels and antioxidants tips toward the oxidant side, ROS will accumulate, which leads to damage to many biomolecules, such as DNA, protein and lipids69. It has been reported that disorders of ROS are associated with some diseases, including Alzheimer's disease and Parkinson's disease70. However, ROS also contribute to the anticancer activity of some chemotherapy71.
It has been reported that ROS can activate caspases, which induce apoptosis by cleaving substrates, such as cell cycle- and DNA repair-related proteins, and the mediators of apoptosis72. ROS can induce the collapse of the mitochondrial membrane potential (MMP), leading to the release of factor cytochrome c from the inner mitochondrial membrane into the cytosol, which activates the apoptosis executioner caspase-3 through activation of the apoptosis initiator caspase-973. Moreover, the release of cytochrome c enhances the accumulation of ROS74.
The structure of GA includes an α,β-unsaturated ketone, and structure-activity relationship analysis implied that the α,β-unsaturated ketone present in GA is relevant to its cytotoxicity75. As some drugs that contain unsaturated ketones were able to induce apoptosis via the accumulation of ROS76,77, we presumed that GA could also induce apoptosis in multiple myeloma RPMI8226 cells via ROS generation.
In our study, we found that GA induced RPMI8226 cell apoptosis and inhibited proliferation in a dose-dependent manner, and these changes were accompanied by the activation of caspse-3 and cleavage of PARP-1. At the same time, the concentration of ROS was also deregulated in RPMI8226 cells treated with GA in a dose-dependent manner. To verify the hypothesis that ROS accumulation contributes to the apoptosis caused by GA, the ROS scavenger N-acetylcysteine (NAC) was used. Strikingly, NAC significantly blocked the ability of GA to induce RPMI8226 cell apoptosis and alleviated the accumulation of ROS. The activation of caspase-3 and the cleavage of PARP1 caused by GA were also inhibited by the addition of NAC. This result was consistent with reports that GA could induce apoptosis in human hepatoma SMMC-7721 cells via ROS accumulation5.
Conclusions
The natural product GA is a promising novel antitumor agent that acts via various mechanisms in solid tumors and hematological malignancies. GA can be exploited in different malignancies that are refractory to standard care because it acts through numerous antitumor mechanisms. GA has the potential to selectively kill neoplastic cells but spare normal cells4 and is currently in clinical trials in China. In a phase I human tolerability trial of GA injection, Wang et al reported that the maximal tolerated dose (MTD) of a single injection was 55 mg/m2, and the main dose-limiting toxicities (DLTs) were liver dysfunction and pain78. Another phase II clinical trial, in which all 50 cases were advanced solid tumor patients with tumors that were not sensitive to conventional chemotherapy, was conducted. The patients were treated with 45 mg/m2 GA (qd or qod) five times in a 14-d cycle. After two cycles, 47 cases were eligible for final analysis, and the responses were PR 3, SD 29, and PD 1579. As GA has the potential to induce apoptosis in various cell lines derived from respective hematological malignancies, we believe that GA can also be exploited for curing patients with various hematological malignances.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81070429).
