Abstract
Osteolysis of bone following total hip replacements is a major clinical problem. Examination of the areas surrounding failed implants has indicated an increase in the bone-resorption-inducing cytokine, interleukin 1β (IL-1β). NALP3, a NOD-like receptor protein located in the cytosol of macrophages, has been shown to signal the cleavage of pro-IL-1β into its mature, secreted form, IL-1β. Here we show that titanium particles stimulate the NALP3 inflammasome. We demonstrate that titanium induces IL-1β secretion from macrophages and this response is dependent on the expression of components of the NALP3 inflammasome, including NALP3, ASC, and Caspase-1. We also show that titanium particles trigger the recruitment of neutrophils and that this acute inflammatory response is dependent on the expression of the IL-1 receptor and IL-1α/β. Moreover, administration of the IL-1 receptor antagonist (IL-1Ra) diminished neutrophil recruitment in response to titanium particles. Together, these results suggest that titanium particle-induced acute inflammation is due to activation of the NALP3 inflammasome, which leads to increased IL-1β secretion and IL-1-associated signaling, including neutrophil recruitment. Efficacy of IL-1Ra treatment introduces the potential for antagonist based-therapies for implant osteolysis.
Keywords: Titanium, inflammasome, neutrophils, IL-1, NALP3
INTRODUCTION
With over one million total joint replacements performed every year (1), joint replacement surgery is a major advance in treatment of people with arthritis (2). It is estimated that six million total hip and knee replacements will be performed per year in the US by 2015 making this a significant health issue. Long-term studies of hip and knee replacements have indicated that loosening of joint replacements, as well as bone-loss surrounding the replacement, increases over time. Approximately 10–20% of patients who undergo joint replacement surgery will develop joint loosening requiring replacement of the joint (3, 4). Patients who develop significant inflammation surrounding a fixed implant, if left untreated, will eventually develop joint loosening and, in some cases, bone loss surrounding the joint. This type of aseptic inflammation is associated with activation of macrophages in the tissue surrounding the prosthesis and the destruction of bone. Studies have shown that over time, small wear-particles generated from implants become dislodged and are released into the surrounding area (4) where they can be phagocytosed by circulating monocytes and macrophages (1). This uptake of particles stimulates cells to release pro-inflammatory cytokines such as tumor necrosis factor (TNF), interleukin-6 (IL-6), prostaglandin E2 (PGE2), and interleukin-1β (IL-1β) (5–9). These cytokines, especially IL-1β, have been implicated in mediating osteoclast activation and/or bone resorption (10–12).
NALP3 [NACHT-, LRR-, and pyrin domain (PYD)-containing protein 3] has been shown to be involved in inflammatory responses to particulates including silica, asbestos and monosodium urate (MSU) crystals (13–15). Activation of the NALP3 inflammasome involves a conformational change in NALP3 into its active form, which then associates with its adaptor protein ASC (16) through PYD interactions. This complex leads to the recruitment of pro-caspase-1 through caspase-recruitment domain (CARD) interactions (17–19). Pro-caspase-1 is cleaved into its active form, Caspase-1. Active Caspase-1 cleaves pro-IL-1β into its active, secreted form, IL-1β. Secreted IL-1β can bind to and activate the IL-1 receptor (IL-1R), leading to a more robust pro-inflammatory response. Polymorphisms in NALP3 are linked to a spectrum of auto-inflammatory diseases with unchecked IL-1 production (20).
The role of the NALP3 inflammasome in the response to titanium wear-particles has not previously been characterized. We hypothesized that titanium particles activate a pro-inflammatory response in patients through activation of the inflammasome complex, leading to loosening and failure of the joint replacement. To assess this, we first determined whether injection of titanium particles into mice led to neutrophil recruitment, a hallmark of acute inflammation, and whether this neutrophil influx was dependent on the expression of IL-1R and IL-1α/β. To examine the potential for an antagonist-based therapy, we also tested whether treatment of mice with the IL-1 receptor antagonist (IL-1Ra) could decrease neutrophil recruitment. Finally, in order to determine a role for the NALP3 inflammasome in titanium-induced inflammation, we examined whether titanium particles could activate IL-1β secretion in mouse and human macrophages deficient in key components of the inflammasome signaling complex: NALP3, ASC, and Caspase-1.
