Abstract
Purpose of review
To summarize the epidemiology of food protein-induced enterocolitis syndrome (FPIES).
Recent findings
FPIES is regarded as a rare non-IgE-mediated gastrointestinal allergic disorder. Older nonpopulation-based studies reported an average of 1–15 cases presenting to allergy clinics a year, but recent studies have reported figures as high as 90 cases a year. The yearly incidence of FPIES in one Australian study was one in 10,000 infants less than 2 years of age. Chronic FPIES typically presents in neonates, whereas acute FPIES is primarily a disorder of young infants. FPIES has a slight male predominance; eczema and a family history of atopy are commonly present at diagnosis; almost one in 10 infants have coexistent IgE food allergies and siblings are rarely affected. There is regional variation in common triggering foods, rates of combined cow milk and soy FPIES and multiple food group FPIES. Understanding of the epidemiology of FPIES is limited by the lack of a universally accepted definition and the publication of few prospective population-based case series.
Summary
FPIES is not as rare as once thought, but how common it is, what factors predispose to its development, and why there is regional variation needs to be addressed by future well designed population-based studies?
Keywords: enterocolitis, epidemiology, food hypersensitivity, food protein-induced enterocolitis syndrome
HISTORICAL ASPECTS
Food protein-induced enterocolitis is regarded as a rare non-IgE-mediated food allergy affecting the gastrointestinal tract. Gryboski [1] published the first case series of food protein enterocolitis to cow milk formula in neonates. Unlike cow milk proctocolitis, infants were unwell with prolonged diarrhea and vomiting after drinking cow milk formula for days to weeks. Frank or occult blood in the stools and anemia were noted in all cases, and half of the infants were failing to thrive on presentation [2,3]. Rectal biopsies showed either a slight infiltrate of lymphocytes/plasma cells or profuse polymorphonuclear cell infiltration, crypt abscess formation and mucosal injury [1].
Almost 10 years later, Powell [2,3] described the same condition following cow milk formula introduction in a smaller cohort, most of whom were neonates. Additionally, she noted some cases had abdominal distension, hypothermia, peripheral blood neutrophilia, and stools frequently contained a mixture of inflammatory cells, including lymphocytes, neutrophils and eosinophils. Both authors reported if reexposure to cow milk formula occurred after a brief period of abstinence, some infants presented acutely with profuse vomiting and diarrhea, cardiovascular collapse and neutrophilia usually 2 h after ingestion [1,3]. Powell [2,3] further recognized many infants with chronic cow milk enterocolitis also had an acute or chronic enterocolitis reaction once exposed to soy formula.
McDonald et al.[4] first coined the term food protein-induced enterocolitis (FPIE), recognizing cow milk/soy protein likely triggered the reaction and that chronic exposure to the food protein was required to cause intestinal injury. Two years later the same authors reported three cases of acute egg enterocolitis in infants with cow milk/soy enterocolitis, but with no prior history of egg ingestion [5]. Some 20 years earlier, Ikola [6] had published a case of an infant with acute rice and wheat enterocolitis, following initial introduction of these foods. These early studies highlighted that acute reactions could occur without previous chronic exposure or enterocolitis and other food proteins apart from cow milk/soy could trigger acute reactions.
Box 1.
no caption available
Sicherer et al.[7] suggested the disorder be called a syndrome, recognizing the disease was characterized by a constellation of shared clinical and laboratory features. Thus, FPIE became FPIES. Two distinct phenotypes of the disorder are recognized, acute and chronic FPIES, with the latter representative of the disorder first reported by Gryboski [1]. The differences between these two clinical phenotypes have been reviewed elsewhere [8]. To date, only cow milk/soy have been documented to cause chronic FPIES [1–3,9,10], whereas a range of foods has been reported to cause acute FPIES.
EPIDEMIOLOGICAL LIMITATIONS
Epidemiology is ‘the study of how often diseases occur in different groups of people and why’ [11]. Despite more than 1000 cases of FPIES being published, many epidemiological aspects of the disorder remain inadequately studied. Drawing firm conclusions from the current epidemiological data is difficult because no universal accepted definition of FPIES exists, with studies often using different criteria, mixing cases of acute/chronic FPIES [9] and subdividing acute FPIES into typical/atypical [7]; published studies are usually case reports or retrospective case series derived from specialized allergy clinics, potentially favoring the reporting of more severe, complex individuals and the majority of published literature is derived from only a handful of countries.
METHODS
In this article, we aim to summarize the epidemiological aspects of FPIES with these limitations in mind. We searched Medline via Ovid with a combination of terms for FPIES including MeSH terms, text words for FPIES and combined this with a sensitive filter for prognostic studies as developed by the SIGN association [12]. We then supplemented this by searching reference lists of previous review articles and Google scholar (to retrieve gray literature).
Studies were included only if a case definition of FPIES was provided and there were a consecutive set of cases presenting to healthcare setting(s). Single case reports and case series that did not fulfil the above criteria were excluded [3–5,13–23]. We examined 19 published series on FPIES, constituting more than 1000 cases (Table 1) [1,7,9,10,22,24–27,28▪▪,29,30▪,31▪,32–34].
