Abstract
Aim:
To investigate the role of LKB1 in regulation of mTOR signaling in non-small cell lung cancer (NSCLC) cells.
Methods:
LKB1 protein expression and phosphorylation of AMPK, 4E-BP1 and S6K in the cells were assessed using Western blotting in various NSCLC cell lines (A549, H460, H1792, Calu-1 and H1299). Energy stress was mimicked by treating the cells with 2-deoxyglucose (2-DG). Compound C was used to inhibit AMPK activity. Cell growth was measured using the MTS assay.
Results:
LKB1 protein was expressed in LKB1 wild-type Calu-1, H1299 and H1792 cells, but it was undetected in LKB1 mutant A549 and H460 cells. Treatment of the LKB1 wild-type cells with 2-DG (5, 10 and 25 mmol/L) augmented the phosphorylation of AMPK in dose- and time-dependent manners. In the LKB1 wild-type cells, 2-DG dramatically suppressed the phosphorylation of two mTOR targets, 4E-BP1 and S6K, whereas the LKB1 mutant A549 and H460 cells were highly resistant to 2-DG-induced inhibition on mTOR activity. In addition, stable knockdown of LKB1 in H1299 cells impaired 2-DG-induced inhibition on mTOR activity. Pretreatment of H1299 and H1792 cells with the AMPK inhibitor compound C (10 μmol/L) blocked 2-DG-induced inhibition on mTOR activity. 2-DG inhibited the growth of H1299 cells more effectively than that of H460 cells; stable knockdown of LKB1 in H1299 cells attenuated the growth inhibition caused by 2-DG.
Conclusion:
In non-small cell lung cancer cells, LKB1/AMPK signaling negatively regulates mTOR activity and contributes to cell growth inhibition in response to energy stress.
Keywords: non-small cell lung cancer, LKB1/AMPK/mTOR, 2-deoxyglucose
Introduction
Lung cancer is the leading cause of cancer mortality worldwide, with approximately 15% 5-year patient overall survival. Loss-of-function mutations of tumor suppressor genes contribute to lung cancer initiation and progression. LKB1, also known as serine/threonine kinase 11 (STK11), belongs to the calcium/calmodulin-regulated kinase group. Germline mutations in LKB1 give rise to Peutz-Jeghers syndrome, a disorder characterized by benign hamartomas of the gastrointestinal tract1 and a predisposition to certain cancers2,3. Although LKB1 mutation is rare in most types of sporadic cancers, biallelic inactivation of LKB1 is present in 30% of non-small cell lung cancer (NSCLC) primary tumors and cell lines4,5,6 and ranks as the third-highest mutated gene in lung adenocarcinoma after p53 and Ras7. LKB1 encodes a serine/threonine protein kinase and functions as a master upstream kinase of a group of AMP-activated protein kinase (AMPK)-related kinases and thus is involved in the management of cellular energy, inhibition of cell growth and regulation of cell polarity8,9,10. Among these substrate kinases, the most studied is AMPK, a central metabolic switch.
2-Deoxyglucose (2-DG) is a well characterized glycolysis inhibitor11. It is converted by hexokinase to phosphorylated 2-DG (2-DG-P), which cannot be metabolized in glycolysis12. The inhibition of glycolysis by 2-DG treatment leads to a decrease in intracellular ATP concentration and an increase in intracellular AMP concentration13,14. AMP can bind to AMPK and alter its conformation, resulting in AMPK activation by LKB1 via phosphorylation of AMPK at Thr17215,16. Activated AMPK then phosphorylates tuberin protein (TSC2) and activates its GTP activating protein (GAP) activity to inhibit the Ras homolog enriched in brain (Rheb)-mediated mammalian target of rapamycin (mTOR) signaling. This results in repressed phosphorylation of key translational regulators such as eukaryotic translation initiation factor 4e-binding protein 1 (4E-BP1) and ribosomal S6 kinase (S6K1)17.
The role of LBK1 in AMPK activation and mTOR repression has been demonstrated in the in vitro phosphorylation assay18, in LKB1-deficient cancer cells (such as HeLa) and in mouse embryonic fibroblasts (MEFs) from LKB1 knockout mice19. In the present study, we attempted to investigate the relevance of LKB1 signaling in lung carcinogenesis with several lung cancer cell lines expressing wild-type LKB1 or inactivating mutations in LKB1. Here, we report that LKB1 mediates activation of AMPK, inhibition of mTOR and suppression of cell growth in response to energy stress in lung cancer cells.
