Abstract
Purpose of review
Because of the paucity of reports and variability in the diagnostic criteria utilized, little is known regarding the natural outcome of patients with food protein-induced enterocolitis syndrome (FPIES). Data extracted from referenced manuscripts, as well as allergists’ unpublished observations from across the globe, were used to form a cohesive opinion regarding its natural outcome.
Recent findings
All authors concur that there is a generally high rate of recovery for FPIES. The most common foods causing FPIES are milk and soy. Depending upon which study is analyzed, by the age of 3–5 years, approximately 90% of patients recover from their disease. Recovery from FPIES to solid foods, occurs at a later age, but may reflect a later stage of introduction of the food into the diet. An important clinical outcome, although not common, is a shift from FPIES food hypersensitivity to an IgE-mediated food allergy. This necessitates a change in the oral food challenge protocol, if IgE-mediated sensitization is detected.
Summary
Over the past several years, there has been an increasing awareness of FPIES. This knowledge should lead to a more timely diagnosis and should reassure parents and practitioners alike regarding its favorable course.
Keywords: food hypersensitivity, food protein-induced enterocolitis syndrome, repetitive vomiting
INTRODUCTION
The first published descriptions of the food protein-induced enterocolitis syndrome (FPIES) were reported by Rubin [1] and Gryboski [2] respectively. It was not only until 19 years later that diagnostic criteria were suggested for FPIES [3]. These criteria were subsequently modified by Sicherer [4]. Although there were disagreements, historically, regarding the diagnostic criteria [5,6], most authors currently agree on the definition of this disease [6,7▪,8▪▪,9,10▪▪].
In addition to the variable definitions of FPIES, the difficulty in establishing criteria for the disease was hindered by the paucity of reports. Recently, however, several sizable series have been reported from across the globe, including Korea [11], Australia [9], Israel [10▪▪], Italy [12▪] and a very large group from the United States [8▪▪]. These reports have enabled us to summarize the natural history of this syndrome.
CLINICAL MANIFESTATIONS
FPIES is a non-IgE mediated reaction to food, manifested primarily in the gastrointestinal system. For the purpose of this review, the definition of FPIES utilized is repetitive vomiting usually with lethargy or pallor that appears 30–240 min after the offending food. In addition, by definition, other IgE-mediated associated symptoms, such as rash, urticaria and respiratory symptoms are absent. These criteria were adopted by Ruffner et al.[8▪▪], and are in accordance with the two most recent reviews in the field [6,7▪]. Although some authors describe a condition defined as chronic FPIES [13–15], the literature regarding the outcome of this entity is almost nonexistent and as such we have not included this entity in our review.
Box 1.
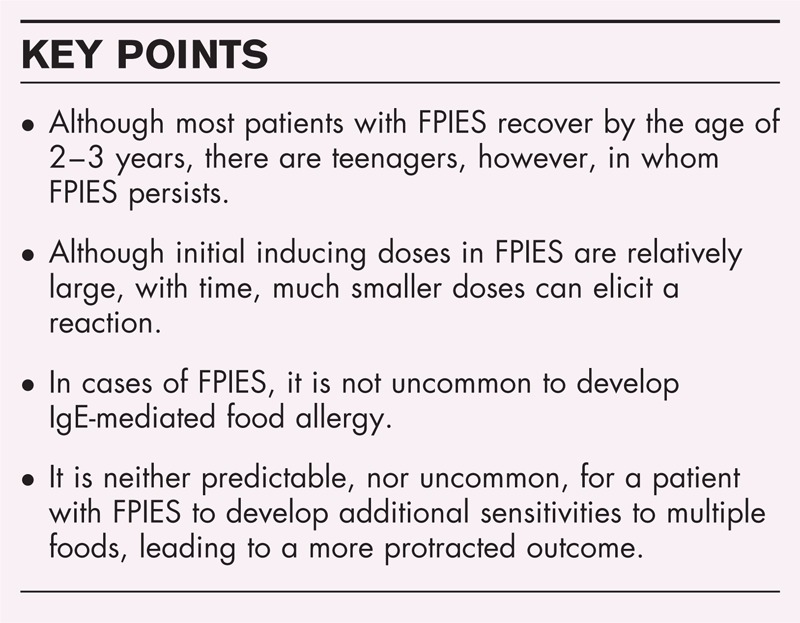
no caption available
The most common reported foods to cause FPIES are cow's milk (Table 1) [4,8▪▪,9,10▪▪,11,12▪,13–25,26▪▪] and soy (Table 2) [4,8▪▪,11,12▪,14,16,18,27–30]. The exception is Australia in which the food most likely to elicit a FPIES reaction was rice (Table 3) [4,8▪▪,9,12▪,13,15,18,24,26▪▪,31–37]. It is unclear whether Australia is unique because of the particular feeding habits or for some other unknown reason. It is interesting to note, however, that in response to a survey, six out of eight of the cases reported from Singapore, a country where early rice feeding is common, were to milk, as well (E. Thame and B.H. Lee, personal communication). However, the list of foods causing FPIES continues to grow. Included are foods that commonly cause IgE-mediated food allergy, such as egg (Table 4) [8▪▪,12▪,18,26▪▪,38,39], peanut (Table 5) [8▪▪,26▪▪] and seafood (Table 6) [8▪▪,9,12▪,13,36,40,41]. But in addition, the list contains many foods that are not commonly reported to induce IgE-mediated reactions such as grains (Table 7) [8▪▪,9,12▪,16–18,26▪▪,36,42], fruits (Table 8) [8▪▪,9,12▪,40,43–45], vegetables and legumes (Table 9) [4,8▪▪,9,12▪,16,28,37,46,47] and poultry and meat (Table 10) [3,4,8▪▪,9,12▪,13,16,28,48].
Table 1.
