Abstract
Airway tissue ischemia and hypoxia in human lung transplantation is a consequence of the sacrifice of the bronchial circulation during the surgical procedure and is a major risk factor for the development of airway anastomotic complications. Augmented expression of hypoxia-inducible factor (HIF)-1α promotes microvascular repair and alleviates allograft ischemia and hypoxia. Deferoxamine mesylate (DFO) is an FDA-approved iron chelator which has been shown to upregulate cellular HIF-1α. Here, we developed a nanoparticle formulation of DFO that can be topically applied to airway transplants at the time of surgery. In a mouse orthotopic tracheal transplant (OTT) model, the DFO nanoparticle was highly effective in enhancing airway microvascular perfusion following transplantation through the production of the angiogenic factors, placental growth factor (PLGF) and stromal cell-derived factor (SDF)-1. The endothelial cells in DFO treated airways displayed higher levels of p-eNOS and Ki67, less apoptosis, and decreased production of perivascular reactive oxygen species (ROS) compared to vehicle-treated airways. In summary, a DFO formulation topically-applied at the time of surgery successfully augmented airway anastomotic microvascular regeneration and the repair of alloimmune-injured microvasculature. This approach may be an effective topical transplant-conditioning therapy for preventing airway complications following clinical lung transplantation.
Keywords: Transplantation, Oxygenation, Surface treatment, Nanoparticle, Wound healing, Bioabsorption
1. Introduction
Lung transplantation continues to be the definitive therapy for many end-stage pulmonary diseases [1]. However, post-operative survival may be limited by serious airway complications, including anastomotic dehiscence, infection and airway stenosis, as a result of donor bronchus ischemia [2]. These complications continue to occur despite improvements in surgical technique and the development of more effective immunosuppressive medications. Because airway ischemia is thought to be a crucial factor in the development of these anastomotic problems, therapies to improve allograft perfusion may be an effective approach for decreasing these transplant-related complications.
Neovascularization is primarily regulated by the transcription factor hypoxia-inducible factor (HIF)-1 [3]. HIF-1 consists of a constitutively expressed HIF-1β subunit and an oxygen-regulated HIF-1α subunit [4]. In the presence of oxygen, two proline residues in HIF-1α are hydroxylated by the prolyl hydroxylase, PHD2. This facilitates the binding of the von Hippel–Lindau (VHL) complex and HIF-1α degradation. In hypoxic conditions, PHD2 is inactivated and HIF-1α is stabilized, HIF-1α then dimerizes with the β subunit, translocates to the nucleus, and induces gene transcription by binding to the hypoxia response elements (HRE) of oxygen-sensitive genes [5]. HIF-1-mediated transcriptional responses induce the expression of proangiogenic growth factors that directly activate resident endothelial cells and recruit circulating angiogenic cells [6,7].
DFO, a bacterial siderophore, is an FDA-approved therapy for iron overload-related diseases [8,9]. DFO also has been extensively studied in other disease models. DFO stabilizes HIF-1α by inhibiting the activity of PHDs through the depletion of Fe2+ [10,11], and induce the transcriptional activity of HIF-1α in tumors [12]. DFO also has been shown to promote pancreatic β cell function through upregulation of HIF-1α in a murine model of diabetes [13]. In a rat median nerve injury model, local administration of DFO-loaded lipid particles promoted end-to-end nerve reconstruction [14]. By stabilizing the HIF-1α protein, DFO potentiates the homing of mesenchymal stem cells to sites of injury to promote target tissue regeneration [15]. In a mouse hind limb ischemia model, DFO enhanced vascular repair and decreased tissue ischemia [16]. Lastly, numerous recent studies suggest that DFO may have a role as a neuro-protective agent through both HIF-1α-dependent and -independent mechanisms [17].