Table 1.
Case series examining acute and chronic food protein-induced enterocolitis syndrome
| References | Data on all food triggers | Study design | Definition | Country | No. | Duration (years) | Center | Male (%) | Eczema (%) | FHx atopy (%) | IgE FA (%)a |
| Ruffner et al. [30▪] | Yes | Retrospective | Their own | USA | 462 | 5 | One hospital | 60 | 34 | – | – |
| Frith et al. [25] | Yes | Prospective | Their own | Australia | 90 | 1 | Australia wide | 48 | 46 | 53 | 14 |
| Garcia and Jimenez Diaz [26] | Yes | Retrospective | Sicherer et al. [7] (PC) | Spain | 16 | 12 | Unknown | 63 | – | – | – |
| Jarvinen-Seppo et al. [27] | Yes | Retrospective | Sicherer et al. [7] | USA | 76 | 8 | Single allergy clinic | – | – | – | – |
| Sopo et al. [31▪] | Yes | Retrospective | Their own/Powell [34] | Italy | 66 | 7 | Three allergy clinic | 61 | 9 | 20 | 2 |
| Mehr et al. [29] | Yes | Retrospective | Sicherer et al. [7] | Australia | 35 | 16 | Single allergy clinic | 57 | 51 | – | 11 |
| Fogg et al. [24] | Yes | Prospective | Sicherer et al. [7] | USA | 19 | 1.5 | Single allergy clinic | 53 | 11 | – | – |
| Nowak-Wegrzyn et al. [9]b | Yes | Retrospective | Sicherer et al. [7] | USA | 44 | 5 | Two allergy clinic | 59 | 34 | 77 | – |
| Sicherer et al. [7] | Yes | Retrospective | Their own | USA | 20 | 6 | Single allergy clinic | 44 | 31c | 56c | 15 |
| Katz et al. [28▪▪] | CM only | Prospective | Sicherer et al. [7] | Israel | 44 | 2 | One hospital | 52 | 7 (PC) | – | – |
| Nomura et al. [10]b | CM only | Retrospective | Powell [34] | Japan | 30 | 3 | Japanese database | 50 | – | – | – |
| Hwang et al. [33] | CM/soy only | Prospective | Powell [34] | Korea | 23 | 4 | One hospital | 70 | 0 | – | – |
| Fukuie et al. [32]b | CM/soy/wheat/rice | Not stated | Powell [34] | Japan | 10 | 4 | One hospital | – | 10 | – | – |
| Chung et al. [19] | Not stated | Prospective | Sicherer et al. [7] | Korea | 28 | – | One hospital | – | – | – | – |
| Levy and Danon [22] | Solid food only | Retrospective | Their own | Israel | 6 | 6 | One hospital | 67 | – | – | – |
| Burks et al. [23] | CM/soy | Prospective | Their own | USA | 22 | 1.5 | One hospital | – | – | – | – |
| McDonald et al. [5]b | CM/soy | Prospective | Powell [34] | USA | 10 | – | One hospital | – | – | – | – |
| Powell [3]b | CM/soy | Retrospective | Their own | USA | 9 | – | One hospital | – | – | – | – |
| Gryboski [1]b | CM | Retrospective | None set | USA | 21 | 16 | One hospital | 90 | 14 | 62 | – |
CM, cow milk; FHx, family history; –, not available; PC, personal communication with corresponding author.
aIgE FA = IgE-mediated food allergy (that is positive ssIgE- and IgE-mediated clinical reaction to a separate food protein not causing FPIES).
bIn these series, chronic FPIES or a combination of cases of acute/chronic FPIES was reported.
cData only available for infants with typical FPIES (n = 16).
PREVALENCE AND INCIDENCE OF FOOD PROTEIN-INDUCED ENTEROCOLITIS SYNDROME
Non-IgE-mediated food allergy is not as common as IgE-mediated food allergy [28▪▪]. How common FPIES is remains largely unknown. Older studies report an average of 1–15 cases a year presenting to allergy clinics, whereas recent data suggest the disorder is more common than previously thought, with one center reporting an average of 90 cases a year (Table 1).
Whereas earlier studies focused on chronic FPIES [1–3], the majority of published series now describe the acute phenotype. Chronic FPIES may have become a less common entity, because of improved and earlier recognition by pediatricians that cow milk/soy can induce allergic gastrointestinal reactions in newborns and the availability of hydrolyzed formulas for treatment of such presentations.
Based on cases presenting to pediatric allergy outpatients, the yearly prevalence of FPIES in two series was approximately 1% [30▪,31▪]. Katz et al.[28▪▪] were the first to perform a population-based case study. The prevalence of cow milk FPIES over a 2-year period in this Israeli population was 0.34%. Cow milk FPIES would, therefore, not be regarded as rare based on prevalence definitions of rare disease used in the USA (one in 1500 individuals) and Europe (one in 2000 individuals) [35]. Other studies have reported an increase in FPIES cases presenting to allergy clinics over the past 2 decades [29,31▪]. Whether this is due to a true increase in disease burden or improved recognition of the disorder by physicians/community remains unclear.