Reported cases of milk-food protein-induced enterocolitis syndrome
| Year | Ref. no. | No. of cases | Symptoms | Age onset (m) | Outcome (a = accidental, c = challenge) | No. of other food FPIES | No. of PD | Remarks | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | |||||||
| 1982 | [13] | 1 | 1 | 1 | 1 | 1.25 | 14 | NA | Ch(1) | 0 | Chronic CM? |
| 1993 | [14] | 1 | + | − | + | 1 | NA | NA | S(1) | 0 | Methemoglobinemia, fever |
| 1998 | [4] | 13 | 13 | 4 | 9 | 0.25–5 | 6(6–25) | 7(12–84) | S(5) | NA | 5 methemoglobinemia. 1 reacted to EHCF, 2 atypical |
| 2000 | [15] | 1 | + | + | + | 0.5 | NA | 72 | NA | Tolerated soy | |
| 2003 | [16] | 4 | 4 | 2 | 3 | 0.1–6 | 28 (a,c), | 25, 16? 19m | O (3) S(3) Ba(2), R(3) | 1 | Most with FPIES to multiple foods; 3 had chronic FPIES |
| 2005 | [17] | 1 | + | + | ± | 3 | NA | 30 | 3 | Tolerated soy; sepsis treatment | |
| 2006 | [18] | 8 | 7 (>1in 6) | NA | 6 | 0.5–4 | NA | NA | R+S(1) | NA | |
| 2007 | [19] | 1 | + | + | + | 8 | 24 | 9 | None | 1 | Sepsis work up |
| 2009 | [11] | 23 | + | + | 33% | 0.5–2 | 100% 20 m | NA | R(1), Be, E (1), F+ shellfish (1) | NA | Hypotension (11%), diarrhea later 3/23 methemoglobinemia |
| 2009 | [9] | 7 | 7 | 4 | 2 | 4.1 ± 1.8 | Most by 24 m | None | 2 | ||
| 2010 | [20] | 1 | − | + | + | 48 | NA | NA | NA | NA | Gastrostomy, hypotension |
| 2010 | [21] | 1 | + | + | − | 1 day | NA | NA | NA | NA | Very delayed diagnosis |
| 2011 | [10▪▪] | 44 | 44 | 40 | 11 | 0.1–6 | 90% by 36 | 1 at 44 | NA | NA | Study restricted to CMP |
| 2012 | [22] | 1 | + | + | − | 2 | NA | 5 | None | 3 | Breast fed, chronic FPIES |
| 2012 | [12▪] | 44 | 98% | 80% | 54% | 3.5 ± 2.4 | 24 ± 8 | B(2), F(3), P(3), R(2), W(2) | 2.7 | 10/44 with multiple food FPIES | |
| 2011 | [23] | 2 | + | + | + | 1.1, 8 | Converted to IgE | ||||
| 2013 | [24] | 1 | + | + | + | 4 | NA | NA | R | NA | |
| 2013 | [25] | 1 | + | + | + | 3 | NA | 3(c) | NA | 0 | Started with IgE, lost SPT |
| 2013 | [26▪▪] | 1 | + | + | − | NA | NA | 12y(c) | NA | NA | Ondansetron |
| 2013 | [8▪▪] | 310 | All* | All* | ∼ 50% | 6.3 ± 0.7 | 32 ± 24.1 | 80% by 5 | >50% to 2 or more | NA | 2 teenagers with persistent FPIES. *Diagnostic criteria |
B, banana; Ba, barley; Be, beef; Ch, chicken; D, diarrhea; E, egg; EHCF, extensively hydrolyzed cow's milk formula; F, fish; FPIES; food protein-induced enterocolitis syndrome; L/P, lethargy or pallor; NA, not available; O, oat; P, poultry; PD, prediagnosis episodes; R, rice; Rep V, repetitive vomiting; S, soy; T, turkey; To, tomato; W, wheat.
Table 2.
Reported cases of soy-food protein-induced enterocolitis syndrome
| Year | Ref. no. | No. of cases | Symptoms | Age onset (m) | Outcome (a = accidental, c = challenge) | Other food FPIES (n) | No. of PD | Remarks | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | |||||||
| 1993 | [14] | 2 | 2 | 2 | 2 | 2, 1.5 | NA | NA | CM (1) | 0 | 1+ milk, 2 methemoglobinemia |
| 1998 | [4] | 14 | 13 | 2 | 11 | 0.25–7 | 2 (24–36) | 9 + 3 lost to FU | CM(7), P(1) | NA | |
| 1998 | [27] | 7 | 7 | 6 | 6 | 2–5 | 12–24 in 5, 2 NA | NA | None | NA | |
| 2003 | [16] | 7 | 7 | 3 | 4 | 0.5–6 | 2 (24–31) | 5 (16–63) | R(6), CM(3), O(5), Ba(1), SB(1), [1+ CM, O, R, B, SB} | 1–3 | 7/7 multiple food FPIES; 1/7 with chronic FPIES |
| 2003 | [28] | 2 | 2 | 1 | 1 | 1–2 | 0 | 24, 90, one 19 years (Pt #1)(a) | P,T(1), CM, C(1) | Several | Methemoglobinemia |
| 2006 | [29] | 2 | 2 | 2 | 2 | 1 | NA | EHCF (tolerated neocate) | A | Chronic? Sepsis work up methemoglobinemia | |
| 2006 | [18] | 4 | 4 | NA | 2 | 0.25–4 | NA | NA | CM(2), R(1) | N | 2 with multiple food FPIES |
| 2009 | [11] | ? | + | 33% | 33% | ? | |||||
| 2009 | [9] | 12 | 12 | 10 | 2 | 5.4 ± 3.1 | NA | NA | R(2) | 2 | |
| 2012 | [12▪] | 3 | 98% | 80% | 54% | 10.6 ± 6.7 | 22 | None | 2 | Described with other FPIES cases | |
| 2012 | [30] | 1 | + | + | + | 5 | NA | 6(a) | NA | 3 | Maternal soy ingestion, transferred through breast milk |
| 2013 | [8▪▪] | 189 | All* | All* | ∼ 50% | 7.7 ± 8.9 | 33.9 ± 23 | NA, one 16 y/o(c) | Most | *Diagnostic criteria | |
?, probably; B, banana; Ba, barley; C, corn; Ch, chicken; CM, cow's milk; D, diarrhea; EHCF, extensively hydrolyzed cow's milk formula; FPIES; food protein-induced enterocolitis syndrome; FU, follow-up; L/P, lethargy or pallor; NA, not available; O, oat; P, poultry; PD, prediagnosis episodes; R, rice; Rep V, repetitive vomiting; S, soy; SB, string bean; T, turkey; W, wheat.
Table 3.
