Abstract
Aim:
To investigate the antitumor actions of the Crotalus durissus neurotoxin (crotoxin) on human esophageal carcinoma (Eca-109) cells in vitro and transplanted esophageal Eca-109 tumors in nude mice.
Methods:
The growth-inhibitory effect was analyzed in Eca-109 cells using MTT assay. Cell morphology changes in nuclei were observed using Hoechst 33342 staining, while apoptosis and cell cycle distribution were examined by flow cytometry. RT-PCR was used to measure the Bcl-2, p15, and caspase-3 p17 gene expression levels. A tumor transplantation model was established by inoculation of Eca-109 cells were into female Balb/c nude mice. Crotoxin (25, 50, and 100 mg/kg) was subcutaneously injected into the transplanted tumors every 2 d for a total of 10 injections. Tumor size and weight were measured. Bcl-2, p15, and caspase-3 p17 protein expression in transplanted tumors was analyzed using Western blotting.
Results:
Crotoxin (25, 50, and 100 μg/mL) inhibited the growth of Eca-109 cells in a dose-dependent manner with inhibition rates of 22.9%, 35.8%, and 57.2%, respectively. Hoechst 33342 staining revealed apoptotic cells with pyknotic nuclear chromatin after crotoxin treatment. In Eca-109 cells, crotoxin induced apoptosis and G1 block, significantly upregulated the expression of p15 and caspase-3 p17 genes and downregulated the expression of Bcl-2 gene. Furthermore, crotoxin inhibited the growth of Eca-109 tumors in nude mice in a dose-dependent manner. Western blotting showed that crotoxin increased p15 and caspase-3 p17 protein levels and reduced Bcl-2 protein level in tumor specimens.
Conclusion:
Crotoxin inhibits the growth of Eca-109 cells in vitro via apoptosis induction and G1 block. Local administration of crotoxin inhibits the growth of subcutaneously transplanted Eca-109 cells in nude mice, possibly via increasing p15 and caspase-3 p17 protein expression and reducing Bcl-2 protein expression.
Keywords: Crotalus durissus neurotoxin (Crotoxin), human esophageal Eca-109 cells, apoptosis, p15, caspase-3 p17, Bcl-2, G1 phase block, transplant tumor
Introduction
Esophageal tumors are one of the most common malignant tumors. Treatment usually involves surgery, but the cure rate remains unsatisfactory. Therefore, much attention has been focused on the development of drug treatments for esophageal cancer, leading to important advances, including the identification of components of natural substances with antitumor activities1,2,3,4,5. Crotalus durissus neurotoxin (crotoxin) is a nerve toxin isolated from South American diamondback snake subfamilies. Recent studies have indicated a potential antitumor effect of crotoxin6,7, although its specific mechanism remains unclear. In this study, we therefore investigated the antitumor effects of crotoxin in human esophageal carcinoma (Eca-109) cells and in a nude mouse tumor transplantation model. We also discuss the possible molecular mechanisms responsible for crotoxin's observed actions in order to provide a theoretical basis for future clinical applications.
Materials and methods
Cell culture
Human esophageal carcinoma (Eca-109) cells were purchased from the Shanghai Institutes for Biological Sciences and cultured in RPMI-1640 medium containing 10% fetal bovine serum at 37 °C in a 5% CO2 incubator. Cells were grown as an adherent monolayer and were digested with 0.25% trypsin and passaged when they reached 80%–90% confluence. Experiments were conducted using cells in the logarithmic phase of growth.
Drugs, reagents, and instruments
Crotoxin was a gift from the Pharmacy Department of Suzhou University Medical College (Suzhou, China). Dimethyl sulfoxide (DMSO) and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO, USA). The flow cytometer was from BD (Franklin Lakes, NJ USA). The microplate reader was from Bio-Rad (Hercules, CA, USA).