METHODS AND MATERIALS
Reagents
Lipopolysaccharide (LPS), Nigericin, and poly(dA:dT) sodium salt were from Sigma-Aldrich (St. Louis, MO). CA-074-Me, Phorbol 12-myristate 13-acetate (PMA), and GeneJuice transfection reagent were from EMD Chemicals (Gibbstown, NJ). Commercially pure titanium particles (Alfa Aesar, Ward Hill, MA) were autoclaved in H2O, washed with 100% ethanol, dried under UV light in a laminar flow hood, and resuspended in sterile PBS for subsequent studies. Particles were determined to have <1EU/ml of endotoxin using the Pyrochrome LAL endotoxin assay (Associates of Cape Cod, East Falmouth, MA). Particles (n=6708) had a median diameter of 1.86 μm with a mean diameter of 3.805 μm (SD = 6.065 μm) as determined by confocal microscopy and (NIH) ImageJ software.
Mice
C57BL/6 (WT) and IL-1R-deficient (IL-1R KO) mice were from Jackson Laboratories (Bar Harbor, ME). CD14 KO and MD2 KO mice were a gift from D. Golenbock (UMass Medical School). IL-1α/β KO mice were a gift from Y. Iwakura (University of Tokyo). All mouse strains, age and sex-matched with appropriate controls, were bred and maintained in the animal facilities at the University of Massachusetts Medical School. All experiments involving live animals were in accordance with guidelines set forth by the University of Massachusetts Medical School Department of Animal Medicine and the Institutional Animal Care and Use Committee.
Intraperitoneal (i.p.) injections
Mice were injected i.p. with sterile PBS (500 ul), 4% Thioglycollate (1 ml), or 30 mg titanium particles (600 μl at 50 mg/ml). For IL-1Ra studies, mice were injected subcutaneously with 200 μl (1 mg/kg final dose) of IL-1Ra (Anakinra; Amgen, Thousand Oaks, CA) in 2% Hyaluronic Acid carrier (Calbiochem, EMD, Gibbstown, NJ) 2 h prior to and 1h following titanium injections. Mice were sacrificed by isoflurane inhalation followed by cervical dislocation. Peritoneal exudate cells (PECs) were isolated 16–18 h after injections as previously described (21).
Flow cytometric analysis
To enumerate neutrophils, PECs (1×106) were incubated with anti CD16/CD32 monoclonal antibody (clone 2.4G2; BD Biosciences, San Jose, CA) for 30 minutes to block FcγRIIB/III receptors and stained with Ly6G-FITC (BD Biosciences) and 7/4-Alexa647 (AbD Serotec, Raleigh, NC) for 30 minutes at 4°C. Following staining, cells were washed with PBS and analyzed on a LSRII (BD Biosciences). Neutrophil numbers in PECs were calculated by multiplying total cell numbers by the percentage of Ly6G+, 7/4+ cells. Data were acquired by DIVA (BD Biosciences) and were analyzed with FlowJo 8.8.6 software (Tree Star Inc., Ashland, OR).
Cell culture
Immortalized mouse macrophages from WT, NALP3-deficient, ASC-deficient, and Caspase 1-deficient mice were made as previously described (13) and grown in DMEM supplemented with 10% fetal calf serum (FCS), 1% L-glutamine, and 1% Penicillin/streptomycin at 37°C with 5% CO2. Cells were plated in granulocyte macrophage colony-stimulating factor (GM-CSF) (1 ng/ml; eBioscience, San Diego, CA)-containing media for 18 h prior to stimulations. THP-1 cells (ATCC, Manassas, VA) were differentiated into macrophages with 10 nM PMA for 48 h and grown in RPMI-1640 supplemented with 10% FCS, 1% L-glutamine, 1% Penicillin/streptomycin, 50 μM β-Mercaptoethanol, and 10 mM HEPES at 37°C with 10% CO2.
Cell stimulations
For particle stimulations, mouse and human macrophages (4–5 × 105) were primed for 3 h with LPS (100 ng/ml) to up-regulate pro-IL-1β expression or left unprimed (Media), then stimulated with titanium particles (Ti) at given concentrations (mg/ml), transfected with 400 ng of poly(dA:dT) (dA:dT) using GeneJuice, or treated with Nigericin (5 mM) for an additional 6 h (mouse) or 18 h (human).