Reported cases of rice-food protein-induced enterocolitis syndrome
| Year | Ref. no. | No. of cases | Symptoms | Age onset (m) | Outcome (a = accident, c = challenge) | No. of other food FPIES | No. of PD | Other | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | |||||||
| 1963 | [31] | 1 | − | − | + | 1 | 11 | NA | NA | Chronic at the beginning | |
| 1982 | [13] | 1 | + | − | + | 6 | 30 | CM | NA | Chronic diarrhea, malnutrition, resolved with EHCF, FPIES? | |
| 1992 | [32] | 1 | + | + | + | 1.25 | NA | 11 | NA | 4 | |
| 1996 | [33] | 4 | 4 | 3 | − | 3, 4, 2, 5 | NA | NA | NA | 1–3 | Hypotension, first introduction of food |
| 1998 | [4] | 1 | 1 | 1 | − | 6 | NA | 16 | S(1) | NA | |
| 2000 | [15] | 10 | 10 | 5 | 4 | 3–6 (5 cases described) | 4 (21–45) | 5 (12–63) | O(5), CM (3), P(1),S(6), SP(1), Ba(2) | 1–>4 | 1 case with chronic FPIES, 8/10 with multiple food FPIES |
| 2003 | [16] | 9 | 9 | 7 | 5 | 4–6 | 3 (21–45) | 6 (12–63) | B (1), CM(3), O(5), Pe(1), S(6), SB(2), SP(1) | Additional 1 – immediate response | |
| 2004 | [34] | 1 | 1 | 1 | − | 11 | NA | Ba | |||
| 2006 | [35] | 5 | 5 | 5 | 5 | 5–6 | 2, 36 m | 2, +1 lost to FU | None | >1 | 1 patient had sepsis work up performed twice |
| 2006 | [18] | 2 | 2 | NA | 1 | 4–9 | NA | NA | CM(1), O(1), S(1) | NA | None alone |
| 2009 | [9] | 14 | 14 | 13 | 3 | 5.2 ± 0.8 | 80% 36 m | S(2), SP(1), B(1), O(1) | 4 | (36%) other food | |
| 2012 | [12▪] | 3 | 98% | 80% | 54% | 10.6 ± 6.7 | 53 ± 17 | R(2), CM(1) | 3 | ||
| 2012 | [36] | 1 | + | + | 4 | Still/4y | Can have cooked | None | 4 | 10g first reaction, few grains last | |
| 2013 | [8▪▪] | 88 | All* | All* | ∼50% | 7.3 ± 5.1 | 43.1 ± 5.1 | NA | Most | *Diagnostic criteria | |
| 2013 | [37] | 1 | + | + | + | 5.5 | NA | 7(c) | SP(1) | 2 | Oral mucosal contact |
| 2013 | [26▪▪] | 1 | + | + | − | NA | NA | 10y(c) | NA | NA | Ondansetron |
| 2013 | [24] | 1 | + | + | + | 8 | NA | CM | 0 | Hypotension | |
B, banana; Ba, barley; CM, cow's milk; D, diarrhea; EHCF, extensively hydrolyzed casein formula; FPIES; food protein-induced enterocolitis syndrome; FU, follow-up; L/P, lethargy or pallor; NA, NOT available; O, oat; P, poultry; PD, prediagnosis episodes; Pe, peas; R, rice; Rep V, repetitive vomiting; S, soy; SP, sweet potato; T, turkey; W, wheat.
Table 4.
Reported cases of egg-food protein-induced enterocolitis syndrome
| Year | Ref. no. | No. of cases | Symptoms | Age onset (m) | Outcome (a = accident, c = challenge) | Other food FPIES | No. of PD | Remarks | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | |||||||
| 2006 | [18] | 1 | + | NA | + | 9 | NA | NA | None | NA | |
| 2011 | [38] | 1 | + | + | + | 11 | NA | 22(c) | None | 4 | |
| 2012 | [12▪] | 4 | 4 | 3 | 2 | 10.6 ± 6.7 | 53 ± 17 | None | 2.5 | ||
| 2013 | [26▪▪] | 1 | + | + | − | NA | NA | 36(c) | NA | NA | Ondansetron |
| 2013 | [8▪▪] | 51 | All* | All* | ∼50%* | 11.3 ± 9.6 | 41.8 ± 39.2 | NA | *Diagnostic criteria | ||
| 2013 | [39] | 4 | 4 | 4 | 2 | 6, 9,48, 6, | 24(1) | 3(24–72) | None | 1–12 | 2 with positive SPT. Worst outcome to patient with negative SPT to egg |
D, diarrhea; FPIES; food protein-induced enterocolitis syndrome; L/P, lethargy or pallor; NA, not available; PD, prediagnosis episodes; Rep V, repetitive vomiting; SPT, skin prick test.
Table 5.
Reported cases of peanut food protein-induced enterocolitis syndrome
| Year | Ref. no. | No. of cases | Symptoms | Age onset (m) | Outcome (a = accident, c = challenge) | Other food FPIES | No. of PD | Remarks | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | |||||||
| 2013 | [26▪▪] | 1 | + | + | − | NA | NA | 60 (c) | NA | NA | Ondansetron |
| 2013 | [8▪▪] | 9 | All* | All* | ∼50%* | 7.3 ± 5.1 ? | 42.1 ± 3.8 | NA | NA | NA | *Diagnostic criteria |
?, probably; D, diarrhea; FPIES; food protein-induced enterocolitis syndrome; L/P, lethargy or pallor; NA, not available; PD, prediagnosis episodes; Rep V, repetitive vomiting.
Table 6.
Reported cases of food protein-induced enterocolitis syndrome induced by fish and shellfish
| Year | Ref. no. | No. of cases | Fish | Symptoms | Age onset (m) | Outcome (a = accidental, c = challenge) | Other food FPIES | No. of PD | Remarks | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | ||||||||
| 1982 | [13] | 1 | NA | + | + | + | 4 | NA | 28m | ?CM | Malnutrition | |
| 2005 | [40] | 14 | 7 sole; 10 –hake; small hake – 4; cork float – 4 | 12 | 3 | 5 | 9–12 | 4 tolerant; 3 tolerant to single fish | 2 parent refuse challenge; 2 positive challenge; 2 too early; 1 not challenged | Wa | 3–6 | |
| 2009 | [9] | 1 | ? | + | + | − | 9 | NA | NA | None | ||
| 2012 | [41] | 1 | Scallops, clams | + | + | + | 33 years | 53 | The oldest case of FPIES reported. Persisted through age 53 | |||
| 2012 | [36] | 1 | Cod | + | + | − | 9 | NA | 5 g induced the first reaction to 0.25 g last reaction | |||
| 2012 | [12▪] | 8 | Cod, sole, + other | + | + | 54% | Mean 60 | CM(1), P(2) | 2.8 | Some species of fish tolerated | ||
| 2013 | [8▪▪] | 4 | Salmon and crab | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
?, probably; CM, cow's milk; D, Diarrhea; FPIES; food protein-induced enterocolitis syndrome; L/P, lethargy or pallor; NA, not available; PD, prediagnosis episodes; Rep V, repetitive vomiting; Wa, watermelon.
Table 7.