Cell-growth inhibition experiments (MTT)
Cells were harvested during logarithmic growth and digested with 0.25% trypsin to yield a single-cell suspension containing 1.0×105 cells/mL. Cells were then inoculated into 96-well plates at a density of 100 μL/well. Six parallel wells were used for each experiment. Cells were allowed to adhere for 24 h, after which the culture medium was replaced with medium containing 25, 50, or 100 μg/mL crotoxin (experimental groups); 5, 10, or 20 μg/mL cisplatin (positive control groups); medium alone (negative control group); or nothing (blanks). After 24 h, MTT (5 mg/mL, 20 μL) was added to each well followed by incubation for another 4 h. DMSO (150 μL) was then added to each well, and the plates were vibrated gently for 10 min. Absorption at 570 nm (OD) was measured using a microplate reader. Each experiment was repeated three times, and the average values were calculated. Cell proliferation inhibition (%)=(1−experimental group average OD value/control group average OD value)×100%.
Hoechst 33342 staining of Eca-109 cells
Adherent Eca-109 cells in the logarithmic growth phase were digested with 0.25% trypsin to produce a single-cell suspension. The cell density was adjusted to 1.0×105cells/mL, and a total of 5×104 cells were inoculated into each well of a 24-well plate and cultured overnight in RPMI-1640 containing 10% fetal calf serum. After the cells had adhered, the original medium was removed and replaced with medium containing 25, 50, or 100 μg/mL crotoxin in 1 mL complete RPMI-1640 medium (a total of 9 wells) and with complete RPMI-l640 without crotoxin in three negative-control wells. The cells were cultured for an additional 24 h and adjusted to pH 7.0, and 1 mg/mL Hoechst 33342 was added to a final concentration of 20 μg/mL. Cells were incubated at 37 °C for 30 min, followed by the addition of 4% polyphosphate formaldehyde mixed with culture medium in a 3:1 ratio, yielding a final formaldehyde concentration of 1%. Cells were fixed in this solution for 5–10 min, and the wells were then examined and photographed under a fluorescence microscope.
Detection of effects of crotoxin on cell cycle by flow cytometry
Eca-109 cells were treated in one of five ways: negative control group (no drug intervention), crotoxin group 1 (25 μg/mL), crotoxin group 2 (50 μg/mL), crotoxin group 3 (100 μg/mL), and cisplatin group (10 μg/mL). Eca-109 cells from each group were digested with 0.25% of trypsin and centrifuged at 1500 r/min for 5 min to produce a clear supernatant. A total of 0.5 mL of sediment was oscillated to separate the cells, and the cells were then fixed in 70% ethanol at −20 °C for 24 h. Before each experiment, the fixed cells were centrifuged at 1500 r/min for 10 min and washed twice in phosphate-buffered saline. A total of 200 μL RNaseA (1 g/L) was added, and the samples were digested at 37 °C in a constant-temperature water bath for 30 min and immediately immersed in ice water to terminate RNase activity. One milliliter of propidium iodide (50 μg/mL) was added, and the samples were incubated at 4 °C for 30 min while protected from light. The cell cycle distribution was examined using a flow cytometer.
Analysis of Bcl-2, p15, and caspase-3 p17 gene expression levels by real-time quantitative fluorescence transcriptase polymerase chain reaction (RT-PCR)
Eca-109 cells in each experimental group were cultured with crotoxin at final concentrations of 25, 50, and 100 μg/mL, while negative control cells were cultured in RPMI-1640 without crotoxin for 48 h. Total RNA was extracted according to standard RT-PCR protocol and resolved by 2.5% agarose gel electrophoresis. p15, Bcl-2, and caspase-3 p17 DNA was amplified using the corresponding primers: 5′-TTA GCA CTT GGG TGA CGG-3′ and 5′-TCG CTT GCA CAT CCT CTC-3′ for p15, 5′-GGG AGA ACA GGG TAC GAT AA-3′ and 5′-CCA CCG AAC TCA AAG AAG G-3′ for Bcl-2, 5′-GTG CGA GTG TCT AAC GG-3′ and 5′-CGG ATC AGT CTT TGG GTC-3′ for caspase-3 p17, and 5′-GTG GAC ATC CGC AAA GAC-3′ and 5′-GAA AGG GTG TAA CGC AAC T-3′ for β-actin.