Materials and methods
Materials
Mouse monoclonal antibody against LKB1 was purchased from Abcam(Cambridge, MA, UK). Antibodies against AMPK, phospho-AMPK α-Thr172, phospho-S6K-Thr389, and phospho-4E-BP-Ser65 were purchased from Cell Signaling Technology(Beverly, MA, USA). Mouse anti-GAPDH antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). 2-Deoxyglucose (2-DG) was purchased from Sigma (St Louis, MO, USA). Compound C was purchased from Calbiochem (Billerica, MA, USA).
Cell lines and cell culture
Two lung adenocarcinomas cell lines (A549 and H1792), two large cell lung cancer cell lines (H460 and H1299), one squamous lung cancer cell line Calu-1 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI 1640 medium purchased from Invitrogen (Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 U/mL streptomycin and 100 mg/mL penicillin) at 37 °C in a humidified atmosphere with 5% CO2. LKB1 stable knockdown cell line H1299-LKB1shRNA and control cell line H1299-pLK0.1 were established previously by our lab20.
Western blot analysis
Cells were seeded in 6-well plates and incubated for 24 h prior to treatment with different concentrations of 2-DG for different periods of time. Cells were lysed in a lysis buffer containing 20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1% Triton X-100, 0.5 mmol/L EDTA, 1 mmol/L PMSF, 1 mmol/L NaF, and 1 μg/mL leupeptin at 4 °C. Protein concentration was determined by Bradford assay. Equal amounts of protein from each cell lysate (50 μg/lane) were subjected to 10% SDS-polyacrylamide gel electrophoresis (SDS/PAGE) and transferred onto polyvinylidene difluoride membranes (Millipore, New Bedford, MA, USA). The membranes were blocked for 1 h at room temperature and then probed with primary antibodies against LKB1 (dilution 1:3000), AMPK (dilution 1:1000), phospho-AMPK (dilution 1:1000), phospho-S6K (dilution 1:1000), and phospho-4E-BP (dilution 1:1000) or GAPDH (dilution 1:3000) in Tris-buffered saline containing 0.2% Tween 20 and 5% fat-free dry milk overnight at 4 °C. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (dilution 1:5000) for 1 h at room temperature. Specific proteins were visualized with enhanced chemiluminescence detection reagent according to the manufacturer's instructions (Pierce Biotechnology, Rockford, IL, USA).
Cell growth inhibition assay
Cells were seeded in 96-well cell culture plates at a density of 5000 cells per well. After attachment, cells were treated with indicated concentrations of 2-DG for 48 or 72 h. Cell growth inhibition assay was determined with the Cell Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega, WI, USA) according to the manufacturer's instructions.
Statistical analysis
Most of our results are representative of at least three independent experiments and are presented as the mean±standard deviation (SD) of triplicate samples. Error bars represent standard deviations between experiments.
Results
LKB1 expression in various NSCLC cell lines
To investigate the relevance of LKB1 in lung carcinoma, we studied several NSCLC cell lines with and without LKB1-inactivating mutations. According to our previous work6, LKB1 has a nonsense mutation at codon 37 in H460 and A549 cell lines. Consistent with this, LKB1 was undetectable in A549 and H460 cells, while it was readily detectable in Calu-1, H1299, and H1792 cells, demonstrating that these cells are LKB1 wild-type cells (Figure 1).
Figure 1.
LKB1 protein expression in NSCLC cell lines. Cell lysates were collected, and anti-LKB1 antibody was used to detect LKB1 expression. GAPDH was used as the loading control.
AMPK activation mediated by LKB1
We next evaluated the phosphorylation of AMPK in these cells. ATP depletion using 2-DG resulted in the augmentation of AMPK phosphorylation in LKB1 wild-type cells (Calu-1, H1299, and H1792) in a time- and dose-dependent manner. Specifically, 2-DG-induced AMPK phosphorylation was readily detectable at 30 min after the addition of 2-DG (Figure 2A) and was sustained for at least 24 h (Figure 2B). In addition, 5 mmol/L 2-DG was sufficient to substantially increase AMPK phosphorylation (Figure 2C). In contrast, in LKB1-mutant H460 cells and LKB1 stable knockdown cell line H1299-LKB1shRNA, AMPK activation by 2-DG was severely attenuated20, confirming the indispensable role of LKB1 in AMPK activation in lung cancer cells.
Figure 2.