Reported cases of food protein-induced enterocolitis syndrome induced by grains
| Year | Ref. no. | No. of cases | Grain | Symptoms | Age onset (m) | Outcome (a = accidental, c = challenge) | No. of other food FPIES | No. of PD | Remarks | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | ||||||||
| 2003 | [16] | 8 | O | 8 | 8 | 7 | 3–6 | 3 (18–36) | 5 (19–63) | R(5), CM(3), S(5), SB(2), SP(1), P(1) | 1–5 | 1 case with chronic FPIES, 1 case to grain alone |
| 2005 | [17] | 1 | 1 | + | + | 6 | 36 | None | 2 | Treated for sepsis | ||
| 2006 | [18] | 1 | 1 | − | − | 4 | NA | NA | R(1) | NA | ||
| 2009 | [9] | 2 | 2 | + | + | 5–6.4 | NA | NA | R(2) | 2 | ||
| 2013 | [8▪▪] | 74 | All* | All* | ∼50% | 9.3 ± 6.2 | 37 ± 25.6 | NA | *Diagnostic criteria | |||
| 2012 | [36] | 1 | W | 1 | + | + | 6 | 39(c) | None | NA | 15 g in first reaction to 0.5 g last | |
| 2012 | [12▪] | 2 | NA | NA | NA | NA | NA | CM (2) | NA | |||
| 2013 | [26▪▪] | 1 | 1 | + | + | − | NA | 7y (c) | NA | NA | ||
| 2013 | [8▪▪] | 46 | All* | All* | ∼50% | 11.7–14.5 | 31.1.3 ± 14.5 | NA | NA | *Diagnostic criteria | ||
| 2003 | [16] | 2 | Ba | 2 | 1 | − | 4–6.5 | 1 (24) | 1 (21) | O, CM, (1); CM, S, R, SB, P (1) | 1 | None alone |
| 2013 | [8▪▪] | 18 | All* | All* | ∼50% | 11.7–14.5 | 55.3 ± 51.9 | NA | NA | *Diagnostic criteria | ||
| 2012 | [12▪] | 2 | C | 2 | 80% | 54% | NA | NA | NA | R(2) | 1 | |
| 2012 | [42] | 1 | 1 | + | + | + | 7 | NA | None | 2 | ||
| 2013 | [8▪▪] | 37 | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | ||
Ba, barley; CM, cow's milk; C, corn; D, diarrhea; FPIES; food protein-induced enterocolitis syndrome; L/P, lethargy or pallor; NA, not available; O, oat; PD, prediagnosis episodes; R, Rice; Rep V, repetitive vomiting; S, soy; SB, string bean; W, wheat.
Table 8.
Reported cases of food protein-induced enterocolitis syndrome induced by fruits
| Year | Ref. no. | No. of cases | Fruit | Symptoms | Age Onset (m) | Outcome (a = accidental, c = challenge) | Other food FPIES | No. of PD | Remarks | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | ||||||||
| 2009 | [9] | 1 | B | + | + | − | 6 | ? | 1 = R | |||
| 2012 | [12▪] | 2 | + | + | ? | NA | NA | CM(2) T(1) | ||||
| 2013 | [43] | 1 | + | + | + | 6 | NA | 19 (a) | NA | 3 | Only | |
| 2013 | [8▪▪] | 16 | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | ||
| 2013 | [8▪▪] | 8 | A | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2013 | [8▪▪] | 7 | Pea | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2008 | [44] | 1 | Peach | + | + | − | 4 | 12 | CM | 1 | ||
| 2013 | [8▪▪] | 4 | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | ||
| 2013 | [8▪▪] | 4 | Plum | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2013 | [8▪▪] | 4 | St | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2013 | [8▪▪] | 4 | Wa | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2005 | [40] | 1 | NA | NA | NA | 13 Years | NA | Fish | NA | |||
| 2013 | [8▪▪] | 3 | Av | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2012 | [45] | 1 | Or | + | + | + | 10 | NA | 24 | None | 5 | |
| 2013 | [8▪▪] | 11 | other | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
A, apple; Av, avocado; B, banana; CM, cow's milk, FPIES; food protein-induced enterocolitis syndrome; Or, orange; D, diarrhea; L/P, lethargy or pallor; NA, not available; PD, prediagnosis episodes; Pea, pear; Rep V, repetitive vomiting; St, strawberry; Wa, watermelon.
Table 9.
Reported cases of food protein-induced enterocolitis syndrome induced by vegetables and legumes
| Year | Ref. no. | No. of cases | Vegetable | Symptoms | Age onset (m) | Outcome | Other food FPIES | No. of PD | Remarks | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | ||||||||
| 1994 | [47] | 1 | SP | + | + | − | 5 | NA | NA | Sq | None | Sepsis work up |
| 2003 | [16] | 1 | + | + | − | 6 | 34 | 13 | O, R, S, SB, | 2 | ||
| 2007 | [8▪▪] | 19 | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | ||
| 2009 | [9] | 2 | 2 | 1 | 1 | 7.6 | NA | O(1), R(1) | ||||
| 2013 | [37] | 1 | 1 | + | + | 5.5 | 7 (c) | NA | R(1) | 2 | ||
| 1998 | [4] | 1 | P | + | − | − | 5 | NA | Not tested | S(1) | NA | |
| 2003 | [16] | 1 | + | − | − | 4.5 | 14 (a) | 4.5 | CM, S, R, O, Sq | 1 | ||
| 2003 | [28] | 1 | + | + | + | 8 | NA | 80 | ||||
| 2013 | [8▪▪] | 13 | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | ||
| 2003 | [28] | 1 | Lentils | + | + | − | 8 | NA | S, T(1) | |||
| 2013 | [8▪▪] | 8 | Potato | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2013 | [8▪▪] | 7 | Carrot | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 1994 | [46] | 1 | Sq | + | + | − | 5 | NA | SP | None | Hypotension | |
| 2013 | [8▪▪] | 6 | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | ||
| 2003 | [16] | 2 | SB | + | − | − | 6 | 24 | 46 | S(2) (and other) | ||
| 2013 | [8▪▪] | 3 | Kidney bean | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2013 | [8▪▪] | 2 | Green bean | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2012 | [12▪] | 1 | To | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2013 | [8▪▪] | 5 | Other | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
B, banana; CM, cow's milk; D, diarrhea; FPIES; food protein-induced enterocolitis syndrome; L/P, lethargy or pallor; NA, not available; O, oat; PD, prediagnosisepisodes; R, rice; Rep V, repetitive vomiting; S, soy; SB, string bean; SP, sweet potato; Sq, squash; To, tomato.
Table 10.