A prospective, population-based case series on FPIES in Australia is being conducted through a national rare disease register: the Australian Paediatric Surveillance Unit (APSU). Australian general and subspecialist pediatricians (e.g. allergists, gastroenterologists, others) report new cases of FPIES on a monthly basis. Preliminary information from the first 12 months of the study (January–December 2012) is available in abstract form [25]. The incidence of FPIES to all food triggers was one in 10,000 infants less than 2 years of age, which closely corresponds to the Australian reported incidence of eosinophilic esophagitis in children of 0.9 per 10,000 [36]. Although approximately 1400 pediatricians report on a monthly basis to the APSU, this incidence is likely to be an underestimate. Not all pediatricians report to the APSU; reporting of cases is voluntary and some cases may never be referred to a pediatrician or may remain undiagnosed.
An international classification of diseases code for FPIES will be introduced in 2015. This will assist in the study of FPIES presentations to hospitals. However, almost half of cases of FPIES do not attend a hospital and misdiagnosis of FPIES is frequent in emergency departments [29]. Miscoding of cases will invariably occur. Nevertheless, the code will give future studies the ability to link population-based data with emergency presentations to improve case ascertainment.
GENDER AND AGE OF ONSET
Most case series report only a slight male predominance (50–60%; Table 1). FPIES reactions to the offending food protein in breast milk are rare [10,37,38], suggesting direct oral ingestion of larger quantities of food proteins are required to cause a reaction in the majority of individuals. The age of onset of FPIES, therefore, appears to be influenced by when food proteins are directly introduced into an infant's diet.
Chronic FPIES is essentially a disorder of newborns following the introduction and repeated exposure to cow milk or soy protein formulas. The median age of onset of chronic cow milk enterocolitis reported by Powell [3] and Gryobksi [1] was 1 week (range 4 days–3 weeks) to 4 weeks (range 3 days–4 months), respectively.
Acute FPIES often presents in infants between 4 and 6 months of age, and in approximately 75% of cases reactions occur after their first or second known ingestion of the causative food [29]. This 4–6-month window of peak presentations corresponds to the age when solids are most commonly introduced [39]. Not surprisingly, those with cow milk and soy FPIES present earlier than those with solid food FPIES (Table 2), given such formulas are introduced earlier than solids. Infants with acute FPIES to rice/oats present at a younger age compared with those with FPIES to wheat, fish or egg (Table 2). This again likely reflects feeding practices, with rice cereals being among the commonest weaning foods in westernized countries, such as Australia and the USA, whereas foods such as wheat, fish and eggs tend to be introduced later [40–42]. Based on recent data, chicken FPIES occurred earlier in an Australian study compared with a large USA case series (Table 2). Chicken is recommended in Australian feeding guidelines to be tried as one of the first solid foods [43], whereas in one USA study [40], only 28% of infants had eaten chicken by 6 months of age.
Table 2.
Onset of food protein-induced enterocolitis syndrome in months.a
| Study | All triggers | Cow milk and soy | Solids | Individual solids |
| Ruffner et al. [30▪] (USA) | – | 7 (0.7) | 12.1 (1.1) | Rice 7.4 (5.1) |
| Oats 9.3 (6.2) | ||||
| Egg 11.3 (9.6) | ||||
| Wheat 11.9 (9.5) | ||||
| Chicken 17.6 (12.3) | ||||
| Nowak-Wegrzyn et al. [9] (USA) | – | 1 (2 days–12 months)a | 5.5 (3–7 months) | – |
| Frith et al. [25] (Australia; unpublished data) | 6.2 (3.4) | 3.8 (2.6) | 7.5 (3.3) | Rice 6.3 (3.3) |
| Chicken 6.7 (1.3) | ||||
| Oats 7.1 (1.4) | ||||
| Wheat 7.5 (0.7) | ||||
| Fruits 7.8 (3.0) | ||||
| Vegetables 9 (2.8) | ||||
| Egg 8.4 (2.1) | ||||
| Fish 10.9 (3.6) | ||||
| Mehr et al. [29] (Australia) | 5.6 (2.7) | 4.9 (2.6) | 6.1 (1.7) | Rice 5.2 (0.8)b |
| Oat 5.7 [1] | ||||
| Sopo et al. [31▪] (Italy) | 5.7 (5.1) | 3.5 (2.4)c | 10.6 (6.7) | – |
–, not available.
aData presented from studies in which age of onset recorded and triggers included to cow milk, soy and solid foods. Data presented as either mean onset of age (standard deviation) or median onset of age (age range).
bData for other solid foods not presented as <3 cases per solid food.
cFigure relates to cow milk FPIES only. The authors included three children with soy FPIES with other foods.
