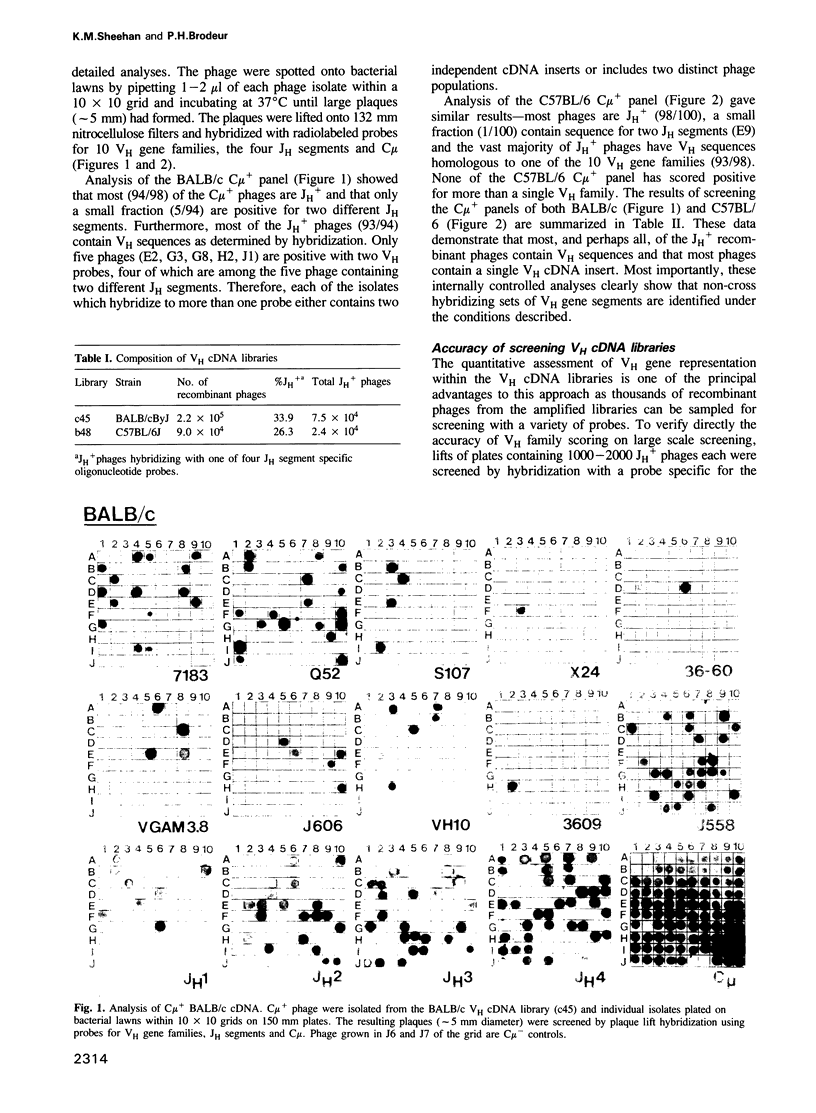
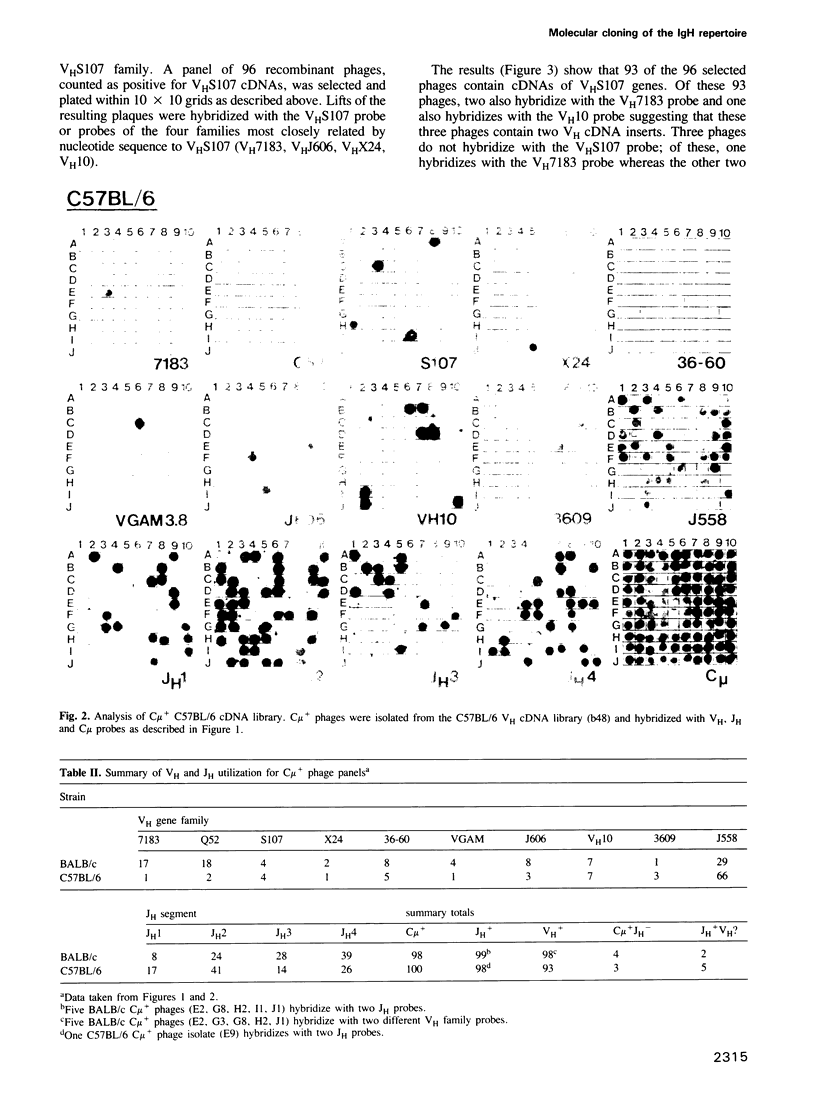
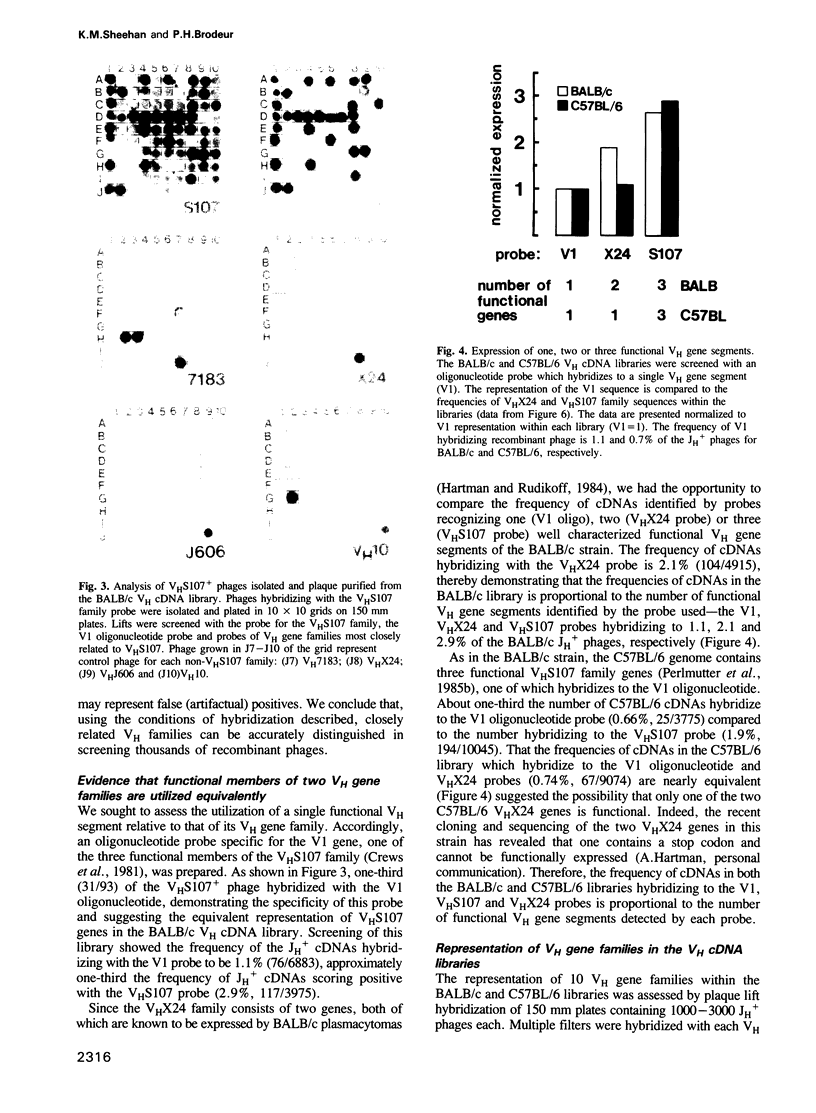
Abstract
The generation of the primary antibody repertoire requires the somatic rearrangement of germline gene segments. It is not known, however, whether all functional V and J gene segments have an equal probability of contributing to this initial set of antibody specificities. To address this issue, we have examined the relative utilization of VH and JH gene segments of the mouse. We have constructed VH cDNA phage libraries from C mu transcripts obtained from polyclonally activated spleen cells of the BALB/c and C57BL/6 strains. We show that probes specific for either one, two or three functional VH gene segments hybridize to cDNAs at frequencies directly proportional to the number of functional germline VH genes detected by each probe. In contrast, the representation of 10 VH gene families within each library indicates that certain families are under-represented relative to their estimated germline gene number. These families must either have extraordinary proportions of nonfunctional genes or are influenced by as yet unidentified regulatory mechanisms or constraints on rearrangement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. I. Distribution in different lymphoid organs from different inbred strains of mice at different ages. J Exp Med. 1977 Jun 1;145(6):1511–1519. doi: 10.1084/jem.145.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baumann B., Potash M. J., Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985 Feb;4(2):351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blankenstein T., Bonhomme F., Krawinkel U. Evolution of pseudogenes in the immunoglobulin VH-gene family of the mouse. Immunogenetics. 1987;26(4-5):237–248. doi: 10.1007/BF00346518. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Brodeur P. H., Osman G. E., Mackle J. J., Lalor T. M. The organization of the mouse Igh-V locus. Dispersion, interspersion, and the evolution of VH gene family clusters. J Exp Med. 1988 Dec 1;168(6):2261–2278. doi: 10.1084/jem.168.6.