Reported cases of food protein-induced enterocolitis syndrome induced by poultry and meat
| Year | Ref. no. | No. of cases | Food | Symptoms | Age onset (m) | Outcome (a = accidental, c = challenge) | Other food FPIES | No. of PD | Remarks | |||
| Rep V | L/P | D | Recovery age (m) | Persistent (last known age, m) | ||||||||
| 1982 | [13] | 2 | Ch | 2 | 1 | 2 | 1.25, 6 | 1–19 | 1–25 | 2-CM, 1-R | NA | |
| 1994 | [47] | 1 | + | + | + | 5 | NA | NA | None | 4 | ||
| 1998 | [4] | 1 | − | + | + | 24 | 9y | T(1) | NA | Atypical | ||
| 2003 | [16] | 1 | + | − | − | 6 | 12y(c) | T | NA | |||
| 2003 | [28] | 4 | 4 | 3 | 1 | 3, 4.5, 7, 12 | 1– 3 years | 3 cases (24, 36, 24) | S(1), CM(2), T(1) | Several | 3/4 with multiple food FPIES; hypotension reported | |
| 2009 | [9] | 1 | + | + | ? | 8 | 0 | 36 | None | NA | ||
| 2012 | [36] | 1 | + | − | − | 8 | 60 or earlier | None | NA | 5g to 0.25 g | ||
| 2013 | [8▪▪] | 21 | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | ||
| 1998 | [4] | 1 | − | + | + | 24 | 9 years | |||||
| 2003 | [16] | 1 | T Beef | + | − | − | 7 | 12y | Ch(1) | 2 | Unchallenged | |
| 2003 | [28] | 2 | 2 | 1 | − | 6, 11 | NA | 36, 80 | Ch(1), P(1), S(1) | |||
| 2013 | [8▪▪] | 19 | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | ||
| 2009 | [8▪▪] | 11 | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | ||
| 2009 | [8▪▪] | 7 | Pork | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
| 2012 | [12▪] | 2 | P | 98% | 80% | 53% | 10.6 ± 6.7 | 53 ± 17 | F(2) | 4 | ||
| 2009 | [9] | 1 | Lamb | + | + | − | 11.2 | |||||
| 2013 | [8▪▪] | 2 | Lamb | All* | All* | ∼50%* | 14.5 ± 13.5 | 60.4 ± 33.7 | NA | NA | *Diagnostic criteria | |
Ch, chicken; D, diarrhea; F, fish; FPIES, food protein-induced enterocolitis syndrome; L/P, lethargy or pallor; NA, not available; O, oat; P, poultry; PD, prediagnosis episodes; R, rice; Rep V, repetitive vomiting; S, soy; T, turkey.
LOW BUT INCREASING AWARENESS OF FOOD PROTEIN-INDUCED ENTEROCOLITIS SYNDROME
The first manifestation of FPIES usually begins with the first or second introduction of the offending food [4,9,10▪▪,12▪,15,30,32]. Not uncommonly, however, it is only after repeated episodes that the actual diagnosis is finally made (see Tables 1–10 for the number of prediagnosis episodes). Until then, unfortunately, unnecessary procedures or medical treatments may be performed. For example, a sepsis work up was performed at many centers (A. Fiocchi, personal communication, 19–23, 49) ranging up to 28% [9] and 50% of cases [16]. Furthermore, up to 22% of cases had a surgical consultation [9]. The only population-based prospective study [10▪▪] estimated the incidence of milk FPIES as 0.34% of the newborn population. This compared with 0.5% for that described for IgE-mediated cow's milk protein (CMP) allergy, in the same population [10▪▪]. The results of a 1-year surveillance study [24] of FPIES in Australia, however, noted an incidence of 0.01%. The most likely explanation accounting for the differences in these two studies may relate to the low level of awareness and familiarity with this condition, among physicians at large. In fact, there were several regions of Australia in which there was not even a single report described (S. Mehr, personal communication). The low degree of awareness may be related to the lack of mortality in this disease and the high rate of spontaneous recovery. In addition, the challenge procedure is time consuming, as the reaction might be delayed up to 3–4 h after the last dose and may continue for up to 48 h [6,49]. This makes it difficult to carry out a FPIES oral food challenge (OFC) in an office-based or even a day care hospital setting. This limited availability of performing an OFC, coupled with the dramatic nature of the reaction lead to a tendency to postpone the challenge. Even in the presence of a very suggestive history, if the challenge is performed by 18 months; for example it is not uncommon that most challenges are negative (S. Mehr, personal communication). One interesting aspect of this low familiarity to FPIES is that it may explain why foods perceived to have a low allergenic profile such as rice have a greater number episodes occurring prior to diagnosis, compared with milk and soy (4 versus 2, for rice versus milk or soy, respectively) (Tables 1–3).
In recent years, however, there appears to be an increased interest in FPIES. This increased interest has translated into an increased number of publications. More than 29 publications appeared between 2011 and 2013, compared with between 2008 and 2010. Furthermore, at least two multicenter registries, in Italy (Sopo, personal communication) and Australia [50], were recently initiated. Finally, educational intervention programs successfully implemented in Italy (A. Fiocchi, personal communication) should reduce the time to diagnosis and decrease unnecessary procedures to be performed. These trends will hopefully provide important and validated information in the next several years.
DURATION OF DISEASE AND RATE OF RECOVERY
All experts agree that there is a high and rapid rate of recovery from FPIES. Optimally, the loss of reactivity to the offending food would be determined by a scheduled OFC. In real life, however, many patients experience an accidental exposure without a reaction and report subsequently, usually not voluntarily, that they are not sensitive any more. In Tables 1–10, the available information regarding the various foods causing FPIES, the age of first presentation and the outcome to the best of our knowledge are presented. In our cohort of milk FPIES [10▪▪], over 90% lost their reactivity by the age of 3 years and only a single patient had a challenged-proven reaction after the age of 3 years. Similar results were obtained in Korea (11 and Hwang, personal communication), Australia [9] and Italy [12▪]. The largest group of cow's milk FPIES, consisting of 310 patients identified by retrospective chart review, yielded different and less favorable results [8▪▪]. Only 35% outgrew their FPIES disease by the age of 2, and only 70% outgrew their sensitivity by 3 years. However, by school age (5 years old) more than 85% lost their reactivity to milk. It is possible that the differences noted with regard to the age of resolution between the studies reflect the variability in the mode of collection of data.
It would appear that the age of recovery of FPIES to soy is similar to that of milk. Among the children with FPIES to soy who were challenged, five out of six lost their reactivity by the age of three [9]. There is no information regarding the other six soy allergic children in that study, but it is reasonable to assume that the patients who were not challenged lost their sensitivity at even a higher rate. In another study [27], five out of seven soy allergic children lost their sensitivity by the age of 2 years, whereas the other two were lost to follow-up. Although other series similarly noted that the age of recovery for FPIES to soy was similar to that of milk [8▪▪,12▪], there are case reports in which FPIES persists to an older age [8▪▪,26▪▪,27–51]. To our knowledge, the two cases in which FPIES persisted the longest were to soy, at age 18 [8▪▪] and 19 years old (Y. Levy, Personal communication). In addition, a patient with a late onset of FPIES at age 33 persisted through age 53 [41].
FPIES to solid foods tend to appear at a later age, likely reflecting the later age of introduction of these foods to the diet [8▪▪,16]. Furthermore, in the Italian cohort, the age of resolution of FPIES to solid foods was later than that of milk or soy [12▪]. The median age of resolution for FPIES to fish and egg was exceptionally high, at approximately 60 months (Tables 4 and 6). On the contrary, Ruffner et al.[8▪▪] found no difference in the age of resolution between FPIES reactions mediated by solid foods and liquids (42.1 ± 3.8 versus 32.9 ± 0.95, respectively). Confounding the data is the low catchment for the age of resolution for those reported in this study [8▪▪]. In many of the studies ([11,16,28,52], A.S. Bansal, personal communication), resolution of FPIES was determined by reports after accidental exposure that could have been many months after the true resolution.