Nude mouse tumor transplantation model
Eca-109 cells undergoing logarithmic growth were adjusted to a cell density of l.0×108 cells/mL. Twenty-eight 4–6 weeks old female Balb/c nude mice (weight 16–20 g, average weight 17.5 g) were used. Anesthesia was induced by intraperitoneal injection of 2 mg/kg lidocaine. The shoulder skin was disinfected and each animal received a subcutaneous injection of a 125-μL cell suspension containing 1.25×107 cells. After inoculation, animals were maintained in specific pathogen-free conditions, and their activity, mental state, feeding and defecation were observed daily. Tumor size was measured using Vernier calipers, and the mice were weighed twice per week.
Calculation of tumor inhibition by crotoxin
All experimental animals developed nodules of approximately 4–6 mm in diameter within 15 d of inoculation. The animals were then divided randomly into a high-dose group (100 mg/kg crotoxin), a medium-dose group (50 mg/kg crotoxin), a low-dose group (25 mg/kg crotoxin) and 4 negative control groups (which received equivalent doses of 0.9% NaCl). Each group contained 7 mice. The experimental groups received subcutaneous injections of crotoxin into the transplanted tumors every 2 d for a total of 10 injections, while the negative control mice received subcutaneous injections of the same volume of saline. Mice were euthanized after 4 weeks, blood samples were taken, and the subcutaneous tumors were removed and weighed. The tumor inhibition rates were calculated as follows: tumor inhibition rate (%)=(1−weight of experimental group tumor/weight of control group tumor)×100%. Tumor tissues were processed for immunohistochemistry using conventional methods.
Western blot analysis of Bcl-2, p15, and caspase-3 p17 protein expression in transplanted tumors
Approximately 100 mg of transplanted tumor tissue was removed from liquid nitrogen, quickly placed into 2 mL lysate and fully homogenized. The mixture was transferred to an EP tube on ice and subjected to ultrasonic irradiation for 30 min, followed by centrifugation at 12 000 r/min for 5 min at 4 °C. The concentration of proteins in the supernatant was determined using the BCA method. The samples were boiled, and the proteins were separated by SDS-PAGE. Blots were transferred to nitrocellulose membranes and hybridized. The primary antibodies used were mouse anti-human p15 monoclonal antibody, mouse anti-human caspase 3-p17 monoclonal antibody, and mouse anti-human Bcl-2 monoclonal antibody, with β-actin as an internal control. Secondary antibodies were labeled with horseradish peroxidase. Protein expression was analyzed by chemiluminescence.
Statistical analysis
Measurements are expressed as the mean±SD. Differences between experimental and control groups were compared using two-sample mean difference t-tests with SPSS (Statistical Package for the Social Sciences) 15.0 statistical software for single-factor analysis of variance. P<0.05 was considered to represent a significant difference.
Results
Effects of crotoxin on growth of esophageal carcinoma Eca-109 cells
All concentrations of crotoxin significantly inhibited the growth of Eca-109 cells compared to the control group (P<0.05). The growth-inhibitory activity of crotoxin was dose-dependent with inhibition rates of 22.9%, 35.8%, and 57.2%, respectively (Table 1). Cisplatin also inhibited the growth of Eca-109 cells compared to the control group (P<0.05), with inhibition rates of 12.0%, 18.3%, and 32.4%, respectively (Table 2).