Although FPIES is recognized primarily as a pediatric condition, a case of adult-onset FPIES has been reported to molluscs [44]. It is likely other cases of adult-onset FPIES exist, but have not been reported.
ATOPY
FPIES is pathophysiologically a non-IgE-mediated disorder, but atopic disease and a family history of atopy are frequently present (Table 1). Eczema is the most common atopic disorder at diagnosis. Rates of eczema and family history of atopy vary between studies, with higher rates often reported in studies from the USA and Australia compared with those from Israel and Italy (Table 1). Asthma, allergic rhinitis and eosinophilic esophagitis are less frequent, most likely reflecting the later onset of these conditions [29,30▪].
Coexistent IgE-mediated food allergies have also been reported in some series (2–15% cases; Table 1). Unlike the phenomenon described by Sicherer et al.[7], in which infants with atypical FPIES have IgE sensitization to the triggering food, in these cases infants have FPIES to one food (e.g. rice) but IgE sensitization and a history of immediate IgE reaction to a separate food (e.g. cow milk) [29]. This combination of non-IgE gut allergy and IgE-mediated food allergy has also been recognized in children with eosinophilic esophagitis [45].
SIBLINGS
There have only been three reports of siblings with FPIES. All were twins (two sets of fraternal and one identical twins) [9,46]. In all cases, the twins reacted to the same food protein (soy in one set, cow milk in the other two). There are no reports of parents of affected infants having had childhood or adult-onset FPIES. This is in contrast to eosinophilic esophagitis, in which there is a strong familial association in both parents and siblings [47,48].
RISK FACTORS
Katz et al.[28▪▪] reported those with cow milk FPIES were more likely to be delivered by cesarean section (C/S; relative risk 2, P = 0.0023) than those born by vaginal delivery. The same authors reported Jewish mothers were more likely to have babies with FPIES than non-Jewish mothers; however, Jewish mothers were also more likely to have a C/S (personal communication, Katz). Delivery by C/S can disrupt microbiota composition, and such flora has been associated with eczema predisposition [49]. These findings by Katz et al.[28▪▪] need to be confirmed by others, and stool microbiota studies in infants with FPIES are required. We speculate that intestinal microbiota colonization, modified by factors such as birth mode and feeding practices, may be important in promoting FPIES development. No other risk factors for FPIES have been studied or described, particularly other life-style changes associated with an altered gut microbiota milieu, such as antibiotic use or birth order.
TRIGGERS
Like IgE-mediated food allergy, the bulk of reactions in FPIES are caused by a restricted number of foods. The majority of FPIES reactions are caused by cow milk, soy, rice/oats and egg [7,9,24,25,29,30▪,31▪].
Cow milk and grains are among the commonest causes of acute FPIES; however, geographic differences exist (Fig. 1). Cow milk is the most frequent cause of FPIES in series from the USA [7,9,24,27,30▪] and Italy/Spain [26,31▪]. Soy is a common trigger in the USA but uncommon in Australia [25,29], Israel [28▪▪] and Italy/Spain [26,31▪] (Fig. 1). Combined soy and cow milk FPIES (Table 3) and FPIES to multiple food triggers (Fig. 2) are much more commonly reported in the USA than other countries.
FIGURE 1.
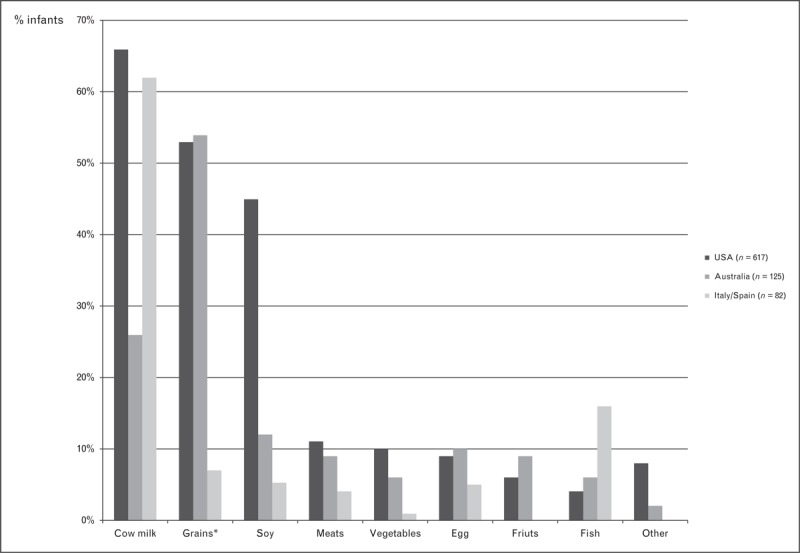
Causative triggers of food protein-induced enterocolitis syndrome in infants (%). Data derived from case series from the USA [7,9,24,27,30▪], Australia [25,29] and Italy/Spain [31▪]. Only case series examining acute FPIES reactions to all foods included. Cases from Italy (n = 66) and Spain (n = 16) combined. Some children had FPIES to more than one food trigger. ∗Rice was the commonest grain trigger (Australia 44%, USA 19% and Italy/Spain 4%), followed by oats (Australia 7%, USA 16% and Italy/Spain 0%).