References
- Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–62. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- Fulda S. Betulinic acid: a natural product with anticancer activity. Mol Nutr Food Res. 2009;53:140–6. doi: 10.1002/mnfr.200700491. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Prasad S, Pandey MK, Yadav VR, Aggarwal BB. Gambogic acid inhibits STAT3 phosphorylation through activation of protein tyrosine phosphatase SHP-1: potential role in proliferation and apoptosis. Cancer Prev Res (Phila) 2011;4:1084–94. doi: 10.1158/1940-6207.CAPR-10-0340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nie F, Zhang X, Qi Q, Yang L, Yang Y, Liu W, et al. Reactive oxygen species accumulation contributes to gambogic acid-induced apoptosis in human hepatoma SMMC-7721 cells. Toxicology. 2009;260:60–7. doi: 10.1016/j.tox.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Wu ZQ, Guo QL, You QD, Zhao L, Gu HY. Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1 cells in vivo and in vitro and represses telomerase activity and telomerase reverse transcriptase mRNA expression in the cells. Biol Pharm Bull. 2004;27:1769–74. doi: 10.1248/bpb.27.1769. [DOI] [PubMed] [Google Scholar]
- Guo QL, You QD, Wu ZQ, Yuan ST, Zhao L. General gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude mice. Acta Pharmacol Sin. 2004;25:769–74. [PubMed] [Google Scholar]
- Xie H, Qin YX, Zhou YL, Tong LJ, Lin LP, Geng MY, et al. GA3, a new gambogic acid derivative, exhibits potent antitumor activities in vitro via apoptosis-involved mechanisms. Acta Pharmacol Sin. 2009;30:346–54. doi: 10.1038/aps.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang L, You QD, Nie FF, Gu HY, Zhao L, et al. Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett. 2007;256:259–66. doi: 10.1016/j.canlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer. 2004;4:106–17. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- Jamali T, Jamali Y, Mehrbod M, Mofrad MR. Nuclear pore complex: biochemistry and biophysics of nucleocytoplasmic transport in health and disease. Int Rev Cell Mol Biol. 2011;287:233–86. doi: 10.1016/B978-0-12-386043-9.00006-2. [DOI] [PubMed] [Google Scholar]
- Xu S, Powers MA. Nuclear pore proteins and cancer. Semin Cell Dev Biol. 2009;20:620–30. doi: 10.1016/j.semcdb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume C, Nakano H, Yoshida K, Wong RW. Characterization of the role of the tumor marker Nup88 in mitosis. Mol Cancer. 2010;9:119. doi: 10.1186/1476-4598-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti KG, et al. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 1997;16:807–16. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth P, Xylourgidis N, Sabri N, Uv A, Fornerod M, Samakovlis C. The Drosophila nucleoporin DNup88 localizes DNup214 and CRM1 on the nuclear envelope and attenuates NES-mediated nuclear export. J Cell Biol. 2003;163:701–6. doi: 10.1083/jcb.200304046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xylourgidis N, Roth P, Sabri N, Tsarouhas V, Samakovlis C. The nucleoporin Nup214 sequesters CRM1 at the nuclear rim and modulates NFkappaB activation in Drosophila. J Cell Sci. 2006;119:4409–19. doi: 10.1242/jcs.03201. [DOI] [PubMed] [Google Scholar]
- Schneider J, Moragues D, Martinez N, Romero H, Jimenez E, Ponton J. Cross-reactivity between Candida albicans and human ovarian carcinoma as revealed by monoclonal antibodies PA10F and C6. Br J Cancer. 1998;77:1015–20. doi: 10.1038/bjc.1998.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould VE, Orucevic A, Zentgraf H, Gattuso P, Martinez N, Alonso A. Nup88 (karyoporin) in human malignant neoplasms and dysplasias: correlations of immunostaining of tissue sections, cytologic smears, and immunoblot analysis. Hum Pathol. 2002;33:536–44. doi: 10.1053/hupa.2002.124785. [DOI] [PubMed] [Google Scholar]
- Agudo D, Gomez-Esquer F, Martinez-Arribas F, Nunez-Villar MJ, Pollan M, Schneider J. Nup88 mRNA overexpression is associated with high aggressiveness of breast cancer. Int J Cancer. 2004;109:717–20. doi: 10.1002/ijc.20034. [DOI] [PubMed] [Google Scholar]