RNA interference
Differentiated THP-1 macrophages were transfected with siGENOME SMARTpools (40 nM) against human NALP3 or ASC or non-targeting (NT) siRNA #2 using DharmaFECT 4 transfection reagent for 72 h. Cells were then stimulated as described above. All siRNA reagents were obtained from Dharmacon (Thermo Fisher Scientific, Lafayette, CO).
ELISA
Cell culture supernatants were assayed for murine or human IL-1β or IL-6 with ELISA kits from BD Biosciences according to the manufacturer’s instructions.
Scanning Electron Microscopy (SEM)
Macrophages were plated at 50–60% confluency in plastic tissue culture dishes. The following day, titanium particles (100 μg/ml) were added to cells for 30 minutes at 37°C. Cells were then fixed and prepared as described in (22).
Statistical Analysis
An unpaired, two-tailed Student’s t-test was used to determine statistical significance of independent experiments where two groups were compared (Figures 1a–e). When more than two groups were compared, a one-way ANOVA (Figure 1f, 2, 3, 4b, 4c) or two-way ANOVA (Figure 4a) followed by Bonferroni’s correction for post test comparisons was used. Values of p < 0.05 were considered significant. Statistics were performed using GraphPad (Prism v5.0a) software.
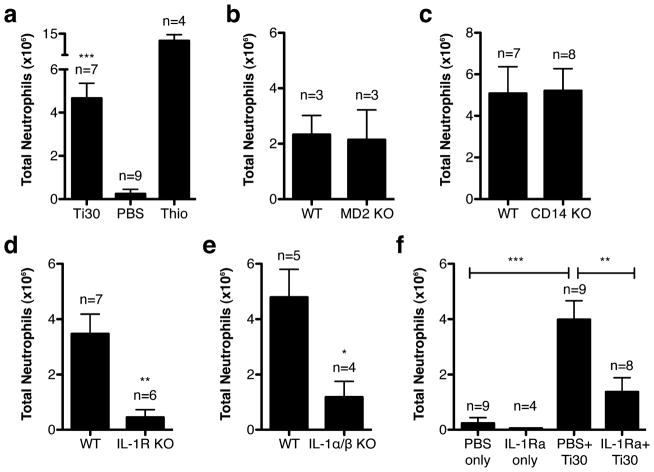
Figure 1.
Neutrophil recruitment following titanium injections in various mouse strains. (a) WT (b) MD2 KO (c) CD14 KO (d) IL-1R KO and (e) IL-1α/β KO. (f) Effect of IL-1Ra treatment on neutrophil recruitment. Graphs show the mean ± s.e.m. from the total number of mice indicated. P-values are shown as *** ≤ 0.0001; ** < 0.01; * < 0.05
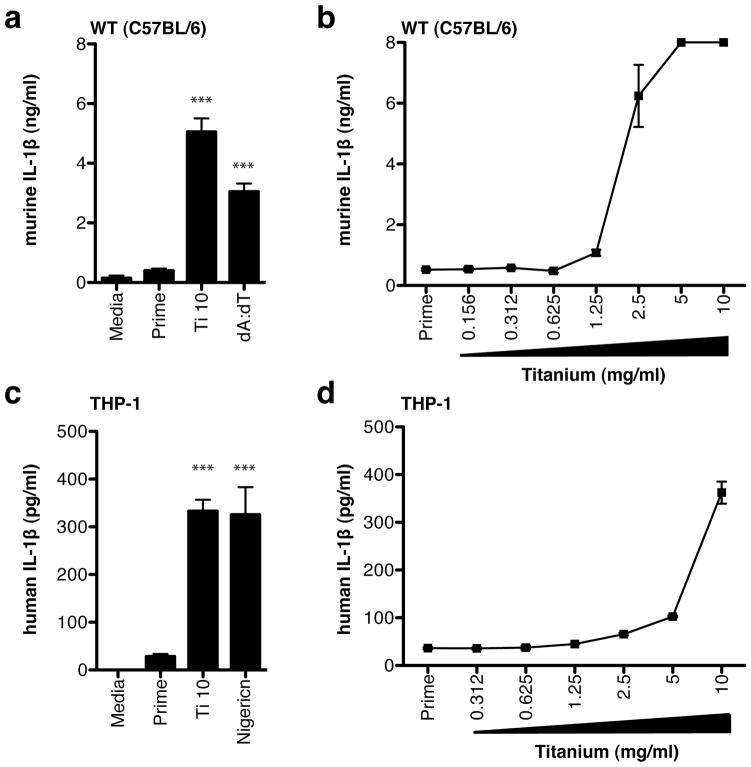
Figure 2.