Using a mouse OTT model, our group discovered that enhanced expression of HIF-1α in airway grafts by adenovirus-mediated gene therapy increased the recruitment of Tie2 expressing angiogenic cells and prolonged tissue perfusion [18]. We further demonstrated that increasing HIF-1α expression through VHL knockdown in recipient Tie2 expressing angiogenic cells accelerated microvascular repair of the donor airway [19]. Based on these recent findings and the beneficial effects of DFO in various disease models, we hypothesized that enhancing HIF-1α expression through local administration of DFO would accelerate anastomotic microvascular regeneration, alleviating tissue ischemia and hypoxia with the potential to promote the health of the anastomosis and to limit post-transplant airway complications. To test this, we created a lipid nanoparticle formulation of DFO that may be applied topically to airway anastomoses and studied its effect in the mouse OTT model.
To improve the bioavailability of DFO to the donor trachea and the anastomotic ends of the recipient trachea, we formulated the compound in lecithin nanoparticles, a mixture of phospholipids and excipients, and used propylene glycol as the carrier [20]. Drug-loaded lipid nanoparticles have emerged as a promising strategy for the treatment of various diseases, because of their small size, biodegradability, versatility [21] and enhanced bioavailability within target tissues [22]. Propylene glycol is a small, hepatically-metabolized molecule, commonly used in food production and is “generally recognized as safe” (GRAS) by the FDA [23–25]. First, we characterized the physical and chemical properties of the formulation with atomic force microscopy (AFM) and scanning electron microscopy (SEM), aided by Raman spectroscopy. Nanoparticle penetration into the tracheal tissue was assessed by fluorescent confocal microscopy and mass spectroscopy of tissue sections. Lastly, we examined the in vivo effect of the nanoparticle formulation on anastomotic airway microvascular regeneration and promotion of allograft perfusion in the mouse OTT model. The main objective of this study was to determine whether peritransplant tissue ischemia could be improved by topical administration of HIF-1α-promoting nanoparticles at the time of surgery.
2. Material and methods
2.1. Preparation of nanoparticle formulations
Analytical grade DFO was purchased from Sigma (St. Louis, MO). Lecithin was obtained from the soft-gels nutritional supplement made by Finest Natural and distributed by Walgreens. Diagnostic grade probumin was purchased from Millipore (Billerica, MA). All solvents used were reaction grade. To prepare the DFO dry powder, equal amounts of DFO and lecithin (48.49% each, by weight) were mixed with a 0.5% aqueous solution of probumin (3.02% by weight). The solution was stirred vigorously until a fine suspension was achieved; this suspension was then lyophilized. A control formulation containing only the vehicle was prepared by making a fine suspension of lecithin (94.14% by weight) in a 0.5% aqueous solution of probumin (5.86% by weight). The liquid suspension was then lyophilized. The final nanoparticle solution was prepared by mixing the dry powders with a 1:9 (w/v) ratio of 40% propylene glycol in deionized water.
2.2. Mice
All animal procedures were approved by Stanford’s Administrative Panel on Laboratory Animal Care (APLAC) and/or the VA Palo Alto Institutional Animal Care and Utilization Committee (IACUC). C57BL/6J (B6; H-2b) and Balb/C (H-2d) mice were used and were purchased from Jackson Laboratory.
2.3. Scanning electron microscopy (SEM)
2.3.1. Characterization of dry powders
All fixatives used in the preparation of samples for scanning electron microscopy were obtained from Electron Microscopy Sciences (Hatfield, PA). Nanoparticle formulations in propylene glycol solution were drop-casted onto an SEM sample stub with a double-sided carbon tab and then air dried at room temperature. The deposited powder was then sputter-coated with an Au–Pd film (7 nm in thickness) in a Denton Desk II machine (Denton Vacuum, NJ), and imaged with a Hitachi S-3400N VP-SEM (Hitachi High Technologies, TX), using secondary electron (SE) detection, operated at 10–15 kV.
2.3.2. Assessment of the tracheal microstructure following incubation in nanoparticle formulations
Whole tracheas were harvested from BALB/c mice and transferred to 1× PBS on ice. The tracheas were incubated in nanoparticle solutions at 37 °C for 10 min in a humidified chamber. The tracheal sections were rinsed in 1× PBS twice, blot dried and fixed overnight in 4% paraformaldehyde with 2% glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.4). Tissues were gently washed twice with the same buffer, and then post-fixed in 1% aqueous osmium tetroxide (OsO4) for one hour. Samples were then washed twice in purified water and dehydrated in an increasing ethanol series (50%, 70%, 90%, 100% (2×) 15 min each). Finally, the specimens were critical-point dried (CPD) in liquid CO2, in a Tousimis 815B critical-point dryer (Tousimis, MD). CPD-dried samples were mounted on 45° angled SEM stubs with adhesive copper tape and sputter-coated with 4 nm of Au–Pd, as described above. The adventitial and mucosal layers of the sections were examined with a Zeiss Sigma field emission SEM (FESEM) (Carl Zeiss Microscopy, NY) operated at 2–3 kV, using InLens SE detection.