Time- and dose-dependent of AMPK phosphorylation induced by 2-DG. (A) Calu-1, H1299, and H1792 cells were treated with 25 mmol/L 2-DG for the indicated time (0, 0.5, 1, and 2 h). (B) Calu-1, H1299 and H1792 cells were treated with 25 mmol/L 2-DG for longer time points (4, 8, and 24 h). (C) Calu-1, H1299, and H1792 cells were treated with the indicated concentration of 2-DG (0, 5, 10, and 25 mmol/L) for 2 h. Anti-p-AMPK antibody was used to detect AMPK phosphorylation, and total AMPK was used as the loading control.
LKB1 negatively regulates mTOR activity
To define the relevance of LKB1 in mTOR regulation, we tested the ability of LKB1 mutant and LKB1 wild-type cells to inhibit mTOR signaling in response to energy stress. Phosphorylation of 4E-BP1 and S6K, two well-characterized targets of mTOR, was dramatically repressed in LKB1 wild-type cells (Calu-1, H1299, and H1792) upon 2-DG treatment (Figure 3A). In contrast, LKB1 mutant cells (A549 and H460) were highly resistant to mTOR inactivation, as evidenced by the levels of phospho-S6K (Figure 3B), suggesting that LKB1 is required for 2-DG-mediated inhibition of mTOR activity. Moreover, stable knockdown of LKB1 in H1299 cell (H1299-LKB1shRNA) impaired the inhibitory effect of 2-DG on mTOR activity (Figure 3C), further supporting the repressive role of LKB1 in regulating mTOR activity.
Figure 3.
LKB1-mediated inhibition of mTOR activity. (A) Calu-1, H1299, and H1792 cells were treated with 25 mmol/L 2-DG for 2 h. (B) H460 and A549 cells were treated with 25 mmol/L 2-DG for 2 h. (C) H1299-pLKO.1 and H1299-LKB1shRNA cells were treated with 25 mmol/L 2-DG for 2 h. Anti-p-4E-BP1 and anti-p-S6K antibody were used to detect 4E-BP1 and S6K phosphorylation, with GAPDH as the loading control.
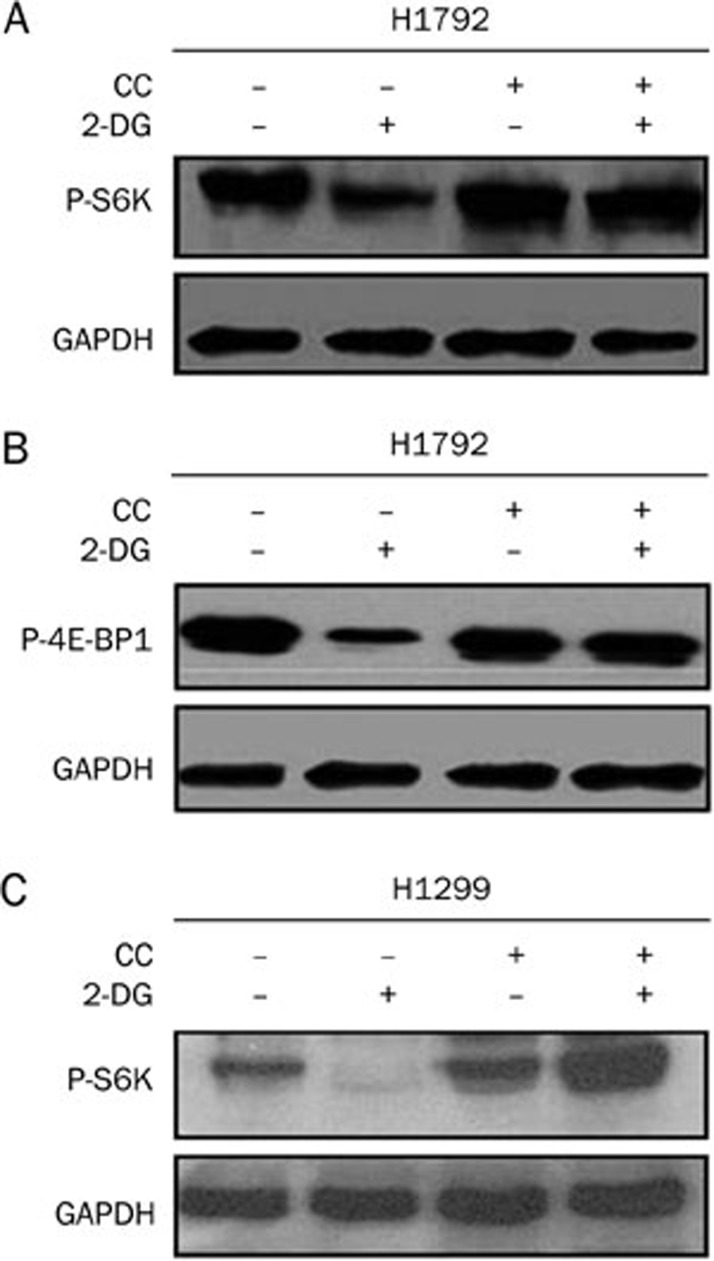
LKB1 functions through AMPK to inhibit mTOR
To explore the functional significance of AMPK in the inhibition of mTOR by LKB1, Compound C (CC), a pharmacological inhibitor of AMPK, was tested. It was observed that the inhibitory effect of LKB1 on S6K phosphorylation was blocked after pretreating LKB1 wild-type H1792 cells with CC for 30 min (Figure 4A). The repression of 4E-BP1 phosphorylation was also reduced (Figure 4B). Similar results were obtained in the LKB1 wild-type H1299 cells (Figure 4C). These data suggest that AMPK functions downstream of LKB1 to inhibit mTOR activity.