Table 1. Effects of crotoxin on the growth of Eca-109 cells. Mean±SD. n=3. bP<0.05, cP<0.01 vs control.
| Concentration of crotoxin (μg/mL) | OD value | P value | Inhibition rate |
|---|---|---|---|
| Control group | 0.732±0.091 | ||
| 25 | 0.574±0.115b | 0.033 | 22.9% |
| 50 | 0.492±0.065c | 0.002 | 35.8% |
| 100 | 0.364±0.055c | 0.001 | 57.2% |
Table 2. Effects of cisplatin on the growth of Eca-109 cells. Mean±SD. n=3. bP<0.05, cP<0.01 vs control.
| Concentration of cisplatin (μg/mL) | OD value | P value | Inhibition rate |
|---|---|---|---|
| Control group | 0.865±0.077 | ||
| 5 | 0.767±0.060b | 0.047 | 12.0% |
| 10 | 0.601±0.062b | 0.018 | 18.3% |
| 20 | 0.569±0.089c | 0.003 | 32.4% |
Effects of crotoxin on Eca-109 cell apoptosis using Hoechst 33342 staining
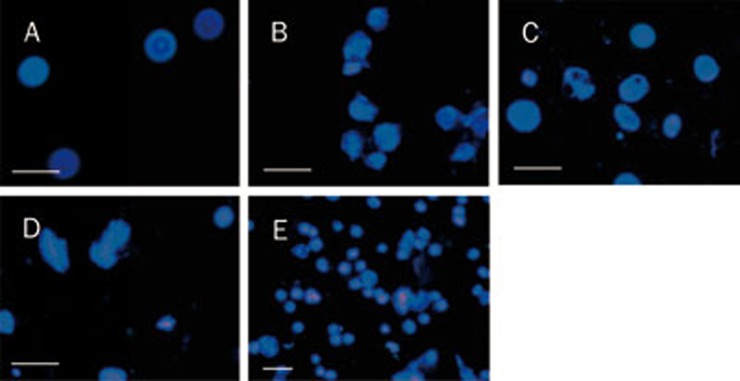
In normal Eca-109 cells, nuclei demonstrated diffused and even fluorescence. However, after crotoxin treatment, cell nuclei became pyknotic and agglomerated, some cells burst, and nuclear debris was scattered between the cells. Large, dense, hyperchromatic, fluorescent particles or pieces were observed in the nuclei or cytoplasm of apoptotic cells (Figure 1).
Figure 1.
Effects of crotoxin and cisplatin on Eca-109 cells using Hoechst 33342 staining. (A) Control; (B) Crotoxin (25 μg/mL); (C) Crotoxin (50 μg/mL); (D) Crotoxin (100 μg/mL); (E) Cisplatin (10 μg/mL). Scale bar=50 μm.
Effect of crotoxin on cell cycle in Eca-109 cells
Exposure of Eca-109 cells to 25 μg/mL of crotoxin for 24 h increased the diploid peak by 7.45%±1.58% compared to the control group (0.71%±0.11%) (P<0.05) and increased the quantity of G1 phase cells by 70.04%±4.52% compared to the control group (54.88%±7.56%) (P<0.05). Treatment with 50 μg/mL of crotoxin for 24 h increased the diploid peak by 8.23%±1.47% and increased the quantity of G1 phase cells by 71.14%±4.86% compared to the control groups (both P<0.05 compared to the control group). Treatment with 100 μg/mL of crotoxin increased the diploid peak by 11.23%±1.51% and increased the quantity of G1 phase cells by 78.19%±4.90% (both P<0.01 compared to the control group). Cisplatin increased the diploid peak by 5.59%±0.13% and increased the G1 phase population by 65.46%±5.47% compared to the negative control group (both P<0.05 compared to the control group) (Table 3 and 4).