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Dildrop R., Krawinkel U., Winter E., Rajewsky K. VH-gene expression in murine lipopolysaccharide blasts distributes over the nine known VH-gene groups and may be random. Eur J Immunol. 1985 Nov;15(11):1154–1156. doi: 10.1002/eji.1830151117. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gerster T., Picard D., Schaffner W. During B-cell differentiation enhancer activity and transcription rate of immunoglobulin heavy chain genes are high before mRNA accumulation. Cell. 1986 Apr 11;45(1):45–52. doi: 10.1016/0092-8674(86)90536-2. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hartman A. B., Rudikoff S. VH genes encoding the immune response to beta-(1,6)-galactan: somatic mutation in IgM molecules. EMBO J. 1984 Dec 1;3(12):3023–3030. doi: 10.1002/j.1460-2075.1984.tb02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H. D., Komisar J. L., Kraig E., Teale J. M. Strain-dependent expression of VH gene families. J Immunol. 1988 Apr 1;140(7):2436–2441. [PubMed] [Google Scholar]
- Jeong H. D., Teale J. M. Comparison of the fetal and adult functional B cell repertoires by analysis of VH gene family expression. J Exp Med. 1988 Aug 1;168(2):589–603. doi: 10.1084/jem.168.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäck H. M., Wabl M. Immunoglobulin mRNA stability varies during B lymphocyte differentiation. EMBO J. 1988 Apr;7(4):1041–1046. doi: 10.1002/j.1460-2075.1988.tb02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Perry R. P. Transcriptional and posttranscriptional control of immunoglobulin mRNA production during B lymphocyte development. Nucleic Acids Res. 1986 Jul 11;14(13):5431–5447. doi: 10.1093/nar/14.13.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Wiedemann L. M., Pittet A. C., Strauss S., Nelson K. J., Davis J., Van Ness B., Perry R. P. Nonproductive kappa immunoglobulin genes: recombinational abnormalities and other lesions affecting transcription, RNA processing, turnover, and translation. Mol Cell Biol. 1985 Jul;5(7):1660–1675. doi: 10.1128/mcb.5.7.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler R. A new murine Ig VH gene family. J Immunol. 1988 Jun 1;140(11):4031–4034. [PubMed] [Google Scholar]