2.4. HPLC–MS analysis of drug penetration into tracheas
2.4.1. Sample preparation
2.4.1.1. Determination of the kinetics of DFO absorption into tracheal tissue
Whole tracheas were harvested from BALB/c mice and transferred to 1× PBS on ice. Each trachea (3–4 mg dry weight) was cut evenly into 3 or 4 cross-sectional segments. Tracheal segments were then dipped in DFO formulation for 3 s, blot dried to remove excessive solution and incubated in a humidified chamber at 37 °C for 0, 10, 30 and 60 min. After incubation, the segments were rinsed in 1× PBS twice and digested in 50 μl of 0.75 mg/ml Liberase TL (Roche Applied Science, IN) in H2O at 37 °C overnight. Digested tissues were further homogenized by sonication.
2.4.1.2. Preparation of pig and human trachea for DFO penetration analysis
Pig tracheas were obtained from a local slaughter house; human tracheas were obtained from Stanford hospital, and the usage of human tracheas was approved by IRB. After a 4 h incubation in DFO nanoparticle solution, pig and human trachea sections were prepared by lateral sectioning. Sections (0.5 mm each) were collected and digested with 3 volumes (v/w) of Liberase TL (0.75 mg/ml in H2O) overnight at 37 °C. Samples of tracheal lysate were vortexed and homogenized with a probe sonicator.
For all types of tracheal tissues, 50 μl of acetonitrile (100 μl) was added to the tissue homogenate to extract DFO. The samples were then centrifuged and the supernatant was diluted (1:20 to 1:100) in 50% acetonitrile and transferred to HPLC vials.
2.4.2. HPLC–MS/MS analysis
2.4.2.1. Preparation of HPLC–MS/MS standards
All chemicals and solvents for HPLC–MS/MS were purchased from Sigma (St. Louis, MO) or Fisher Scientific (Hampton, NH). Stock standard solutions of DFO were prepared by dilution of accurately weighed powders in DMSO. Calibration spiking solutions were prepared by diluting the stock solution with methanol:water (1:1, v/v) to final concentrations of 50, 20, 10, 5, 2, 1, 0.500, 0.200, and 0.100 μg/ml of DFO. Standard spiking solutions (30 μl) were added into vehicle treated tracheal section homogenates and processed with each batch of unknown samples. Chromatograms for standards were used to establish characteristic retention times (RTs) of DFO, and verified that the MS signal was linear over the range of 0.1–50 μg/ml in tracheal section homogenates. The peak areas of DFO were calculated and plotted against the concentration of the calibration standards. Calibration curves were generated using the least squares linear regression method with Analyst® 1.5.1 software.
2.4.2.2. HPLC–MS/MS data acquisition
For DFO separation and detection, the flow rate was set at 300 μl/min. Chromatographic separation was performed on an Ascentics ES Cyno column (Sigma, MO). Mobile phase A was 5 mm ammonium acetate/0.1% formic acid in water and mobile phase B was 0.1% formic acid in acetonitrile. A 2.5-min elution was performed with a 20–90% gradient of mobile phase B. After 3 min, %B was changed to 20% and kept for 1 min. The HPLC was directly coupled to an AB SCIEX 4000 QTRAP triple quadrupole mass spectrometer with electrospray ionization. To monitor DFO, the mass spectrometer was operated in the positive multiple reactions monitoring mode, with transitions of 561.17/102.30 and 560.79/201.00 Da. The switching valve diverted HPLC flow to the mass spectrometer at 0.4–3 min. The elution time for DFO was 0.7 min.