Table 3. Effects of crotoxin on cell apoptosis rate in Eca-109 cells. Mean±SD. n=3. bP<0.05, cP<0.01 vs control.
| Group | Diploid peak (%) | P value |
|---|---|---|
| Control group | 0.71±0.11 | |
| Crotoxin 25 μg/mL | 7.45±1.58b | 0.028 |
| Crotoxin 50 μg/mL | 8.23±1.47b | 0.019 |
| Crotoxin 100 μg/mL | 11.23±1.51c | 0.001 |
| Cisplatin 10 μg/mL | 5.59±0.13b | 0.033 |
Table 4. Effects of crotoxin on cell cycle in Eca-109 cells. Mean±SD. n=3. bP<0.05, cP<0.01 vs control.
| Group | G1 phase (%) | P value |
|---|---|---|
| Control group | 54.88±7.56 | |
| Crotoxin 25 μg/mL | 70.04±4.52b | 0.010 |
| Crotoxin 50 μg/mL | 71.14±4.86c | 0.000 |
| Crotoxin 100 μg/mL | 78.19±4.90c | 0.000 |
| Cisplatin 10 μg/mL | 65.46±5.47b | 0.028 |
RT-PCR analysis of the effects of crotoxin on gene expression in Eca-109 cells
Gene expression levels of p15 and caspase-3 p17 increased in Eca-109 cells after exposure to crotoxin for 24 h in a dose-dependent manner. In contrast, Bcl-2 gene expression levels decreased with increasing concentration of crotoxin (Table 5).
Table 5. Effects of crotoxin on gene expression in Eca-109 cells.
| Concentration of crotoxin | Total number |
p15 (+) |
Caspase 3 p17 (+) |
Bcl-2 (+) |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Control group | 15 | 100 | 1 | 6.7 | 1 | 6.7 | 11 | 73.3 |
| Crotoxin 25 μg/mL | 15 | 100 | 8 | 53.3 | 6 | 40.0 | 4 | 26.7 |
| Crotoxin 50 μg/mL | 15 | 100 | 10 | 66.7 | 9 | 60.0 | 3 | 20.0 |
| Crotoxin 100 μg/mL | 15 | 100 | 13 | 86.7 | 12 | 80.0 | 2 | 13.3 |
Tumor-inhibitory effects of crotoxin on transplanted esophageal Eca-109 tumors in nude mice
All mice developed palpable, subcutaneous round or oval nodules at the injection site 4–6 d after subcutaneous inoculation of human esophageal carcinoma Eca-109 cells. Nodules were 2–3 mm in diameter with clear boundaries, and they could be removed without skin adhesion. Body weight and activity of the transplanted mice were initially unaffected, but the activity levels decreased slightly with increasing tumor size. There were no significant differences in body weight between the experimental and control groups. Tumor diameter was approximately 5–6 mm at 12–16 d after inoculation. The transplanted tumors grew more slowly in animals treated with local crotoxin injections, and their mood remained good. The weight change remained small 20 d after inoculation, with no signs of angularity and no deaths. Tumors in the control group grew more rapidly, and 30 d after inoculation, the control animals appeared angular with weight loss, sluggishness, and reduced activity, although no deaths occurred.
Mice were euthanized one day after completion of drug administration, and the subcutaneous nodules were removed. Tumors showed local nodular or lobulated growth. Tumor size was decreased in the crotoxin-treated groups, and these tumors appeared harder, with no obvious new blood vessels on the surface, no obvious surrounding tissue adhesion, and no signs of protrusion into the muscle and rib cage, indicating a degenerative state of the tumors. Tumors in the control saline-treated mice were significantly larger than those in the crotoxin-treated group, with a greater quantity of new blood vessels on the surface and in sections, indicating a rich blood supply. Anatomical observation revealed adhesion of the tumors to the surrounding tissues. No distant metastases were detected in any animals. The tumor-inhibition rates were 10.8%, 35.7%, and 91.7% in the low-, medium- and high-crotoxin-dose groups, respectively. The average weights of tumors in the medium- and high-crotoxin-dose groups were significantly reduced (P<0.01, Table 6).