Figure 4.
AMPK inhibitor Compound C blocked 2-DG induced mTOR inhibition. A) H1792 cells were pretreated with Compound C (10 μmol/L) for 30 min prior to addition of 25 mmol/L 2-DG for 2 h. Anti-p-S6K antibody was used to detect S6K phosphorylation. B) H1792 cells were pretreated with Compound C (10 μmol/L) for 30 min prior to addition of 25 mmol/L 2-DG for 2 h. Anti-p-4E-BP1 antibody was used to detect p-4E-BP1 phosphorylation. C) H1299 cells were pretreated with Compound C (10 μmol/L) for 30 min prior to addition of 25 mmol/L 2-DG for 2 h. Anti-p-S6K antibody was used to detect S6K phosphorylation, with GAPDH as the loading control.
LKB1 is required for cell growth inhibition in response to energy stress
Given that mTOR is a critical modulator of protein synthesis and cell growth, we sought to determine the effect of 2-DG on cell growth. Although both LKB1 wild-type H1299 cells and LKB1 mutant H460 cells show decreased cell viability upon 2-DG treatment, H1299 cells were more sensitive to 2-DG treatment than H460 cells (Figure 5A). As previously mentioned, 2-DG sensitive H1299 cells demonstrated enhanced AMPK activation and decreased mTOR activity while 2-DG insensitive H460 cells exhibited comprised AMPK-mTOR signaling, consistent with their diverse cell viability upon 2-DG treatment. However, the differing sensitivity of H460 and H1299 cells to 2-DG cannot be attributed simply to LKB1 mutation status because the overall genetic backgrounds of H460 and H1299 cells are also different. Therefore, to further validate the results, the cell viability of isogenic cell lines H1299-LKB1shRNA and H1299-PLKO.1 was tested. As expected, stable knockdown of LKB1 attenuated growth inhibition induced by 2-DG (Figure 5B), further supporting the requirement of LKB1 in mediating cell growth inhibition in response to energy stress in lung cancer.
Figure 5.
LKB1 is required for 2-DG mediated cell growth inhibition. A) H460 and H1299 cells were seeded in 96-well plates and treated with the indicated concentration of 2-DG (0, 2.5, 5, 10, and 25 mmol/L). Plates were subjected to MTS assay after 48 or 72 h. Reactions were conducted in quadruplicate. B) H1299-pLKO.1 and H1299-LKB1shRNA cells were seeded in 96-well plates and treated with 5 mmol/L 2-DG. Plates were subjected to MTS assay after 48 h. Reactions were conducted in quadruplicate.
Discussion
In our study, we confirmed that LKB1 phosphorylates and activates AMPK in a time- and dose-dependent manner upon 2-DG treatment in NSCLC cells. LKB1 mediates the prolonged activation of AMPK following energy stress, thus serving as a metabolic checkpoint and enabling cell growth to be coupled to the availability of fuel supplies.
Three lines of evidence from our investigation support the role of LKB1/AMPK signaling in 2-DG-mediated mTOR inhibition in lung cancer cells. First, mTOR inactivation was observed only in LKB1 wild-type cells but not in LKB1 inactivated mutant cells. Consistently, stable knockdown of LKB1 led to deficiency in mTOR inactivation. Moreover, inhibition of AMPK by Compound C alleviated mTOR suppression. Therefore, LKB1 deficiency or AMPK inhibition impairs inhibition of mTOR activity under energy stress in lung cancer, defining the negative regulation of mTOR by LKB1/AMPK signaling.