MULTIPLE FOODS CAUSING FOOD PROTEIN-INDUCED ENTEROCOLITIS SYNDROME
Not uncommonly, a patient may have FPIES reactions to multiple foods. Thus, an important question arises as to what the replacement food should be and as to how other foods should be introduced into the diet. This problem is reinforced by the fact that there is no laboratory test such as a skin prick test (SPT) or a sIgE that could exclude the possibility of sensitivity. For example, the most common food to cause FPIES is cow's milk, a situation in which a soy-based formula would be considered as a natural candidate for replacement. But, many using a ‘common knowledge’ assume that patients with FPIES to cow's milk are reactive to soy as well [11]. With regard to this issue, there is a sharp discrepancy in the reported prevalence of FPIES to soy in cases of milk-mediated FPIES. Although in the cohorts described from Australia [9], Israel [10▪▪] and Italy [12▪] not a single case of milk-mediated FPIES reacted to soy, in reports emanating from the United States, soy-mediated FPIES was not uncommon among patients with FPIES to milk [8▪▪]. Although in one study [16] this was noted in a tertiary highly selected population, in a more recent and largest report of FPIES to date, 29% of patients with milk mediated FPIES also had FPIES to soy [8▪▪]. In this last study, those that were positive to soy were sensitive to other foods and not only milk. The issue of soy reactivity among cow's milk-mediated FPIES patients, is only one of the questions that will have to be answered by future large-scale population-based multicenter international prospective studies.
In solid food-mediated FPIES, it is common to have FPIES to an additional food. In the recent data from the United States over 40% of patients with grain FPIES had sensitivity to two or more grains and 20% with grain FPIES additionally reacted to soy, milk or both [8▪▪]. In the study [9] from Australia, patients with FPIES-mediated reactivity to both rice and oat were common. On the contrary, in the few reports of FPIES to egg and peanut, no other FPIES-type reactions were mentioned to other foods (Tables 4 and 5).
To date, no risk factors were identified for the appearance of FPIES. Factors analyzed included breastfeeding versus no breastfeeding [8▪▪,10▪▪], the presence or absence of family history and whether the presence of an infant with FPIES increased the risk for a sibling to have FPIES [50].
SEVERITY OF FOOD PROTEIN-INDUCED ENTEROCOLITIS SYNDROME
In many reports, the description of a FPIES reaction during a natural exposure or challenge is quite dramatic. As discussed above, in many cases, infants with accidental exposures to inducing foods prior to diagnosis were treated as having sepsis because of the life-threatening nature of their reaction. Furthermore, there are cases with documented decreases in blood pressure [11,24] and even shock [47]. This led to the common practice at least in some centers to perform an OFC to diagnose FPIES with an intravenous line in place [6]. However, unlike in IgE-mediated food allergic reactions [53,54], no cases of mortality have been reported in FPIES. This may be related to the early age of presentation and resolution for FPIES; no fatal IgE-mediated food allergic reactions were ever reported in infants younger than 2 years old.
Acquired methemoglobinemia, perhaps secondary to dehydration, is mentioned as evidence for the severity of reactions in this disease [14]. The data are sparse on this issue and the three reports that describe this phenomenon are to both milk and soy [9,14,29,]. They occurred both following accidental and OFC exposure [11]. Similarly, the assumption that the presence of evidence of IgE sensitization is an ominous sign [4,16,49] has not been validated [39].
ELICITING DOSE IN FOOD PROTEIN-INDUCED ENTEROCOLITIS SYNDROME
In the four cases reported by Bansal [36], the quantity of inciting food that caused subsequent reactions was several fold less than the quantities that elicited the original reaction. This was noted for rice, wheat, cod and chicken. We made the same observation for milk and this was confirmed by Sopo (personal communication). Furthermore, this phenomenon was reported in a case of soy-mediated FPIES, as well [30]. The observed increased sensitivity upon subsequent exposures may explain the report by Monti et al.[55] in which an infant's threshold for reaction decreased from 50 ml in the first reaction to one spoonful and subsequently by breast milk. The latter was due, presumably, to cow's milk proteins that passed through the breast milk. However, FPIES induced by breast milk was reported in three cases where no previous FPIES reactions induced directly by a food were noted [56].
SECONDARY IgE-MEDIATED FOOD ALLERGY FOLLOWING FOOD PROTEIN-INDUCED ENTEROCOLITIS SYNDROME MEDIATED FOOD HYPERSENSITIVITY
Several studies reported that patients with FPIES may develop an IgE-mediated allergy to the same food. The first described case was identified in 1998 [4], and the observation was subsequently confirmed by several additional authors ([10▪▪,23,57] and Spergel, personal communication). Although this phenomenon has been demonstrated mainly for milk, we are also aware of three additional cases in which it was seen in patients who initially had FPIES to fish (Sopo, personal communication; J. Bone, personal communication) and an additional one to egg (J. Bone, personal communication). Because of the risk for the development of IgE-mediated CMA in these patients, it is prudent to examine by SPT or sIgE for sensitization to cow's milk before an OFC challenge is carried out. Needless to say, even if sensitization is noted, the patient has to be observed for an extended period of 3–4 h after the last dose, as for FPIES. The shift from a FPIES food hypersensitivity to an IgE-mediated food allergy is puzzling, as in most cases, there were no detectable IgE antibodies to milk at the initial diagnosis of FPIES. Furthermore, there were no classical IgE-mediated symptoms at the original challenge [10▪▪]. One possible explanation is the extended period of avoidance of cow's milk protein from the diet increases the risk of developing secondary IgE-CMA. The reason that this shift was not reported more frequently probably rests with earlier ambiguity in the diagnosis of IgE-mediated milk allergy and non-IgE mediated symptoms, in young infants [58]. Given that now the awareness and proper diagnosis of FPIES are increasing, it is expected that this phenomenon will be recognized more frequently, and likely with other foods, as well.
CONCLUSION
More is unknown than known about the natural history of FPIES. In general, FPIES is a benign condition with a favorable course. The duration of the disease is relatively short lived, with the vast majority growing out of the condition by the age of 3–5 years. Given the occurrence of reactions to more than one type of food in some of these patients, it is prudent to introduce alternative foods under medical supervision. In the case of FPIES to cow's milk protein, the possibility of developing IgE-mediated CMP allergy should be entertained. Finally, the possibility of a decreasing threshold for sensitivity to the offending food should be considered.