- Takahashi N, van Kilsdonk JW, Ostendorf B, Smeets R, Bruggeman SW, Alonso A, et al. Tumor marker nucleoporin 88 kDa regulates nucleocytoplasmic transport of NF-kappaB. Biochem Biophys Res Commun. 2008;374:424–30. doi: 10.1016/j.bbrc.2008.06.128. [DOI] [PubMed] [Google Scholar]
- Shu W, Chen Y, He J, Cui G. Effects of gambogic acid on the regulation of nucleoporin Nup88 in U937 cells. J Huazhong Univ Sci Technolog Med Sci. 2007;27:388–92. doi: 10.1007/s11596-007-0410-9. [DOI] [PubMed] [Google Scholar]
- Shu WX, Chen Y, He J. Effects of gambogic acid on the regulation of nucleoporin Nup88 in HL-60 cells. Zhonghua Zhong Liu Za Zhi. 2008;30:484–9. [PubMed] [Google Scholar]
- Shu W, Chen Y, Li R, Wu Q, Cui G, Ke W, et al. Involvement of regulations of nucleophosmin and nucleoporins in gambogic acid-induced apoptosis in Jurkat cells. Basic Clin Pharmacol Toxicol. 2008;103:530–7. doi: 10.1111/j.1742-7843.2008.00292.x. [DOI] [PubMed] [Google Scholar]
- Yun JP, Chew EC, Liew CT, Chan JY, Jin ML, Ding MX, et al. Nucleophosmin/B23 is a proliferate shuttle protein associated with nuclear matrix. J Cell Biochem. 2003;90:1140–8. doi: 10.1002/jcb.10706. [DOI] [PubMed] [Google Scholar]
- Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6:493–505. doi: 10.1038/nrc1885. [DOI] [PubMed] [Google Scholar]
- Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–66. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Yung BY. Involvement of nucleophosmin/B23 in TPA-induced megakaryocytic differentiation of K562 cells. Br J Cancer. 2003;89:1320–6. doi: 10.1038/sj.bjc.6601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Yung BY. Over-expression of nucleophosmin/B23 decreases the susceptibility of human leukemia HL-60 cells to retinoic acid-induced differentiation and apoptosis. Int J Cancer. 2000;88:392–400. doi: 10.1002/1097-0215(20001101)88:3<392::aid-ijc11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Suen CS, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR, Frail DE. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J Biol Chem. 1998;273:27645–53. doi: 10.1074/jbc.273.42.27645. [DOI] [PubMed] [Google Scholar]
- Gojis O, Rudraraju B, Alifrangis C, Krell J, Libalova P, Palmieri C. The role of steroid receptor coactivator-3 (SRC-3) in human malignant disease. Eur J Surg Oncol. 2010;36:224–9. doi: 10.1016/j.ejso.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Ma G, Ren Y, Wang K, He J. SRC-3 has a role in cancer other than as a nuclear receptor coactivator. Int J Biol Sci. 2011;7:664–72. doi: 10.7150/ijbs.7.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amazit L, Pasini L, Szafran AT, Berno V, Wu RC, Mielke M, et al. Regulation of SRC-3 intercompartmental dynamics by estrogen receptor and phosphorylation. Mol Cell Biol. 2007;27:6913–32. doi: 10.1128/MCB.01695-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–8. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Tanner MM, Grenman S, Koul A, Johannsson O, Meltzer P, Pejovic T, et al. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin Cancer Res. 2000;6:1833–9. [PubMed] [Google Scholar]
- Glaeser M, Floetotto T, Hanstein B, Beckmann MW, Niederacher D. Gene amplification and expression of the steroid receptor coactivator SRC3 (AIB1) in sporadic breast and endometrial carcinomas. Horm Metab Res. 2001;33:121–6. doi: 10.1055/s-2001-14938. [DOI] [PubMed] [Google Scholar]
- He LR, Liu MZ, Li BK, Rao HL, Deng HX, Guan XY, et al. Overexpression of AIB1 predicts resistance to chemoradiotherapy and poor prognosis in patients with primary esophageal squamous cell carcinoma. Cancer Sci. 2009;100:1591–6. doi: 10.1111/j.1349-7006.2009.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivas PD, Tzelepi V, Sotiropoulou-Bonikou G, Kefalopoulou Z, Papavassiliou AG, Kalofonos H. Estrogen receptor alpha/beta, AIB1, and TIF2 in colorectal carcinogenesis: do coregulators have prognostic significance. Int J Colorectal Dis. 2009;24:613–22. doi: 10.1007/s00384-009-0647-9. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chen Q, Li W, Su X, Chen T, Liu Y, et al. Overexpression of transcriptional coactivator AIB1 promotes hepatocellular carcinoma progression by enhancing cell proliferation and invasiveness. Oncogene. 2010;29:3386–97. doi: 10.1038/onc.2010.90. [DOI] [PubMed] [Google Scholar]