Swinnen et al reported that treatment of various cancer cell lines with the AMPK activator AICAR prevented proliferation21. Our study demonstrates that potent growth inhibition by 2-DG is dependent on LKB1 gene status. Although there are other effectors downstream of LKB1/AMPK signaling, such as activation of p53 and upregulation of p219,10, mechanistically, we propose that the major growth regulatory pathways controlled by LKB1/AMPK are mediated by inhibition of mTOR. While elevated AMPK activity may be inhibitory for tumor cell growth, too little AMPK activity may also be detrimental. It has been reported that LKB1 wild-type cells are more resistant to cell death upon glucose withdrawal than their mutant counterparts22 because they may require AMPK activation to restore intracellular ATP for cell survival under conditions such as oncogenic mutations driving imbalances in energy metabolism.
According to our study, LKB1-expressing NSCLC cells may be capable of switching off the mTOR pathway in response to metabolic stresses that activate AMPK. Thus, if it is possible to induce the activation of AMPK with a drug, proliferation of LKB1-expressing cancer cells might be inhibited. Currently, 2-DG is being investigated in Phase I/II clinical trials. The finding that the mTOR signaling pathway is elevated in LKB1-deficient lung cancer cells under low energy conditions suggests that mTOR inhibitors, such as rapamycin and its analogues, would be effective at inhibiting growth of these cancer cells.
In conclusion, our study demonstrates that in lung cancer cells, LKB1 plays a significant role in energy response by activating AMPK, inhibiting mTOR activity and thereby suppressing cell growth, suggesting the potential utility of AMPK and mTOR as targets for lung cancer therapy.
Author contribution
Dr Dian-sheng ZHONG initiated the project; Li-xia DONG, Lin-lin SUN, Xia ZHANG, and Li PAN designed and performed research; Li-xia DONG, Lin-juan LIAN, and Zhe CHEN analyzed data; Lin-lin SUN and Dian-sheng ZHONG wrote the manuscript.
Acknowledgments
The project was supported by grants from the National Natural Science Foundation of China (No 30971307 and No 81071915) and the Tianjin Natural Science Foundation (No 10JCYBJC13700).
References
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–7. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–53. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- Lim W, Hearle N, Shah B, Murday V, Hodgson SV, Lucassenet A, et al. Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br J Cancer. 2003;89:308–13. doi: 10.1038/sj.bjc.6601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–62. [PubMed] [Google Scholar]
- Matsumoto S, Iwakawa R, Takahash K, Kohno T, Nakanishi Y, Matsuno Y, et al. Prevalence and specificity of LKB1 genetic alteration in lung cancer. Oncogene. 2007;26:1–8. doi: 10.1038/sj.onc.1210418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong DS, Guo LZ, de Aguirre I, Liu XJ, Lamb N, Sun SY, et al. LKB1 mutation in large cell carcinoma of the lung. Lung Cancer. 2006;53:285–94. doi: 10.1016/j.lungcan.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Marcus AI, Zhou W. LKB1 regulated pathways in lung cancer invasion and metastasis. J Thorac Oncol. 2010;5:1883–6. doi: 10.1097/JTO.0b013e3181fbc28a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun L, Zhong D. Advances of LKB1-AMPK-mTOR signaling pathway in tumor. Zhongguo Fei Ai Za Zhi. 2011;14:685–8. doi: 10.3779/j.issn.1009-3419.2011.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Ge G, Ji H. LKB1 in lung cancerigenesis: a serine/threonine kinase as tumor suppressor. Protein Cell. 2011;2:99–107. doi: 10.1007/s13238-011-1021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JL, Byekova Y, Elmets CA, Athar M. Liver kinase B1 (LKB1) in the pathogenesis of epithelial cancers. Cancer Lett. 2011;306:1–9. doi: 10.1016/j.canlet.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. Effects of 2-deoxyglucose on carbohydrate metabolism: review of the literature and studies in the rat. Metabolism. 1962;11:1098–112. [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Keenan KP, Carney JM, Fernandes G, Feuers RJ, Floyd RA, et al. Caloric restriction mimetics: metabolic interventions. J Gerontol A Biol Sci Med Sci. 2001;56:20–33. doi: 10.1093/gerona/56.suppl_1.20. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–8. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Sun LL, Zhong DS, Wu S, Bai H, Chen Z. Establishment and gene expression profiling of LKB1 stable knockdown lung cancer cell line. Chin Med J. 2011;124:2028–32. [PubMed] [Google Scholar]
- Swinnen JV, Beckers A, Brusselmans K, Organe S, Segers J, Timmermans L, et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–8. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- J Carretero, PP Medina, R Blanco, L Smit, M Tang, G Roncador, et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26:1616–25. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]