Table 6. Effects of crotoxin on tumor weight and tumor inhibition in a nude mouse subcutaneous transplantable tumor model. Mean±SD. n=7 mice. cP<0.01 vs control.
| Concentration of crotoxin (mg/kg) | Average tumor weight (g) | Tumor-inhibition rates (%) |
|---|---|---|
| Control group | 0.75±0.14 | – |
| 25 | 0.68±0.15 | 10.8 |
| 50 | 0.45±0.10c | 35.7 |
| 100 | 0.06±0.08c | 91.7 |
Based on single-factor analysis of variance, tumor weights in the high- and medium-dose groups were lower than those of the control group (P<0.01), while tumor weight in the low-dose group was also decreased (P>0.05). Tumor weights differed significantly between the high-, medium- and low-dose groups (P<0.05).
Effects of crotoxin on p15, caspase-3 p17 and Bcl-2 protein expression
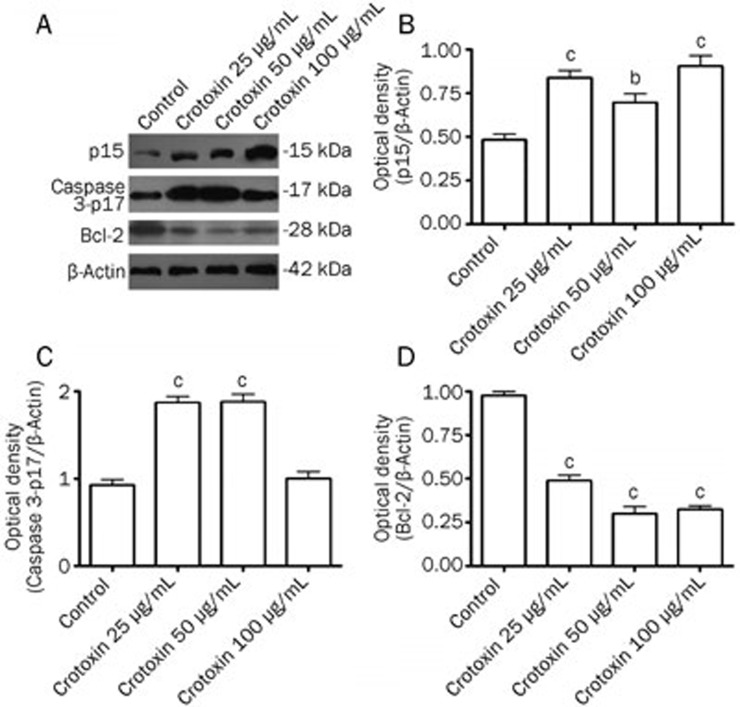
The protein expression of p15, caspase-3 p17 and Bcl-2 was determined with immunoblotting. The results showed that crotoxin induced a robust increase in active caspase-3 p17 protein levels and p15 protein levels after treatment with 25 to 50 μg/mL of crotoxin (Figure 2A–2C). A marked reduction in the Bcl-2 protein level was observed following treatment with 25 to 100 μg/mL crotoxin (Figure 2A and 2D).
Figure 2.
Effects of crotoxin on p15, Caspase 3-p17, and Bcl-2 protein expression in Eca-109 cells. Protein levels were analyzed with immunoblotting. Optical densities of respective protein bands were analyzed with Sigma Scan Pro 5 and normalized with loading control (β-actin). Mean±SD. n=6. bP<0.05, cP<0.01 vs control group.
Discussion
The Crotalus durissus neurotoxin (crotoxin) is composed of two non-covalently bonded subunits, A and B. Crotoxin B is also known as phospholipase A2 and is considered to be the main active unit8,9, which specifically catalyzes hydrolysis of glycerophosphatide, generating lysophospholipids and fatty acids. Recent studies have reported a possible antitumor function for crotoxin10,11,12,13,14, although the specific mechanism responsible for this activity remains unclear. However, it is possible that it may act via the release of cytochrome c and the activation of caspase-3, thus destroying the mitochondrial membrane potential of the tumor cells.
The results of this study demonstrated a clear, dose-dependent inhibition of Eca-109 human esophageal carcinoma cell growth by crotoxin. Fluorescence microscopy also revealed increased apoptosis characterized by nuclear pyknosis, apoptotic-body formation and other morphological features, while flow cytometry detected an obvious apoptosis peak and G1 phase block after treatment of cells with crotoxin for 24 h. These results suggest that the anti-tumor effect of crotoxin is closely related to apoptosis induction and cell cycle blockade.