IL-1β secretion following titanium stimulation in (a) WT immortalized mouse macrophages and (b) THP-1 human macrophages. Dose-response curves to titanium in (c) WT immortalized mouse macrophages and (d) THP-1 human macrophages. Secreted IL-1β levels are mean ± s.e.m. Significance values are shown relative to Prime levels. P-values are shown as *** ≤ 0.0001
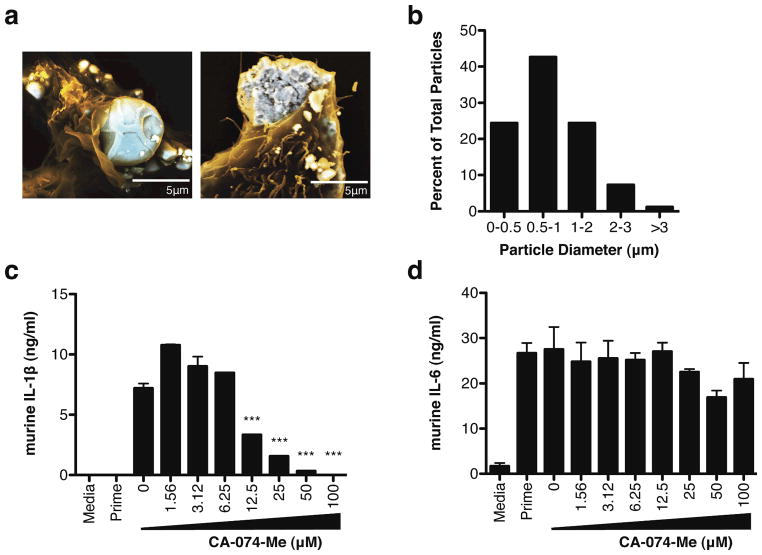
Figure 3.
Response to titanium particle uptake is cathepsin B dependent. (a) SEM image of immortalized mouse macrophages incubated with titanium particles for 30 min. (b) Diameter of macrophage-associated particles (n = 82) as determined by confocal microscopy and NIH ImageJ software. (c) IL-1β and (d) IL-6 secretion following titanium stimulation in WT immortalized mouse macrophages treated with the cathepsin-B inhibitor, CA-074-Me. Secreted cytokine levels are mean ± s.e.m. Significance values are shown relative to untreated (0 uM) samples. P-values are shown as *** ≤ 0.0001; ** < 0.01
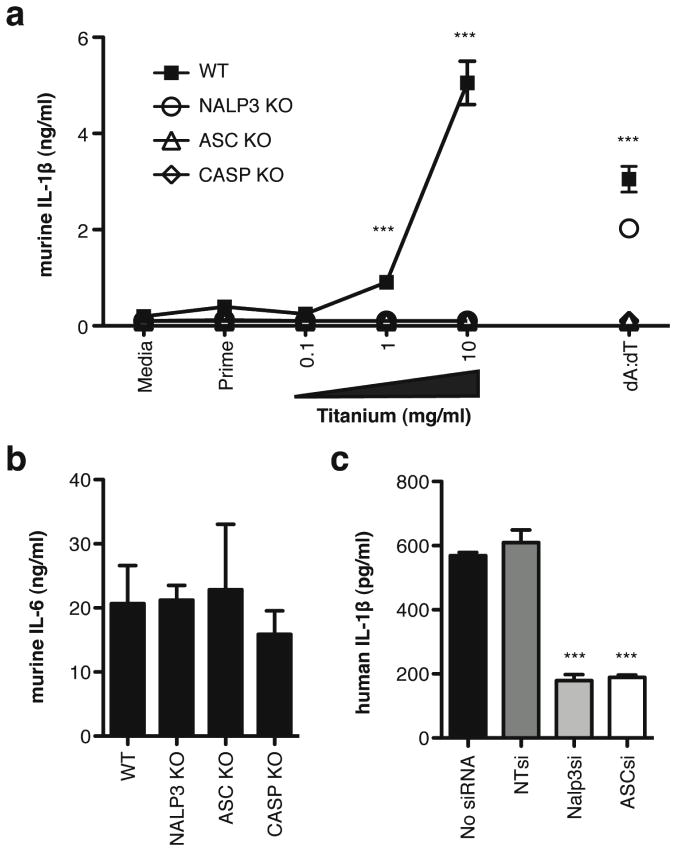
Figure 4.