- Henke RT, Haddad BR, Kim SE, Rone JD, Mani A, Jessup JM, et al. Overexpression of the nuclear receptor coactivator AIB1 (SRC-3) during progression of pancreatic adenocarcinoma. Clin Cancer Res. 2004;10:6134–42. doi: 10.1158/1078-0432.CCR-04-0561. [DOI] [PubMed] [Google Scholar]
- Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6:263–74. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Colo GP, Rosato RR, Grant S, Costas MA. RAC3 down-regulation sensitizes human chronic myeloid leukemia cells to TRAIL-induced apoptosis. FEBS Lett. 2007;581:5075–81. doi: 10.1016/j.febslet.2007.09.052. [DOI] [PubMed] [Google Scholar]
- Esteyries S, Perot C, Adelaide J, Imbert M, Lagarde A, Pautas C, et al. NCOA3, a new fusion partner for MOZ/MYST3 in M5 acute myeloid leukemia. Leukemia. 2008;22:663–5. doi: 10.1038/sj.leu.2404930. [DOI] [PubMed] [Google Scholar]
- Colo GP, Rubio MF, Nojek IM, Werbajh SE, Echeverria PC, Alvarado CV, et al. The p160 nuclear receptor co-activator RAC3 exerts an anti-apoptotic role through a cytoplasmatic action. Oncogene. 2008;27:2430–44. doi: 10.1038/sj.onc.1210900. [DOI] [PubMed] [Google Scholar]
- Li R, Chen Y, Zeng LL, Shu WX, Zhao F, Wen L, et al. Gambogic acid induces G0/G1 arrest and apoptosis involving inhibition of SRC-3 and inactivation of Akt pathway in K562 leukemia cells. Toxicology. 2009;262:98–105. doi: 10.1016/j.tox.2009.04.059. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008;455:1205–9. doi: 10.1038/nature07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzari PL, Cappellini A, Grafone T, Mantovani I, Ricci F, Billi AM, et al. Detection of serine 473 phosphorylated Akt in acute myeloid leukaemia blasts by flow cytometry. Br J Haematol. 2004;126:675–81. doi: 10.1111/j.1365-2141.2004.05121.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Yu CT, Ozen M, Ittmann M, Tsai SY, Tsai MJ. Steroid receptor coactivator-3 and activator protein-1 coordinately regulate the transcription of components of the insulin-like growth factor/AKT signaling pathway. Cancer Res. 2006;66:11039–46. doi: 10.1158/0008-5472.CAN-06-2442. [DOI] [PubMed] [Google Scholar]
- Asnaghi L, Calastretti A, Bevilacqua A, D'Agnano I, Gatti G, Canti G, et al. Bcl-2 phosphorylation and apoptosis activated by damaged microtubules require mTOR and are regulated by Akt. Oncogene. 2004;23:5781–91. doi: 10.1038/sj.onc.1207698. [DOI] [PubMed] [Google Scholar]
- Garcia-Domingo D, Leonardo E, Grandien A, Martinez P, Albar JP, Izpisua-Belmonte JC, et al. DIO-1 is a gene involved in onset of apoptosis in vitro, whose misexpression disrupts limb development. Proc Natl Acad Sci U S A. 1999;96:7992–7. doi: 10.1073/pnas.96.14.7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Domingo D, Ramirez D, Gonzalez de Buitrago G, Martinez AC. Death inducer-obliterator 1 triggers apoptosis after nuclear translocation and caspase upregulation. Mol Cell Biol. 2003;23:3216–25. doi: 10.1128/MCB.23.9.3216-3225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterer A, Campanero MR, Leonardo E, Criado LM, Flores JM, Hernandez JM, et al. Dido gene expression alterations are implicated in the induction of hematological myeloid neoplasms. J Clin Invest. 2005;115:2351–62. doi: 10.1172/JCI24177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen Y, Chen Z, Wu Q, Ke WJ, Wu QL. Gambogic acid induces death inducer-obliterator 1-mediated apoptosis in Jurkat T cells. Acta Pharmacol Sin. 2008;29:349–54. doi: 10.1111/j.1745-7254.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- Packham G. The role of NF-kappaB in lymphoid malignancies. Br J Haematol. 2008;143:3–15. doi: 10.1111/j.1365-2141.2008.07284.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen Y, Chen Z, Ke WJ, Wu QL, He J. Mechanism of gambogic acid-induced apoptosis in Raji cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:88–91. [PubMed] [Google Scholar]