Table 3.
Infants with cow milk food protein-induced enterocolitis syndrome also reacting to soy protein
| Cow milk FPIES (n) | Combined cow milk and soy FPIES (n) | Percentage of cow milk FPIES reacting to soy | |
| Australia | |||
| Frith et al. [25] | 25 | 2 | 10 |
| Mehr et al. [29] | 7 | 0 | 0 |
| Israel | |||
| Katz et al. [28▪▪] | 44 | 0 | 0 |
| Italy/Spain | |||
| Garcia and Jimenez Diaz [26] | 7 | 0 | 0 |
| Sopo et al. [31▪] | 44 | 0 | 0 |
| Korea | |||
| Hwang et al. [33] | 15 | 4 | 21 |
| USA | |||
| Ruffner et al. [30▪] | 310 | 135 | 44 |
| Jarvinen-Seppo et al. [27] | 44 | 20 | 45 |
| Fogg et al. [24] | 13 | 9 | 69 |
| Nowak-Wegrzyn et al. [9] | 29 | 15 | 52 |
| Sicherer et al. [7] | 13 | 8 | 62 |
| Burks et al. [23] | 4 | 6 | 60 |
| Powell [3] | 8 | 5 | 63 |
| Gryboski [1] | 21 | 3 | 14 |
Data included from studies examining FPIES to cow milk/soy. FPIES, food protein-induced enterocolitis syndrome.
FIGURE 2.
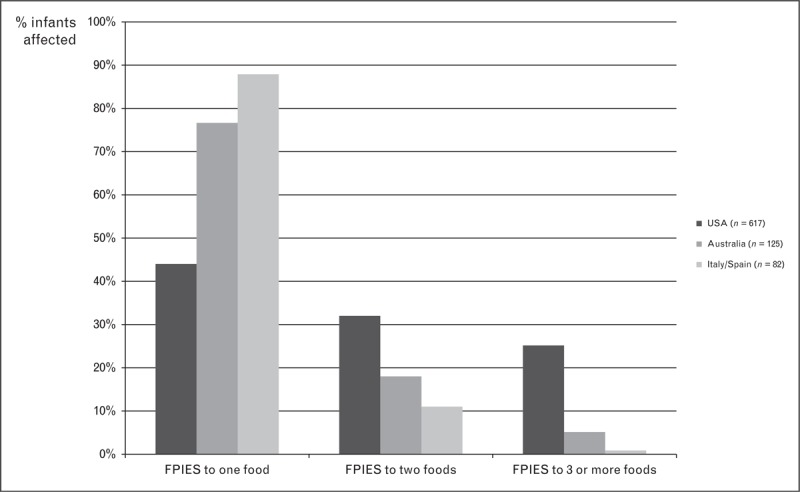
Infants with food protein-induced enterocolitis syndrome to single or multiple different food triggers. Data derived from case series from the USA [7,9,24,27,30▪], Australia [25,29] and Italy/Spain [31▪]. Only case series examining acute FPIES reactions to all foods included. Cases from Italy (n = 66) and Spain (n = 16) combined.
Grains (and, in particular, rice) are the most common solid food triggers in both the USA [7,9,24,27,30▪] and Australia [25,29], but in Australia rice is the most common FPIES trigger overall, causing more reactions than cow milk (Fig. 1). The frequency of other solid food triggers is similar between these two countries. In contrast, fish is the commonest cause of solid food FPIES in Italy/Spain [26,31▪], but relatively uncommon cause in the USA [7,9,24,27,30▪] and Australia [25,29] (Fig. 1).
This variation in FPIES triggers between countries may in part be due to differences in the rates of breast-feeding, frequency of cow milk/soy formula use, populations included in the case series, intestinal microbiota composition and when and what food proteins are introduced into an infant's diet. Based on 2010 data [39], Australia had higher rates of breast-feeding initiation (96%), and continuation at 6 months (60%), compared with the 2010 USA breast-feeding data [50] (75% initiation and 43% continuation at 6 months). By 3 months of age, 46% of Australian infants had received a nonhuman milk formula, compared with 60% in the USA. As most cow milk FPIES occurs in infants less than 3 months of age, the higher rates of cow milk FPIES in the USA compared with Australia may in part be due to the combination of shorter duration of breast-feeding and more frequent use of cow milk formulas.
Soy formulas are also much more commonly used in the USA compared with Australia and Europe. In the USA, 10% of infants at 3 months of age had been fed soy formula [51]. In contrast, only 3% of Australian infants less than 24 months had ever been exposed to soy formulas/milks [39]. Australian [52] and European [53] feeding guidelines do not recommend the use of soy formula under 6 months, whereas American guidelines make no such restrictions [54].