The current study employed a range of methods to confirm the effects of crotoxin in promoting apoptosis in Eca-109 cells. Hoechst 33342 staining and fluorescence microscopy demonstrated apoptotic nuclei and hyperchromatic dense particles within the cytoplasm, further confirming the induction of apoptosis-related morphological changes in Eca-109 cells by crotoxin.
Apoptosis is regulated and restricted by many types of mechanisms, including caspase activation. Caspases are homocysteine aspartate-specific proteases, of which caspase-3 is one of the most important executors of apoptosis. Caspase-3 is responsible for cutting all or part of the key proteins during the implementation stage of apoptosis. Once caspase-3 is activated, cell apoptosis is irreversible15,16. Previous studies have shown that caspase-3 activation is an irreversible step in apoptosis and that apoptosis is closely related to tumor occurrence and inhibition17,18,19. The current RT-PCR results demonstrated that gene expression levels of p15 and caspase-3 p17 were significantly increased in a dose-dependent manner in cells treated with crotoxin for 48 h. This suggests that crotoxin may induce Eca-109 cell apoptosis via caspase-3 signaling pathways.
Eca-109 human esophageal carcinoma cells were inoculated into nude mice to establish a tumor transplant model, and crotoxin was found to inhibit the growth of the transplanted tumors. Western blotting demonstrated that p15 and caspase-3 p17 protein levels were increased in tumors treated with crotoxin, while Bcl-2 protein was significantly reduced. There were no obvious adverse effects associated with tumor inhibition, and weight, mood, diet, and activity were all similar in the crotoxin and control groups.
Conclusions
Crotalus durissus neurotoxin (crotoxin) inhibits the growth of esophageal carcinoma Eca-109 cells in vitro via a mechanism involving apoptosis induction and a G1 phase block. Apoptosis induction may, in turn, be related to caspase-3 activation. Local crotoxin treatment can inhibit the growth of subcutaneously transplanted esophageal Eca-109 tumors in nude mice, and this inhibition may be related to the upregulation of p15 and caspase-3 p17, as well as the downregulation of Bcl-2 protein expression.
Author contribution
Jing-kang HE and Yan XIE designed research; Xiang-sheng WU performed research; Xiang-sheng WU and Yan WANG wrote the manuscript; Zheng-hong QIN and Yan WANG edited the manuscript; Yan WANG and Rong HAN analyzed data.
Acknowledgments
This study was supported by the project of the Jiangsu Province Key Provincial Talents Program (RC2011112).
References
- Shehzad A, Khan S, Sup Lee Y. Curcumin molecular targets in obesity and obesity-related cancers. Futur Oncol. 2012;8:179–90. doi: 10.2217/fon.11.145. [DOI] [PubMed] [Google Scholar]
- Ye F, Zhang GH, Guan BX, Xu XC. Suppression of esophageal cancer cell growth using curcumin, (–)-epigallocatechin-3-gallate and lovastatin. World J Gastroenterol. 2012;18:126–35. doi: 10.3748/wjg.v18.i2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan-Coyne G, O'Sullivan GO, O'Donovan TR, Piwocka K, Mckenna SL. Curumin induces apoptosis-independent death in esophageal cancer cells. Br J Cancer. 2009;101:1585–95. doi: 10.1038/sj.bjc.6605308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GP, Wan X, Xu SP, Wang H, Liu SH, Wang ZG. Antiproliferation and apoptosis induction of paeonol in human esophageal cancer cell lines. Dis Esophaqus. 2008;21:723–9. doi: 10.1111/j.1442-2050.2008.00840.x. [DOI] [PubMed] [Google Scholar]