2.4.2.3. HPLC–MS/MS data analysis
Peak detection, integration and data processing were performed with the AB SCIEX Analyst 1.5.1 software package. Concentrations of DFO were calculated by plotting the peak area of unknown samples against the calibration curve prepared in the corresponding matrix. A 1/× weighted linear regression was used to calculate the unknown DFO concentrations.
2.5. Raman spectroscopy and atomic force microscopy (AFM) imaging
Both Raman and AFM were performed using NTEGRA Spectra combined AFM-Raman system (NT-MDT). For individual particle Raman scanning, dry lyophilized propylene glycol particle cluster was gently tapped against the surface of pre-cleaned Si wafers. Tissue samples for Raman scanning were made by spreading the nanoparticle solution on tissue patches (about 7 × 7 mm), which were fixed to the surface of glass slides and allowed to dry. Raman measurements and confocal scanning of the nanoparticles applied to either Si wafers or tracheal tissues were performed in backscattering geometry with a long-working Mitutoyo objective (100×, 0.7 NA). The illumination light was 473 nm, and the power was kept at ~0.8 mW to minimize sample damage. Raman maps were produced with a step size of 0.5 μm and 1 s exposure. 600 g/mm gratings were used for optimal signal and spectral resolution.
AFM imaging was performed in tapping mode with commercial cantilevers (k = 5.4 N/m, R <10 nm) at 0.7 Hz. This provided surface topography and phase contrast images to discern stiffness of different areas within the islands. The locally equalized topography image was also obtained from the initial topography image by the AFM image analysis software, supplied with the instrument, to allow taller structures to be seen.
2.6. Analysis of nanoparticle cellular localization
Rhodamine B isothiocyanate (RBITC) was purchased from Sigma; 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol)-amine (DSPE–PEG–NH2, Mw = 3400) was purchased from Laysan Bio (Arab, AL) and the PD-10 desalting column was purchased from GE Healthcare. Rhodamine, a fluorescent marker, was linked to an inert lipid (DSPE) in the nanoparticle formulation. The linking reaction was performed by dissolving 34 mg (10 μm) of DSPE–PEG–NH2 and 15.6 mg (29.1 μm) of RBITC in a 1 ml solution of methanol:water (1:9, v/v). The reaction mixture was stirred overnight in a dark room at 4 °C. The solution was then run through a PD-10 desalting column with MilliQ water to remove the unreacted RBITC. The labeled fractions were collected and lyophilized to obtain rhodamine-labeled DSPE. To prepare the fluorescent labeled nanoparticles, DSPE was mixed with 1% lecithin by weight. The labeled nanoparticles were administered on the inside and outside of the walls of tracheal samples, and then incubated at 37 °C for 4 h. After incubation, they were washed 3 times with PBS, then embedded in OCT (Sakura Finetek) to make frozen sections. The tissue blocks were cut to 20 μm sections. Samples were stained with mounting media containing DAPI fluorescent dye and imaged with a Leica SP2 confocal fluorescence microscope.
2.7. Tracheal transplantation
Four to six week old BALB/c mice were used as donors and age and sex matched B6 mice were used as recipients. The surgical procedure of orthotopic tracheal transplantation was performed as previously described [18]. Briefly, both donor and recipient mice were anesthetized with 50 mg/kg of ketamine and 10 mg/kg of xylazine. 5- to 7-ring tracheal segments were removed from donor mice. The donor tracheas were stored in PBS on ice prior to transplantation. A ~2–3 cm incision was made in the midline of the recipient’s neck. The strap muscles were then bluntly dissected and retracted with 3-0 suture to allow clear exposure of the laryngotracheal complex. After the recipient trachea was transected, the donor graft was removed from the PBS, blot dried and then soaked in the nanoparticle formulation for approximately 5 s. The trachea was removed from the solution and blot dried to remove excess nanoparticle solution. The trachea was then sewn in with 10-0 nylon suture as previously described [18,26]. Next, ~100 μl of nanoparticle solution was applied to the outer wall of the donor trachea and anastomoses. The skin was closed with 5-0 silk sutures.