Grain cereals, such as rice, are common weaning foods in both the USA and Australia, and fish is uncommonly introduced into the diets of young infants [40,51], but is commonly eaten by Italian infants [55]. Rice is the commonest weaning solid food in the United Kingdom, Japan and China [42,56], but whether FPIES to rice is common and a more frequent cause than other foods has not been studied
Differences in dietary behaviors cannot completely explain regional differences in food triggers, highlighting a role for other factors. Rice is a common weaning food in Italy [56], and yet in the retrospective case series of Sopo et al.[31▪], rice constituted only 4% of cases of acute FPIES. Italy has similar rates of breast-feeding and formula use as Australia [55], but the frequency of cow milk FPIES is similar to the USA. Katz et al.[28▪▪] exposed 40 of the 44 infants with cow milk FPIES to soy; however, none reacted. It is likely that the combination of genetic, coexistent atopic disease, intestinal microbiota, breast-feeding and dietary practices may all be important in influencing these regional differences.
CONCLUSION
FPIES can no longer be regarded as a rare disorder. Some aspects of its epidemiology are well documented, such as its occurrence predominantly in infants, slight male predominance, lack of sibling concordance and common food triggers. However, most of these data are derived from retrospective case note studies from allergy clinics and a different picture may emerge from population-based cohorts. The best way to understand the epidemiologically of FPIES is by the establishment of well designed population-based registries/studies. International collaboration is required to establish an agreed definition of FPIES, and this will allow sharing and direct comparison of country-specific data. These steps will improve the understanding of FPIES and will provide insights that are required for those who study, advocate for and manage patients with FPIES.
Acknowledgements
None.
Conflicts of interest
The authors received funding from Nestle and the Allergy Fund at the Children's Hospital at Westmead to perform the Australian FPIES Surveillance study.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Gryboski JD. Gastrointestinal milk allergy in infants. Pediatrics 1967; 40:354–362 [PubMed] [Google Scholar]
- 2.Powell GK. Enterocolitis in low-birth-weight infants associated with milk and soy protein intolerance. J Pediatr 1976; 88:840–844 [DOI] [PubMed] [Google Scholar]
- 3.Powell GK. Milk- and soy-induced enterocolitis of infancy. Clinical features and standardization of challenge. J Pediatr 1978; 93:553–560 [DOI] [PubMed] [Google Scholar]
- 4.McDonald PJ, Powell GK, Goldblum RM. Serum D-xylose absorption tests: reproducibility and diagnostic usefulness in food-induced enterocolitis. J Pediatr Gastroenterol Nutr 1982; 1:533–536 [PubMed] [Google Scholar]
- 5.McDonald PJ, Goldblum RM, Van Sickle GJ, Powell GK. Food protein-induced enterocolitis: altered antibody response to ingested antigen. Pediatr Res 1984; 18:751–755 [DOI] [PubMed] [Google Scholar]
- 6.Ikola RA. Severe intestinal reaction following ingestion of rice. Am J Dis Child 1963; 105:281–284 [DOI] [PubMed] [Google Scholar]
- 7.Sicherer SH, Eigenmann PA, Sampson HA. Clinical features of food protein-induced enterocolitis syndrome. J Pediatr 1998; 133:214–219 [DOI] [PubMed] [Google Scholar]
- 8.Nowak-Wegrzyn A, Sicherer S, TePas E. Food protein-induced enterocolitis syndrome. UpToDate 2013; http://www.uptodate.com/contents/food-protein-induced-enterocolitis-syndrome-fpieshttp://www.uptodate.com/contents/food-protein-induced-enterocolitis-syndrome-fpies. [Accessed 2 February 2014]. Published 28 August 2013 [Google Scholar]
- 9.Nowak-Wegrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics 2003; 111 (4 Pt 1):829–835 [DOI] [PubMed] [Google Scholar]
- 10.Nomura I, Morita H, Hosokawa S, et al. Four distinct subtypes of non-IgE-mediated gastrointestinal food allergies in neonates and infants, distinguished by their initial symptoms. J Allergy Clin Immunol 2011; 127:685–688e1-8 [DOI] [PubMed] [Google Scholar]
- 11.Coggon D, Rose G, Barker DJP. Epidemiology for the uninitiated. BMJ 2014; http://www.bmj.com/about-bmj/resources-readers/publications/epidemiology-uninitiatedhttp://www.bmj.com/about-bmj/resources-readers/publications/epidemiology-uninitiated. [Accessed: 2 February 2014] [Google Scholar]
- 12.Improvement SIGNH. Search Filters, 26 April 2013. http://www.sign.ac.uk/methodology/filters.html [Accessed 2 February 2014] [Google Scholar]