- Pei YX, Heng ZC, Duan GC, Wang MC.The mechanisms and effects of lutein on inducing the cell differentiation of human esophagus cancer EC 9705 Sichuan Da Xue Xue Bao 200738629–32.Chinese. [PubMed] [Google Scholar]
- Sampaio SC, Hyslop S, Fontes MR, Prado-Franceschi J, Zambelli VO, Magro AJ, Brigatte P, Gutierrez VP, Cury Y. Crotoxin: novel activities for a classic beta-neurotoxin. Toxicon. 2010;55:1045–60. doi: 10.1016/j.toxicon.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Yan CH, Liang ZQ, Gu ZL, Yang YP, Reid P, Qin ZH. Contributious of autophagic and apoptotic mechanisms to CrTX-induced death of K562 cells. Toxicon. 2006;47:521–30. doi: 10.1016/j.toxicon.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Pereańez JA, Gómez ID, Patińo AC. Relationship between the structure and the enzymatic activity of crotoxin complex and its phospholipase A2 subunit: an in silico approach. J Mol Graph Model. 2012;35:36–42. doi: 10.1016/j.jmgm.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Samy RP, Thwin MM, Stiles BG, Bow H, Chow VT, Gopalakrishnakone P. Therapeutic potential of peptides with neutralizing ability towards the venom and toxin (CaTX-1) of crotalus adamanteus. Curr Top Med Chem. 2011;11:2540–55. doi: 10.2174/156802611797633384. [DOI] [PubMed] [Google Scholar]
- Wang JH, Xie Y, Wu JC, Han R, Rei PF, Qin ZH, He JK. Crotoxin enhanced the antitumor activity of gefinitib (Iressa) in SKMES-1 human lung squamous carcinoma cells. Oncol Rep. 2012;27:1341–7. doi: 10.3892/or.2012.1677. [DOI] [PubMed] [Google Scholar]
- Ye B, Xie Y, Qin ZH, Wu JC, Han R, He JK. Anti-tumor activity of CrTX in human lung adenocarcinoma cell line A549. Acta Pharmacol Sin. 2011;32:1397–401. doi: 10.1038/aps.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure G, Xu H, Saul FA. Crystal structure of crotoxin reveals key residues involved in the stability and toxicity of this potent heterodimeric β-neurotoxin. J Mol Boil. 2011;412:176–91. doi: 10.1016/j.jmb.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Ferquson EL, Duncan R. Dextrin-phospholipase A2: synthesis and evaluation as a bioresponsive anticancer conjugate. Biomacromolecules. 2009;10:1358–64. doi: 10.1021/bm8013022. [DOI] [PubMed] [Google Scholar]
- Yan CH, Yang YP, Qin ZH, Gu ZL, Reid P, Liang ZQ. Autophagy is involved in cytotoxin effects of crotoxin in human breast cancer cell line MCF-7 cells. Acta Pharmacol Sin. 2007;28:540–8. doi: 10.1111/j.1745-7254.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- Sareen D, Ebert AD, Heins BM, McGivern JV, Ornelas L, Svendsen CN. Inhibition of apoptosis blocks human motor neuron cell death in a stem cell model of spinal muscular atrophy. Plos One. 2012;7:e39113. doi: 10.1371/journal.pone.0039113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aref S, Abdullah D, Fouda M, EI Menshawy N, Azmy E, Bassam A, Menessy A, EI Refaei M. Neutrophil apoptosis in neutropenic patients with hepatitis C infection: Role of caspases 3, 10, and GM-CSF. Indian J Hematol Blood Transfus. 2011;27:81–7. doi: 10.1007/s12288-011-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayora A, Arvelo F. Prostate cancer and apoptosis. Invest Clin. 2011;52:376–96. [PubMed] [Google Scholar]
- Karmakar S, Choudhury SR, Banik NL, Rag SK. Induction of mitochondrial pathways and endoplasmic reticulum stress for increasing apoptosis in ectopic and orthotopic neuroblastoma xenografts. J Cancer Ther. 2011;2:77–90. doi: 10.4236/jct.2011.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–9. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]