Titanium-induced IL-1β secretion is inflammasome dependent. (a) IL-1β and (b) IL-6 secretion in WT, NALP3 KO, ASC KO, and CASP KO immortalized mouse macrophages. (c) IL-1β secretion following titanium stimulation in THP-1 human macrophages transfected with siRNA pools specific for NALP3, ASC, or luciferase (NT). Secreted cytokine levels are means ± s.e.m. P-values are shown as *** ≤ 0.0001; ** < 0.01
RESULTS
Titanium particles induce an IL-1 dependent neutrophil influx in vivo
Mice injected with titanium particles exhibited a significant increase in neutrophils compared to injections of PBS (Figure 1a). As expected, increased neutrophil numbers were seen in mice injected with the positive control, thioglycollate. To control for the possibility that LPS or endotoxin present on the surface of the titanium particles was responsible for the apparent immune response to injections, we used MD2-deficient (MD2 KO) and CD14-deficient (CD14 KO) mice. Both MD2 and CD14 facilitate LPS signaling through TLR4, and deficient mice are unresponsive to LPS (23, 24). Titanium injections were able to induce neutrophil influx in MD2 KO and CD14 KO mice at levels similar to WT control mice (Figure 1b, c), indicating that LPS did not contribute to neutrophil recruitment by titanium.
IL-1R has been shown to be required for neutrophil recruitment following exposure to stimulants in mice (13, 21, 25). To determine whether expression of IL-1R was required for titanium-induced neutrophil influx, IL-1R-deficient (IL-1R KO) mice were injected with titanium and compared to WT mice. Titanium injections were unable to induce a neutrophil influx in IL-1R KO mice (Figure 1d). In order to examine the role of IL-1 production, IL-1α/β KO mice were injected with titanium. Deficient mice exhibited significantly lower numbers of neutrophils (Figure 1e), confirming a role for IL-1 associated signaling in the immune response to titanium wear-particles in vivo. Additionally, WT mice treated with IL-1Ra, the soluble receptor antagonist of IL-1, showed a significant decrease in neutrophil recruitment after injection with titanium, compared to control-treated WT mice (Figure 1f). Taken together, these results indicate a critical role for IL-1 in titanium-induced neutrophil recruitment.
Titanium induces IL-1β production in mouse and human macrophages
WT immortalized mouse macrophages responded to titanium particles in a dose-dependent manner with high levels of secreted IL-1β (Figure 2a, c). Supernatants from macrophages that received an LPS prime only (Prime) did not exhibit an increase in IL-1β production. As a positive control, macrophages were transfected with double-stranded DNA, poly-dA:dT (dA:dT). WT macrophages produced a significant amount of IL-1β production in response to dA:dT stimulation. As additional controls, macrophages incubated with titanium particles alone produced low to un-detectable levels of IL-6, when compared to LPS stimulation, and IL-1β, when compared to primed cells (Figure S1), verifying that endotoxin levels associated with titanium particles did not play a role in observed cytokine responses.
A similar cytokine response to titanium particles was seen in PMA-differentiated THP-1 human macrophages. Titanium particles induced significantly higher levels of IL-1β secretion when compared to un-stimulated controls (Figure 2b). As a control, human macrophages exhibited mature IL-1β secretion in response to Nigericin, a known IL-1β stimulator. Titanium-induced IL-1β production was also dependent on particle concentration (Figure 2d). Together, these results demonstrate that both human and mouse macrophages respond to titanium particle stimulation with mature IL-1β secretion.
Titanium particles are internalized in macrophages
To visualize phagocytosis of titanium particles, immortalized mouse macrophages were incubated with titanium particles then analyzed through SEM. Titanium particles are highly internalized in macrophages (Figure 3a), with the majority of all titanium particles located within cells by 30 minutes. Macrophage-associated particles had a median diameter of 1.02 μm with a mean diameter of 1.21 μm (S.D. = 0.79 μm) (Figure 3b), similar to that seen in failed joints (26).