Acknowledgements
The authors gratefully acknowledge our colleagues for their helpful discussions and for providing their data from published and unpublished cases. A partial list of contributors includes: S.A. Fiocchi, D. Peroni, M. Ben Shoshan, A. Bansal, M. Landi, S.H. Sicherer, S.A. Bock, S. Bahna, E. Tham, B.W. Lee, MS Sopo, S. Mehr, G. Monti, J.B. Hwang, M. Don, Y. Levy and J. Bone. The authors apologize for anyone who was inadvertently omitted.
M.R.G. is funded by a Kamea grant from the Ministry of Health, Israel.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Rubin MI. Allergic intestinal bleeding in the newborn: a clinical syndrome. Am J Med Sci 1940; 200:385–390 [Google Scholar]
- 2.Gryboski JD. Gastrointestinal milk allergy in infants. Pediatrics 1967; 40:354–362 [PubMed] [Google Scholar]
- 3.Powell GK. Food protein-induced enterocolitis of infancy: differential diagnosis and management. Compr Ther 1986; 12:28–37 [PubMed] [Google Scholar]
- 4.Sicherer SH, Eigenmann PA, Sampson HA. Clinical features of food protein-induced enterocolitis syndrome. J Pediatr 1998; 133:214–219 [DOI] [PubMed] [Google Scholar]
- 5.Leonard SA, Nowak-Wegrzyn A. Clinical diagnosis and management of food protein-induced enterocolitis syndrome. Curr Opinion Pediatr 2012; 24:739–745 [DOI] [PubMed] [Google Scholar]
- 6.Jarvinen KM, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome (FPIES): current management strategies and review of the literature. J Allergy Clin Immunol 2013; 1:317–322 [DOI] [PubMed] [Google Scholar]
- 7▪.Sopo SM, Greco M, Monaco S, et al. Food protein-induced enterocolitis syndrome, from practice to theory. Expert Rev Clin Immunol 2013; 9:707–715 [DOI] [PubMed] [Google Scholar]; Proposed the current definition.
- 8▪▪.Ruffner MA, Ruymann K, Barni S, et al. Food protein-induced enterocolitis syndrome: insights from review of a large referral population. J Allergy Clin Immunol 2013; 1:343–349 [DOI] [PubMed] [Google Scholar]; Largest group.
- 9.Mehr S, Kakakios A, Kemp AS. Food protein-induced enterocolitis syndrome: 16-year experience. Pediatrics 2009; 123:e459–e464 [DOI] [PubMed] [Google Scholar]
- 10▪▪.Katz Y, Goldberg MR, Rajuan N, et al. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow's milk: a large scale prospective population-based study. J Allergy Clin Immunol 2011; 127:647–653 [DOI] [PubMed] [Google Scholar]; The only prospective population-based study.
- 11.Hwang JB, Sohn SM, Kim AS. Prospective follow-up oral food challenge in food protein-induced enterocolitis syndrome. Arch Dis Child 2009; 94:425–428 [DOI] [PubMed] [Google Scholar]
- 12▪.Sopo SM, Giorgio V, Iacono ID, et al. A multicenter retrospective study of 66 Italian children with food protein-induced enterocolitis syndrome: different management for different phenotypes. Clin Exp Allergy 2012; 43:1257–1265 [DOI] [PubMed] [Google Scholar]; Large multicenter study.
- 13.Vitoria JC, Camarero C, Sojo A, et al. Enteropathy related to fish, rice and chicken. Arch Dis Child 1982; 57:44–48 [PMC free article] [PubMed] [Google Scholar]
- 14.Murray KF, Christie DL. Dietary protein intolerance in infants with transient methemoglobinemia and diarrhea. J Pediatr 1993; 122:90–92 [DOI] [PubMed] [Google Scholar]
- 15.Sicherer SH. Food protein-induced enterocolitis syndrome: Clinical perspective. J Ped Gastroenter and Nutr 2000; 30:S45–S49 [DOI] [PubMed] [Google Scholar]
- 16.Nowak-Wegrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics 2003; 111:829–835 [DOI] [PubMed] [Google Scholar]
- 17.Sicherer SH. Food protein-induced enterocolitis syndrome: case presentation and management lesson. J Allergy Clin Immunol 2005; 115:149–156 [DOI] [PubMed] [Google Scholar]
- 18.Fogg MI, Brown-Whitehorn TA, Pawlowski NA, Spergel JM. Atopy patch test for the diagnosis of food protein-induced enterocolitis syndrome. Ped Allergy Immunol 2006; 17:351–355 [DOI] [PubMed] [Google Scholar]
- 19.Maloney J, Nowak-Wegrzyn A. Educational clinical case series for pediatric allergy and immunology: allergic proctocolitis, food protein-induced enterocolitis syndrome and allergic eosinophilic gastroenteritis with protein- on non-IgE-mediated cow's milk allergy. Pediatr Allergy Immunol 2007; 18:3607. [DOI] [PubMed] [Google Scholar]
- 20.Jones KDJ, Noimark L, Osborn M, et al. A case of severe atypical food protein-induced enterocolitis syndrome. Allergy 2010; 65:1061–1062 [DOI] [PubMed] [Google Scholar]
- 21.Chaabane M, Bidat E, Chevallier B. A new case of food protein-induced enterocolitis syndrome. Archives de Pediatrie 2010; 17:502–506 [DOI] [PubMed] [Google Scholar]
- 22.Monti G, Castagno E, Liguori SA, et al. Reply. J Allergy Clin Immunol 2012; 129:873–87422296754 [Google Scholar]
- 23.Onesimo R, Dello Iacono I, Giorgio V, et al. Can food protein induced enterocolitis syndrome shift to immediate gastrointestinal hypersensitivity? A report of two cases. Eur Ann Allergy Clin Immunol 2011; 43:61–63 [PubMed] [Google Scholar]
- 24.Caminity L, Salzano G, Crisafulli G, et al. Food protein induced enterocolitis caused by rice beverage. Ital J Pediatr 2013; 39:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banzato C, Piacentini GL, Comberiati P, et al. Unusual shift from IgE-mediated milk allergy to food protein-induced enterocolitis syndrome. Eur Ann Allergy Clin Immunol 2013; 45:209–211 [PubMed] [Google Scholar]
- 26▪▪.Holbrook T, Keet CA, Frischmeyer-Guerrerio PA, Wood RA. Use of ondansetron for food protein-induced enterocolitis syndrome. J Allergy Clin Immunol 2013; 132:1219–1220 [DOI] [PubMed] [Google Scholar]; May suggest a mechanism.