- 13.Murray KF, Christie DL. Dietary protein intolerance in infants with transient methemoglobinemia and diarrhea. J Pediatr 1993; 122:90–92 [DOI] [PubMed] [Google Scholar]
- 14.Hojsak I, Kljaic-Turkalj M, Misak Z, Kolacek S. Rice protein-induced enterocolitis syndrome. Clin Nutr 2006; 25:533–536 [DOI] [PubMed] [Google Scholar]
- 15.Zapatero Remon L, Alonso Lebrero E, Martin Fernandez E, et al. Food-protein-induced enterocolitis syndrome caused by fish. Allergol Immunopathol (Madr) 2005; 33:312–316 [DOI] [PubMed] [Google Scholar]
- 16.Cavataio F, Carroccio A, Montalto G, Iacono G. Isolated rice intolerance: clinical and immunologic characteristics in four infants. J Pediatr 1996; 128:558–560 [DOI] [PubMed] [Google Scholar]
- 17.Beauchamp JN, Gaboury I, Ni A, et al. Solid-food introduction in infants diagnosed as having a cow's-milk protein-induced enterocolitis. J Pediatr Gastroenterol Nutr 2011; 52:639–643 [DOI] [PubMed] [Google Scholar]
- 18.Powell GK, McDonald PJ, Van Sickle GJ, Goldblum RM. Absorption of food protein antigen in infants with food protein-induced enterocolitis. Dig Dis Sci 1989; 34:781–788 [DOI] [PubMed] [Google Scholar]
- 19.Chung HL, Hwang JB, Park JJ, Kim SG. Expression of transforming growth factor beta1, transforming growth factor type I and II receptors, and TNF-alpha in the mucosa of the small intestine in infants with food protein-induced enterocolitis syndrome. J Allergy Clin Immunol 2002; 109:150–154 [DOI] [PubMed] [Google Scholar]
- 20.Jarvinen KM, Caubet JC, Sickles L, et al. Poor utility of atopy patch test in predicting tolerance development in food protein-induced enterocolitis syndrome. Ann Allergy Asthma Immunol 2012; 109:221–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shek LP, Bardina L, Castro R, et al. Humoral and cellular responses to cow milk proteins in patients with milk-induced IgE-mediated and non-IgE-mediated disorders. Allergy 2005; 60:912–919 [DOI] [PubMed] [Google Scholar]
- 22.Levy Y, Danon YL. Food protein-induced enterocolitis syndrome – not only due to cow's milk and soy. Pediatr Allergy Immunol 2003; 14:325–329 [DOI] [PubMed] [Google Scholar]
- 23.Burks AW, Casteel HB, Fiedorek SC, et al. Prospective oral food challenge study of two soybean protein isolates in patients with possible milk or soy protein enterocolitis. Pediatr Allergy Immunol 1994; 5:40–45 [DOI] [PubMed] [Google Scholar]
- 24.Fogg MI, Brown-Whitehorn TA, Pawlowski NA, Spergel JM. Atopy patch test for the diagnosis of food protein-induced enterocolitis syndrome. Pediatr Allergy Immunol 2006; 17:351–355 [DOI] [PubMed] [Google Scholar]
- 25.Frith C, Joshi P, Campbell D, et al. The first 12 months of FPIES surveillance in Australia. Intern Med J 2013; 43 (S4):1–21 [Google Scholar]
- 26.Garcia MR, Jimenez Diaz F. Food protein-induced enterocolitis syndrome (FPIES): our experience. J Allergy Clin Immunol 2012; 129 (2 Suppl):AB34 [Google Scholar]
- 27.Jarvinen-Seppo KM, Sickles L, Nowak-Wegrzyn AH. Clinical characteristics of children with food protein-induced enterocolitis (FPIES). J Allergy Clin Immunol 2010; 125 Suppl 1: AB85 [Google Scholar]
- 28▪▪.Katz Y, Goldberg MR, Rajuan N, et al. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow's milk: a large-scale, prospective population-based study. J Allergy Clin Immunol 2011; 127:647–653e1-3 [DOI] [PubMed] [Google Scholar]; Currently the only published prospective population-based case series on FPIES, focusing on reactions to cow milk. The study demonstrated FPIES is not rare as once thought; no infants who had soy formula reacted and in a proportion of cases transformation of non-IgE cow milk FPIES into an IgE-mediated cow milk allergy occurred.
- 29.Mehr S, Kakakios A, Frith K, Kemp AS. Food protein-induced enterocolitis syndrome: 16-year experience. Pediatrics 2009; 123:e459–e464 [DOI] [PubMed] [Google Scholar]
- 30▪.Ruffner M, Ruymann K, Barni S, et al. Food Protein-induced enterocolitis syndrome: insights from review of a large referral population. J Allergy Clin Immunol Pract 2013; 1:343–349 [DOI] [PubMed] [Google Scholar]; The largest published case series of FPIES. This USA retrospective study also found FPIES is not rare, egg is a common cause of FPIES, and combined reactions to cow milk/soy and multiple food triggers frequently occurred in this cohort.
- 31▪.Sopo SM, Giorgio V, Dello Iacono I, et al. A multicentre retrospective study of 66 Italian children with food protein-induced enterocolitis syndrome: different management for different phenotypes. Clin Exp Allergy 2012; 42:1257–1265 [DOI] [PubMed] [Google Scholar]; First published FPIES series from Italy, demonstrating, that unlike the USA/Australia, rice-induced reactions were rarely reported.