Activation and release of cathepsin B following uptake and lysosomal destabilization is required for optimal inflammasome activation in response to silica crystals, alum, and amyloid-β (13, 27). In order to determine whether cathepsin is required for inflammasome activation and subsequent IL-1β production in response to titanium, WT immortalized mouse macrophages were treated with the cathepsin B inhibitor CA-074-Me. Supernatants from cells pre-treated with CA-074-Me had significantly lower levels of IL-1β following titanium stimulation compared to untreated cells (Figure 3c). Treated and untreated cells produced comparable levels of the inflammasome independent cytokine, IL-6, in response to LPS, confirming that inhibition was inflammasome specific (Figure 3d). Together, these results indicate that optimal titanium-induced IL-1β production requires cathepsin B release.
Immune response to titanium requires the NALP3 inflammasome
Supernatants from immortalized macrophages generated from NALP3- (NALP3 KO), ASC- (ASC KO), and Caspase-1- (CASP KO) deficient mice exhibited undetectable levels of secreted IL-β in response to titanium while IL-1β secretion from WT macrophages was readily detected (Figure 4a). Stimulation with dA:dT induces mature IL-1β production in a NALP3-independent manner (28). As expected, NALP3 KO macrophages were able to respond to dA:dT similar to WT cells, while ASC KO and CASP KO macrophages were unable to respond to dA:dT. As a control, WT and deficient cells were able to produce similar levels of the IL-6 in response to LPS stimulation for 6 hours (Figure 4b).
A similar result was observed in THP-1 human macrophages. Cells transfected with siRNAs against NALP3 and ASC exhibited a significant reduction in the secretion of IL-1β in response to titanium particles (Figure 4c) compared to no-siRNA or non-targeting (NT) siRNA controls. Together, these results implicate a role for components of the NALP3 inflammasome complex in the IL-1 associated response to titanium particles in both mouse and human macrophages.
DISCUSSION
Our results show that titanium wear-particles induce IL-1β cytokine production in both human and mouse macrophages leading to IL-1 associated signaling and subsequent neutrophil recruitment (Figure 5). Although titanium is rarely used in bearing surfaces currently, titanium remains an important alloy used in many hip and knee replacements and understanding the immune response to titanium particles is an important contribution to our understanding the mechanisms of implant loosening and periprosthetic osteolysis. Studies have shown that among failed arthroplasties, patients with cemented titanium-aluminum-vanadium prostheses had the highest amounts of IL-1β in serum, compared to patients with implants comprised of other metals, such as cobalt and chromium (6). Additionally, it has been demonstrated that patients with failed cementless total hip arthroplasties have significantly higher levels of TNF, IL-6 and IL-1β and increased macrophage numbers when osteolysis is evident (29). Our data support these findings and suggest a potential mechanism for increases in IL-1β surrounding failed prostheses.
Figure 5.
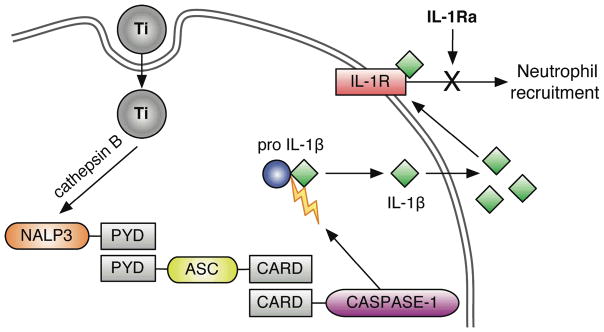
Titanium triggers inflammasome activation. Titanium particle internalization triggers the release of Cathepsin B from lysosomes, which together activate Nalp3. Activated Nalp3 recruits ASC through PYD domain interactions. This complex triggers cleavage and recruitment of activated Caspase-1 through CARD domain interactions. This inflammasome complex then cleaves pro-IL-1β into its active, secreted form IL-1β, which can trigger downstream IL-1 associated signaling, including neutrophil recruitment, through activation of the IL-1R. This acute inflammatory response can be inhibited with IL-1Ra treatment.