- 27.Levy Y, Kornbrot B, Danon YL. Soybean allergy in cow milk-tolerant infants. Pediatr Asthma Allergy Immunol 1998; 12:253–259 [Google Scholar]
- 28.Levy Y, Danon YL. Food protein-induced enterocolitis syndrome: not only due to cow's milk and soy. Pediatr Allergy Immunol 2003; 14:325–329 [DOI] [PubMed] [Google Scholar]
- 29.Anand RK, Appachi E. Case report of methemoglobonemia in two patients with food protein induced enterocolitis. Clin Pediatr 2006; 45:679–682 [DOI] [PubMed] [Google Scholar]
- 30.Tan J, Campbell D, Mehr S. Food protein-induced enterocolitis syndrome in an exclusively breast-fed infant: an uncommon entity. J Allergy Clin Immunol 2012; 129:873. [DOI] [PubMed] [Google Scholar]
- 31.Ikola RA. Severe intestinal reaction following ingestion of rice. Am J Dis Child 1963; 105:281–284 [DOI] [PubMed] [Google Scholar]
- 32.Borchers SD, Li BUK, Friedman RA, McClung HJ. Rice induced anaphylactoid reaction. J Pediatr Gastroenter Nutr 1992; 15:321–324 [DOI] [PubMed] [Google Scholar]
- 33.Cavataio F, Carroccio A, Montalto G, Iacono G. Isolated rice intolerance. J Pediatr 1996; 128:558–560 [DOI] [PubMed] [Google Scholar]
- 34.Gray HC, Foy TM, Becker BA, Knusten AP. Rice-induced enterocolitis in an infant: TH1/TH2 cellular hypersensitivity and absent IgE reactivity. Ann Allergy Asthma Immunol 2004; 93:601–605 [DOI] [PubMed] [Google Scholar]
- 35.Hojsak I, Kijaic-Turkalj M, Misak Z, Kolacek S. Rice protein-induced enterocolitis syndrome. Clin Nutr 2006; 25:533–536 [DOI] [PubMed] [Google Scholar]
- 36.Bansal AS, Bhaskaran S, Bansal RA. Four infants presenting with severe vomiting in solid food-protein induced enterocolitis syndrome. J Med Case Rep 2012; 6:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mane SK, Hollister ME, Bahana SL. Food protein-induced enterocolitis syndrome to trivial oral mucosal contact. Eur J Pediatr 2013; 2501–2502[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Caubet JC, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome to hen's egg. J Allergy Clin Immunol 2011; 128:1386–1389 [DOI] [PubMed] [Google Scholar]
- 39.Hsu P, Mehr S. Egg: a frequent trigger of food protein-induced enterocolitis syndrome. J Allergy Clin Immunol 2013; 131:241–242 [DOI] [PubMed] [Google Scholar]
- 40.Remon ZL, Alonso LE, Martin FE, Martinez MMI. Food protein-induced enterocolitis syndrome caused by fish. Allergol Immunopathol 2005; 33:312–316 [DOI] [PubMed] [Google Scholar]
- 41.Fernandes BN, Boyle RJ, Gore C, et al. Food protein-induced enterocolitis syndrome can occur in adults. J Allergy Clin Immunol 2012; 130:1199–1200 [DOI] [PubMed] [Google Scholar]
- 42.Sopo SM, Filoni S, Viorgio V, et al. Food protein-induced enterocolitis syndrome (FPIES) to corn: a case report. J Investig Allergol Clin Immunol 2012; 22:391–392 [PubMed] [Google Scholar]
- 43.Don M, Longo G. Food protein-induced enterocolitis syndrome due to banana: an uncommon entity. Eur Ann All Clin Immunol 2013; 45:61–62 [PubMed] [Google Scholar]
- 44.Bruni F, Peroni DG, Piacentini GL, et al. Fruit proteins: another cause of food protein-induced enterocolitis syndrome. Allergy 2008; 63:1645–1646 [DOI] [PubMed] [Google Scholar]
- 45.Federely TJ, Ryan P, Dinakar C. Food protein-induced enterocolitis syndrome triggered by orange juice. Ann Allergy Asthma Immunol 2012; 109:472–473 [DOI] [PubMed] [Google Scholar]
- 46.Coates RW, Weaver KR, Lloyd R, et al. Food protein-induced enterocolitis syndrome as a cause for infant hypotension. West J Emerg Med 2011; 12:512–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandenplas Y, Edelman R, Sacre L. Chicken-induced anaphylactoid reaction and colitis. J Pediatr Gastroenterol Nutr 1994; 19:240–241 [DOI] [PubMed] [Google Scholar]
- 48.Andrews T, Tsarouhas N, Spergel J. Food allergy presenting as a ‘septic’-appearing infant. Pediatr Emerg Care 2004; 20:677–679 [DOI] [PubMed] [Google Scholar]
- 49.Nowak-Wegrzyn A, Muraro A. Food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol 2009; 9:371–377 [DOI] [PubMed] [Google Scholar]
- 50.Frith K, Campbell D, Joshi P, et al. The first 12 months of FPIES surveillance in Australia. Intern Med J 2013; 43 (S4):1–21 [Google Scholar]
- 51.Caubet JC, Bencharitiwong R, Masilamani M, Sampson HA. T cell responses to food proteins in acute food protein-induced enterocolitis (FPIES). J Allergy Clin Immunol 2013; 131:AB183 [Google Scholar]
- 52.Mehr SS, Kakakios AM, Kemp AS. Rice: a common and severe cause of food protein-induced enterocolitis syndrome. Arch Dis Child 2009; 94:220–223 [DOI] [PubMed] [Google Scholar]
- 53.Levy MB, Goldberg MR, Nachshon L, et al. Lessons from cases of mortality due to food allergy in Israel: cow's milk protein should be considered a potentially fatal allergen. Isr Med Assoc J 2012; 14:29–33 [PubMed] [Google Scholar]
- 54.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med 1992; 327:380–384 [DOI] [PubMed] [Google Scholar]
- 55.Monti G, Castagno E, Liguori SA, et al. Food protein-induced enterocolitis syndrome by cow's milk proteins passed through breast milk. J Allergy Clin Immunol 2011; 127:679–680 [DOI] [PubMed] [Google Scholar]
- 56.Nomura I, Morita H, Hosokawa S, et al. Four distinct subtypes of non-IgE-mediated gastrointestinal food allergies in neonates and infants, distinguished by their initial symptoms. J Allergy Clin Immunol 2011; 127:685–688 [DOI] [PubMed] [Google Scholar]
- 57.Kessel A, Dalal I. The pendulum between food protein-induced enterocolitis syndrome and IgE-mediated milk allergy. Acta Paediatr 2011; 100:e183–e185 [DOI] [PubMed] [Google Scholar]
- 58.Katz Y, Rajuan N, Goldberg MR, et al. Early exposure to cow's milk protein is protective against IgE-mediated cow's milk protein allergy. J Allergy Clin Immunol 2010; 126:77–82 [DOI] [PubMed] [Google Scholar]