- 32.Fukuie FT, Nomura I, Nakatani K, et al. Food protein induced entercolitis syndrome in neonate: summary of 10 patients. J Allergy Clin Immunol 2008; 121 (Issue 2 Suppl 1):S105 [Google Scholar]
- 33.Hwang JB, Sohn SM, Kim AS. Prospective follow-up oral food challenge in food protein-induced enterocolitis syndrome. Arch Dis Child 2009; 94:425–428 [DOI] [PubMed] [Google Scholar]
- 34.Powell GK. Food protein-induced enterocolitis of infancy: differential diagnosis and management. Compr Ther 1986; 12:28–37 [PubMed] [Google Scholar]
- 35.Rare Disease. Wikepedia. http://en.wikipedia.org/wiki/Rare_disease#cite_note-RDA2002-2 [Accessed 2 February 2014] [Google Scholar]
- 36.Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child 2006; 91:1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monti G, Castagno E, Liguori SA, et al. Food protein-induced enterocolitis syndrome by cow's milk proteins passed through breast milk. J Allergy Clin Immunol 2011; 127:679–680 [DOI] [PubMed] [Google Scholar]
- 38.Tan J, Campbell D, Mehr S. Food protein-induced enterocolitis syndrome in an exclusively breast-fed infant-an uncommon entity. J Allergy Clin Immunol 2012; 129:873. [DOI] [PubMed] [Google Scholar]
- 39.Australian Institute of Health and Welfare Canberra 2010. Australian National Infant Feeding Survey. Indicator results. 2011. http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=10737420925 [Accessed 2 February 2014] [Google Scholar]
- 40.Grummer-Strawn LM, Scanlon KS, Fein SB. Infant feeding and feeding transitions during the first year of life. Pediatrics 2008; 122 Suppl 2:S36–S42 [DOI] [PubMed] [Google Scholar]
- 41.Gabriel R, Pollard G, Suleman G, et al. Infant and child nutrition in Queensland 2003. August 2005. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.180.2298&rep=rep1&type=pdf [Accessed 2 February 2014] [Google Scholar]
- 42.Inoue M, Binns CW. Introducing solid foods to infants in the Asia pacific region. Nutrients 2014; 6:276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Health and Medical Research Council. Eat for Health Infant Feeding Guidelines Summary. 2013. http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/n56b_infant_feeding_guideline_summary.pdf [Accessed 2 February 2014] [Google Scholar]
- 44.Fernandes BN, Boyle RJ, Gore C, et al. Food protein-induced enterocolitis syndrome can occur in adults. J Allergy Clin Immunol 2012; 130:1199–1200 [DOI] [PubMed] [Google Scholar]
- 45.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med 2004; 351:940–941 [DOI] [PubMed] [Google Scholar]
- 46.Shoda T, Isozaki A, Kawano Y. Food protein-induced gastrointestinal syndromes in identical and fraternal twins. Allergol Int 2011; 60:103–108 [DOI] [PubMed] [Google Scholar]
- 47.Blanchard C, Rothenberg ME. Basic pathogenesis of eosinophilic esophagitis. Gastrointest Endosc Clin N Am 2008; 18:133–143x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malerba G, Lauciello MC, Scherpbier T, et al. Linkage analysis of chromosome 12 markers in Italian families with atopic asthmatic children. Am J Respir Crit Care Med 2000; 162 (4 Pt 1):1587–1590 [DOI] [PubMed] [Google Scholar]
- 49.Penders J, Gerhold K, Stobberingh EE, et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol 2013; 132:601–607e8 [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. Breastfeeding Report Card – United States, 2010. August 2010. http://www.cdc.gov/breastfeeding/pdf/BreastfeedingReportCard2010.pdf [Accessed 2 February 2014] [Google Scholar]
- 51.Centers for Disease Control and Prevention. Infant Feeding Practices Study II. Chapter 3. Infant feeding. 2005. http://www.cdc.gov/ifps/pdfs/data/IFPS2_tables_ch3.pdf [Accessed 2 February 2014] [Google Scholar]
- 52.Kemp AS, Hill DJ, Allen KJ, et al. Guidelines for the use of infant formulas to treat cows milk protein allergy: an Australian consensus panel opinion. Med J Aust 2008; 188:109–112 [DOI] [PubMed] [Google Scholar]
- 53.Agostoni C, Axelsson I, Goulet O, et al. Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2006; 42:352–361 [DOI] [PubMed] [Google Scholar]
- 54.American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000; 106 (2 Pt 1):346–349 [PubMed] [Google Scholar]
- 55.Giovannini M, Riva E, Banderali G, et al. Feeding practices of infants through the first year of life in Italy. Acta Paediatr 2004; 93:492–497 [DOI] [PubMed] [Google Scholar]
- 56.Caroli M, Mele RM, Tomaselli MA, et al. Complementary feeding patterns in Europe with a special focus on Italy. Nutr Metab Cardiovasc Dis 2012; 22:813–818 [DOI] [PubMed] [Google Scholar]