A role for IL-1 associated pro-inflammatory cytokine production following titanium rod implantation has been demonstrated in a murine intramedullary model (9). Our studies support these observations and suggest that neutrophil recruitment may also play a role in inflammation. Previous reports have also shown that implantation of 4 × 108 titanium particles (30 mg) onto the calvarium of mice leads to significant pro-inflammatory cytokine production and bone resorption after only one week (30). Our results show that peritoneal injection of 30 mg of titanium particles induces acute inflammation, as seen by neutrophil recruitment in mice within 16 h through IL-1 associated signaling. These results are consistent with other groups’ findings that neutrophils are recruited/activated following stimulation with particles including silica, asbestos, and monosodium urate crystals (13, 14, 21, 25, 31). Our results support the hypothesis that titanium wear-particles activate a pro-inflammatory response in humans through IL-1 associated signaling (Figure 5). Additionally, we have shown that neutrophil recruitment following titanium injections is significantly reduced in mice treated with the recombinant IL-1 receptor antagonist, IL-1Ra. IL-1Ra, also known as Anakinra™, has proven to be highly effective at decreasing bone erosion and joint space narrowing in rheumatoid arthritis patients (32–34). Studies have shown that retroviral delivery of IL-1Ra was able to decrease inflammation and inflammatory cytokine production in a murine air pouch model of osteolysis (35). It has also been shown that IL-1Ra treatment diminishes the amount of bone loss in ovariectomized rats and mice (36, 37). Polymorphisms within the IL-1 gene cluster associated with increased IL-1Ra mRNA expression have also been associated with decreased susceptibility to osteolysis after total hip arthroplasty (38). Along with these results, the ability of IL-1Ra to decrease neutrophilia following titanium injections in mice introduces the potential for antagonist-based therapies for titanium and other wear-particle induced inflammation.
We further demonstrate that titanium particles induce IL-1β secretion through activation of the NALP3 inflammasome complex (Figure 5). The role of the NALP3 inflammasome in these events is consistent with previous observations in responses to other implant materials (39). Interestingly, a growing number of systemic inflammatory diseases, characterized by fever, anemia, and elevated levels of acute-phase proteins, have been linked to abnormalities in NLR signaling pathways. Previous studies have shown that certain patients have increased inflammation secondary to particulate debris and are more likely than others to develop osteolysis and subsequent mechanical loosening of the implant ultimately requiring revision joint replacement. Defining the underlying causes of osteolysis will influence the appropriate treatment and management of particulate induced osteolysis and possibly identify those patients at high risk for this condition pre-operatively before the primary total joint replacement. Taken together, our results increase the understanding of how implant materials, and particulate material in general, interact with the host immune system and provide further insight into the development of treatments for titanium-particulate debris induced inflammation.
Supplementary Material
Acknowledgments
We would like to thank the following individuals at UMass: E. Latz and K. Fitzgerald for deficient macrophage cell lines, G. Hendricks for scanning electron microscopy imaging and analysis, and A. Cerny for animal husbandry and genotyping. This work was supported by NIH grants R01AI64349 to RWF and P01AI083215-01 to RWF and EKJ, NIH/NIAID grant U54AI057159 to RWF, and JDRF grant to RWF. Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used.