2.8. Blood perfusion monitoring by laser Doppler flowmetry
The procedure has been described in detail in Ref. [27]. In short, the transplanted mice were placed under general anesthesia and the tracheal grafts were carefully exposed using stay sutures to gently retract the strap muscles, revealing the anterior wall of the trachea. Perfusion monitoring was performed with a fiberoptic LDF probe connected to the OxyLab laser Doppler flowmetry (LDF) monitor (Oxford Optronix). This provides a continuous digital readout of blood perfusion units (BPUs) by real-time measurements of red blood cells in flux that is proportional to the red blood cell perfusion. The probe is connected to a micromanipulator and is gently lowered onto the outer surface of tracheal grafts. BPU measurements were recorded.
2.9. Tissue preparation for perfusion studies and immunohistochemistry
For whole-mount tracheal microvascular analysis, mice were injected with 100 μl of FITC-conjugated tomato lectin (Vector Laboratories) at a concentration of 1 mg/ml. After 5 min of circulation, the mice were perfused with 1% PFA diluted in PBS for about 2 min until the outflow of the solution turned clear. The tracheas were then harvested, fixed in 1% PFA for 1 h at 4 °C, and then washed 3 times with PBS. Whole tracheas were mounted on glass slides in Vectashield H-1200 mounting medium (Vector Laboratories). Assessment of the percentage of the perfused area was carried out as previously described [19]. Briefly, the whole tracheal allograft (every cartilaginous and inter-cartilaginous region) was examined and each area was scored either a 1 if it was perfused or 0 if it was not perfused. The percent perfusion was then calculated as follows: total score/total regions examined. Frozen sections were used for other immunohistochemistry analysis. Tracheal samples were snap-frozen in OCT solution, and the samples were stored at −80 °C. Eight micron thick sections were used for immunofluorescence staining. Anti-CD31 antibody (1:200; BD Pharmingen) and anti-VEGFR2 (1:100, R&D system) were used to stain endothelial cells; anti-PLGF (1:100) and anti-SDF-1 (1:100) were both purchased from R&D systems; anti-Ki67 antibody (1:100; BD Pharmingen) was used to stain proliferating cells; anti-p-eNOS antibody (1:100; Cell Signaling) was used to stain the phosphorylated form of eNOS in endothelial cells. Dihydroethidium (DHE) (20 μM, Invitrogen) was used to detect ROS. The TUNEL assay (Invitrogen, C10245) to stain for apoptotic cells was carried out according to the manufacturer’s protocol. Photomicrographs were taken with a Zeiss LSM 510 laser scanning confocal microscope with Zeiss LSM Image Browser software. Quantification of the staining of Ki67, p-eNOS, DHE and TUNEL were performed with ImageJ software.
2.10. Quantitative real time RT-PCR
Tracheal samples were incubated in RNAlater solution (Invitrogen) overnight at 4 °C. Total RNA was then isolated using the QIAGEN Shredder and RNeasy Mini Kit (QIAGEN) as per the manufacturer’s protocol. Total RNA (1 μg) was reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Invitrogen) and 5 μM random hexamer primers according to the manufacturer’s protocol. 2 μl of 1:10 diluted reverse transcription reactions were added to quantitative real time-PCR (qRT-PCR) reactions with 5 μl of 2× SYBR Green Master Mix (Applied Biosystems) and 100 nm of forward and reverse primers specific for the genes of interest in a total volume of 10 μl. Detection was carried out with the ABI Prism 7700 sequence detector (Applied Biosystems). SDS analysis software (Applied Biosystems) was used to analyze the data. 18S mRNA expression was used to normalize gene expression to account for sample-to-sample variation in input and reverse transcription efficiency. The 2−ΔΔCt method was utilized to calculate fold changes. The primers employed are listed in Supplementary Table 1.
2.11. Statistics
Statistical analysis was performed using a 2-tailed Student’s t test, with a significance level of P <0.05.