- Lu L, Tang D, Wang L, Huang LQ, Jiang GS, Xiao XY, et al. Gambogic acid inhibits TNF-alpha-induced invasion of human prostate cancer PC3 cells in vitro through PI3K/Akt and NF-kappaB signaling pathways. Acta Pharmacol Sin. 2012;33:531–41. doi: 10.1038/aps.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol. 1996;154:91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, Guasti L, et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia. 2002;16:1791–8. doi: 10.1038/sj.leu.2402572. [DOI] [PubMed] [Google Scholar]
- Asher V, Sowter H, Shaw R, Bali A, Khan R. Eag and HERG potassium channels as novel therapeutic targets in cancer. World J Surg Oncol. 2010;8:113. doi: 10.1186/1477-7819-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, et al. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–8. [PubMed] [Google Scholar]
- Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FW, et al. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J Biol Chem. 2002;277:18528–34. doi: 10.1074/jbc.M200592200. [DOI] [PubMed] [Google Scholar]
- Pillozzi S, Brizzi MF, Bernabei PA, Bartolozzi B, Caporale R, Basile V, et al. VEGFR-1 (FLT-1), beta1 integrin, and hERG K+ channel for a macromolecular signaling complex in acute myeloid leukemia: role in cell migration and clinical outcome. Blood. 2007;110:1238–50. doi: 10.1182/blood-2006-02-003772. [DOI] [PubMed] [Google Scholar]
- Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H, et al. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64:606–11. doi: 10.1158/0008-5472.can-03-2360. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wei XL, Jia YS, Zheng JQ. Silencing of herg gene by shRNA inhibits SH-SY5Y cell growth in vitro and in vivo. Eur J Pharmacol. 2008;579:50–7. doi: 10.1016/j.ejphar.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Cui G, Shu W, Wu Q, Chen Y. Effect of Gambogic acid on the regulation of hERG channel in K562 cells in vitro. J Huazhong Univ Sci Technolog Med Sci. 2009;29:540–5. doi: 10.1007/s11596-009-0503-8. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–8. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–15. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Chen Y, St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–35. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol. 2006;174:615–23. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–79. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–56. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J Clin Invest. 2003;111:583–93. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–62. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–92. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasibhatla S, Jessen KA, Maliartchouk S, Wang JY, English NM, Drewe J, et al. A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid. Proc Natl Acad Sci U S A. 2005;102:12095–100. doi: 10.1073/pnas.0406731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Shen SC, Tsai SH. Prostaglandin D2 and J2 induce apoptosis in human leukemia cells via activation of the caspase 3 cascade and production of reactive oxygen species. Biochim Biophys Acta. 2005;1743:291–304. doi: 10.1016/j.bbamcr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Kondo M, Shibata T, Kumagai T, Osawa T, Shibata N, Kobayashi M, et al. 15-Deoxy-Delta(12,14)-prostaglandin J(2): the endogenous electrophile that induces neuronal apoptosis. Proc Natl Acad Sci U S A. 2002;99:7367–72. doi: 10.1073/pnas.112212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang J. Phase I human tolerability trial of gambogic acid. Chin J New Drugs. 2007;16:79–83. [Google Scholar]
- Zhan X. The summary report of Phase IIa clinical trial of gambogic acid injection [dissertation] Peking: Peking Union Medical College; 2008.