References
- 1.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs JJ, Roebuck KA, Archibeck M, et al. Osteolysis: basic science. Clin Orthop Relat Res. 2001;393:71–77. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Saeed S, Revell PA. Production and distribution of interleukin 15 and its receptors (IL-15Ralpha and IL-R2beta) in the implant interface tissues obtained during revision of failed total joint replacement. Int J Exp Pathol. 2001;82:201–209. doi: 10.1046/j.1365-2613.2001.iep0082-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revell PA. The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J R Soc Interface. 2008;5:1263–1278. doi: 10.1098/rsif.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stea S, Visentin M, Granchi D, et al. Cytokines and osteolysis around total hip prostheses. Cytokine. 2000;12:1575–1579. doi: 10.1006/cyto.2000.0753. [DOI] [PubMed] [Google Scholar]
- 6.Granchi D, Verri E, Ciapetti G, et al. Bone-resorbing cytokines in serum of patients with aseptic loosening of hip prostheses. J Bone Joint Surg Br. 1998;80:912–917. doi: 10.1302/0301-620x.80b5.8513. [DOI] [PubMed] [Google Scholar]
- 7.Rader CP, Sterner T, Jakob F, et al. Cytokine response of human macrophage-like cells after contact with polyethylene and pure titanium particles. J Arthroplasty. 1999;14:840–848. doi: 10.1016/s0883-5403(99)90035-9. [DOI] [PubMed] [Google Scholar]
- 8.Boynton EL, Waddell J, Meek E, et al. The effect of polyethylene particle chemistry on human monocyte-macrophage function in vitro. J Biomed Mater Res. 2000;52:239–245. doi: 10.1002/1097-4636(200011)52:2<239::aid-jbm1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Epstein NJ, Warme BA, Spanogle J, et al. Interleukin-1 modulates periprosthetic tissue formation in an intramedullary model of particle-induced inflammation. J Orthop Res. 2005;23:501–510. doi: 10.1016/j.orthres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Stashenko P, Dewhirst FE, Peros WJ, et al. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987;138:1464–1468. [PubMed] [Google Scholar]
- 11.Assuma R, Oates T, Cochran D, et al. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 12.Thomson BM, Mundy GR, Chambers TJ. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987;138:775–779. [PubMed] [Google Scholar]
- 13.Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinon F, Petrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 15.Dostert C, Petrilli V, Van Bruggen R, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masumoto J, Taniguchi S, Ayukawa K, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 17.Mariathasan S, Newton K, Monack DM, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 18.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 19.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Agostini L, Martinon F, Burns K, et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen CJ, Shi Y, Hearn A, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayat MA. Introduction to biological scanning electron microscopy. Baltimore: University Park Press; 1978. p. xviii.p. 323. [Google Scholar]
- 23.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 24.Wright SD, Ramos RA, Tobias PS, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 25.Chen CJ, Kono H, Golenbock D, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 26.Lee JM, Salvati EA, Betts F, et al. Size of metallic and polyethylene debris particles in failed cemented total hip replacements. J Bone Joint Surg Br. 1992;74:380–384. doi: 10.1302/0301-620X.74B3.1587882. [DOI] [PubMed] [Google Scholar]
- 27.Halle A, Hornung V, Petzold GC, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muruve DA, Petrilli V, Zaiss AK, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 29.Chiba J, Rubash HE, Kim KJ, Iwaki Y. The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clin Orthop Relat Res. 1994;300:304–312. [PubMed] [Google Scholar]
- 30.Schwarz EM, Lu AP, Goater JJ, et al. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res. 2000;18:472–480. doi: 10.1002/jor.1100180321. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher HR, Agudelo CA. Intravascular degranulation of neutrophils: an important factor in inflammation? Science. 1972;175:1139–1140. doi: 10.1126/science.175.4026.1139. [DOI] [PubMed] [Google Scholar]
- 32.Abramson SB, Amin A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 2002;41:972–980. doi: 10.1093/rheumatology/41.9.972. [DOI] [PubMed] [Google Scholar]
- 33.Bresnihan B, Alvaro-Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Genant HK, Watt I, et al. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 2000;43:1001–1009. doi: 10.1002/1529-0131(200005)43:5<1001::AID-ANR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Sud S, Yang SY, Evans CH, et al. Effects of cytokine gene therapy on particulate-induced inflammation in the murine air pouch. Inflammation. 2001;25:361–372. doi: 10.1023/a:1012898513512. [DOI] [PubMed] [Google Scholar]
- 36.Kimble RB, Vannice JL, Bloedow DC, et al. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J Clin Invest. 1994;93:1959–1967. doi: 10.1172/JCI117187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baltzer AW, Whalen JD, Wooley P, et al. Gene therapy for osteoporosis: evaluation in a murine ovariectomy model. Gene Ther. 2001;8:1770–1776. doi: 10.1038/sj.gt.3301594. [DOI] [PubMed] [Google Scholar]
- 38.Gordon A, Kiss-Toth E, Stockley I, et al. Polymorphisms in the interleukin-1 receptor antagonist and interleukin-6 genes affect risk of osteolysis in patients with total hip arthroplasty. Arthritis Rheum. 2008;58:3157–3165. doi: 10.1002/art.23863. [DOI] [PubMed] [Google Scholar]
- 39.Caicedo MS, Desai R, McAllister K, et al. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: A novel mechanism for implant debris reactivity. J Orthop Res. 2008;27:847–854. doi: 10.1002/jor.20826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.