3. Results
3.1. Structure and morphology analysis of drug nanoparticles
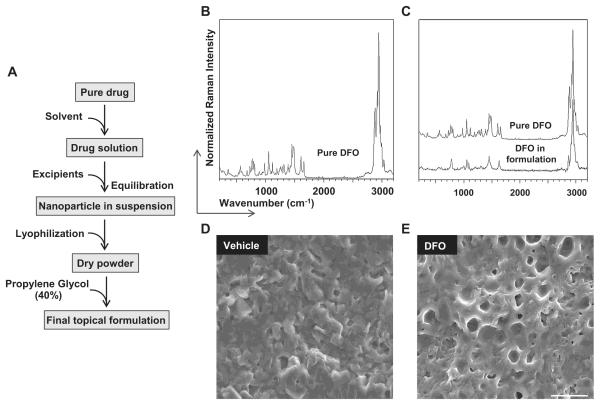
DFO was formulated into encapsulated drug nanoparticles, drug powders and final topical solutions as shown in Fig. 1A. We chose the tracheal membrane-compatible lecithin to encapsulate the drugs to ensure their efficient delivery to the tissue. To assess the encapsulation efficiency, structural analysis of DFO before and after encapsulation was performed using Raman spectroscopy. A Raman spectrum of pure DFO was first examined (Fig. 1B). DFO molecules in the nanoparticle exhibited a spectrum different from that of pure DFO, with many bands merging together and becoming broader, which was likely due to strong hydrodynamic screening of DFO molecules and the disruption of its crystalline structure (Fig. 1C).
Fig. 1.
Analysis of drug molecule structure and morphology study of encapsulated nanoparticles. A. A schematic showing the procedure for nanoparticle formulation. B and C. Drug structure analysis by Raman spectroscopy showing the structures of pure DFO (B) and DFO in the nanoparticle formulation (C). D and E. Nanoparticle morphology analysis shows that the vehicle (D) and DFO dry powders (E) are homogeneous. Scale bar: 20 μm (D, E).
Next, SEM was used to study the morphology of the nanoparticles. To acquire SEM images of dry nanoparticle powder, 40% propylene glycol was first used to make the nanoparticle solution which was then deposited onto generic aluminum SEM sample stubs and air-dried in situ. The blank vehicle showed a generally homogeneous lecithin structure (Fig. 1D), and DFO nanoparticles also showed homogeneous semi-porous networks (Fig. 1E).
3.2. Nanoparticle homogeneity
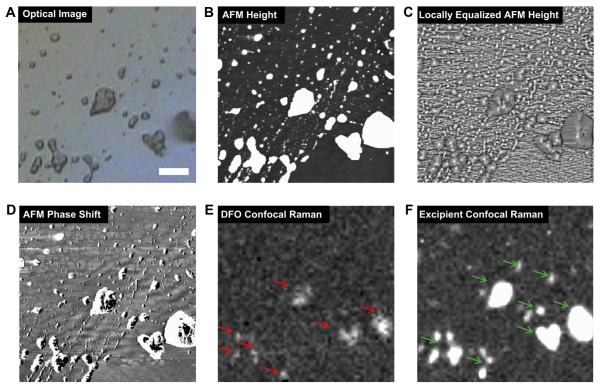
To determine the degree of homogeneity in the distribution of DFO within the nanoparticle, confocal Raman scanning and AFM imaging of small nanoparticle islands on the surface of Si wafers was performed. Sample material was loaded onto the surface of Si wafer to ensure the acquisition of high quality images, and imaging was performed under low power (<1 mW) to avoid sample damage. The optical image of DFO showed that the surface was covered by separate islands (Fig. 2A). The inner structure of the islands was probed by AFM scanning in tapping mode. AFM images showed the morphology and size of smallest nanoparticles, as well as larger nanoparticle aggregates (Fig. 2B–D). Confocal Raman images showed uniform distribution of the excipient and the DFO nanoparticle formulation as well as very good correlation between the distribution of DFO and excipient (Fig. 2E, F). These data together demonstrate that DFO was efficiently encapsulated in the excipient lecithin.
Fig. 2.