- Lawler A. M., Lin P. S., Gearhart P. J. Adult B-cell repertoire is biased toward two heavy-chain variable-region genes that rearrange frequently in fetal pre-B cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2454–2458. doi: 10.1073/pnas.84.8.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livant D., Blatt C., Hood L. One heavy chain variable region gene segment subfamily in the BALB/c mouse contains 500-1000 or more members. Cell. 1986 Nov 7;47(3):461–470. doi: 10.1016/0092-8674(86)90603-3. [DOI] [PubMed] [Google Scholar]
- Maizels N., Bothwell A. The T-cell-independent immune response to the hapten NP uses a large repertoire of heavy chain genes. Cell. 1985 Dec;43(3 Pt 2):715–720. doi: 10.1016/0092-8674(85)90244-2. [DOI] [PubMed] [Google Scholar]
- Manser T. Mitogen-driven B cell proliferation and differentiation are not accompanied by hypermutation of immunoglobulin variable region genes. J Immunol. 1987 Jul 1;139(1):234–238. [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Mason J. O., Williams G. T., Neuberger M. S. The half-life of immunoglobulin mRNA increases during B-cell differentiation: a possible role for targeting to membrane-bound polysomes. Genes Dev. 1988 Aug;2(8):1003–1011. doi: 10.1101/gad.2.8.1003. [DOI] [PubMed] [Google Scholar]
- Pennell C. A., Arnold L. W., Haughton G., Clarke S. H. Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J Immunol. 1988 Oct 15;141(8):2788–2796. [PubMed] [Google Scholar]
- Perlmutter R. M., Berson B., Griffin J. A., Hood L. Diversity in the germline antibody repertoire. Molecular evolution of the T15 VN gene family. J Exp Med. 1985 Dec 1;162(6):1998–2016. doi: 10.1084/jem.162.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Rathbun G. A., Capra J. D., Tucker P. W. Organization of the murine immunoglobulin VH complex in the inbred strains. EMBO J. 1987 Oct;6(10):2931–2937. doi: 10.1002/j.1460-2075.1987.tb02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininger L., Kaushik A., Izui S., Jaton J. C. A member of a new VH gene family encodes antibromelinized mouse red blood cell autoantibodies. Eur J Immunol. 1988 Oct;18(10):1521–1526. doi: 10.1002/eji.1830181008. [DOI] [PubMed] [Google Scholar]
- Schiff C., Milili M., Fougereau M. Functional and pseudogenes are similarly organized and may equally contribute to the extensive antibody diversity of the IgVHII family. EMBO J. 1985 May;4(5):1225–1230. doi: 10.1002/j.1460-2075.1985.tb03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze D. H., Kelsoe G. Genotypic analysis of B cell colonies by in situ hybridization. Stoichiometric expression of three VH families in adult C57BL/6 and BALB/c mice. J Exp Med. 1987 Jul 1;166(1):163–172. doi: 10.1084/jem.166.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter E., Radbruch A., Krawinkel U. Members of novel VH gene families are found in VDJ regions of polyclonally activated B-lymphocytes. EMBO J. 1985 Nov;4(11):2861–2867. doi: 10.1002/j.1460-2075.1985.tb04015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. E., Paige C. J. VH gene family utilization in colonies derived from B and pre-B cells detected by the RNA colony blot assay. EMBO J. 1986 Dec 20;5(13):3475–3481. doi: 10.1002/j.1460-2075.1986.tb04672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. E., Paige C. J. VH gene family utilization is regulated by a locus outside of the VH region. J Exp Med. 1988 Apr 1;167(4):1499–1504. doi: 10.1084/jem.167.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L., Manser T., Gefter M. L. Somatic evolution of variable region structures during an immune response. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1847–1851. doi: 10.1073/pnas.83.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Malynn B. A., Alt F. W. Developmentally regulated and strain-specific expression of murine VH gene families. J Exp Med. 1988 Jul 1;168(1):417–435. doi: 10.1084/jem.168.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Transcriptional regulation of the mu-delta heavy chain locus in normal murine B lymphocytes. J Exp Med. 1984 Aug 1;160(2):564–583. doi: 10.1084/jem.160.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]