Sample homogeneity analysis of DFO in nanoparticle islands on a Si wafer. A–F. DFO sample homogeneity was assessed by optical image (A), AFM height (B), locally equalized AFM height (C), AFM phase shift (D), confocal Raman maps of DFO (red arrows) (E) and the excipient (green arrows) (F). The Raman confocal maps were acquired by integrating the intensities of the following peaks: DFO peak centered at 1620 cm−1 (1600–1640 cm−1) (E) and excipient peak at 1655–1695 cm−1 (F). Scale bar: 5 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Microstructure analysis of nanoparticle treated tracheas
Although the main ingredients used in the nanoparticle formulation are considered safe, we wanted to confirm that the administration of the nanoparticles on the tracheal surface would not adversely affect tracheal microstructures. SEM was used to examine the morphology of the nanoparticle-treated tracheas. The images showed that the adventitial layer of the tracheas treated with vehicle or DFO solution were not significantly different from that of the untreated samples. Similar to the untreated tracheal samples, individual collagen fibrils displayed fine structures with lateral rings clearly visible (Fig. S1A). Also, the mucosal layer of the tracheas treated with vehicle or nanoparticles did not show any visible signs of damage (Fig. S1B). Only a few brushes were observed to be missing from the tops of some cilia bundles in treated samples; a finding likely caused by the capillary forces exerted by water during the nanoparticle solution washing process. Altogether, our data suggest that a 10 min incubation of tracheas in the nanoparticle formulation did not significantly affect the microstructure of the airway.
3.4. Drug penetration into the tracheal tissue
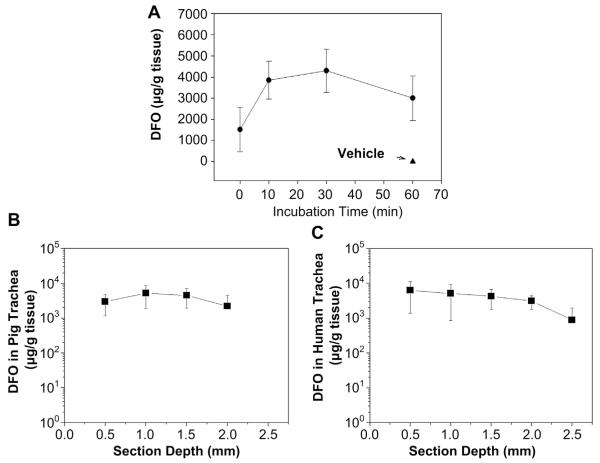
We next assessed the drug nanoparticle penetration into the tracheal tissue. Examination of the penetration kinetics showed that the DFO nanoparticle achieved near-maximum penetration at 10 min of incubation, and reached a plateau when approaching 60 min (Fig. 3A). We then determined the depth of drug penetration and absorption by HPLC–MS/MS. Because mouse tracheas are relatively thin, we chose to use pig and human tracheas for these studies. Although the efficiency of penetration was variable, DFO nanoparticles were able to penetrate the pig and human tracheas (Fig. 3B, C). In the pig trachea, DFO penetrated to and was absorbed to a depth of 2 mm (Fig. 3B). A similar trend was also observed in the human trachea penetration depth analysis (Fig. 3C). These data suggest that the penetration of DFO is efficient in both species of mammalian tracheas examined.
Fig. 3.
Assessment of the nanoparticle penetration into tracheas. A. Kinetics of DFO nanoparticle penetration into mouse tracheas. B and C. Analysis of the depth of the nanoparticle penetration into pig (C) and human (D) tracheas. The DFO concentrations were measured by HPLC (A–C). Data are shown as means ± σ.
3.5. Drug penetration into cytoplasm of the tracheal cells
To test the efficacy of drug absorption, we used confocal microscopy to determine the cellular localization of the drug nanoparticles. Because cells of the subepithelial layer play a more important role in angiogenesis [18], we examined the penetration of drug into these cells. Fluorescence-tagged vehicle was found to be localized in the cytoplasm of cells in tracheas treated with vehicle or DFO nanoparticles (Fig. 4A, B). Quantification showed that the percentages of fluorescence-positive cells were about 70% and 60% for the vehicle and DFO formulation respectively (Fig. 4C). Because the drugs were previously shown to be well-encapsulated by the vehicle (Fig. 1E), the fluorescence signal can be used to estimate the cellular localization of the drug molecules. These images confirmed that the DFO nanoparticle formulation efficiently penetrated the tissue and reached the cells in the subepithelial layer of the trachea.
Fig. 4.
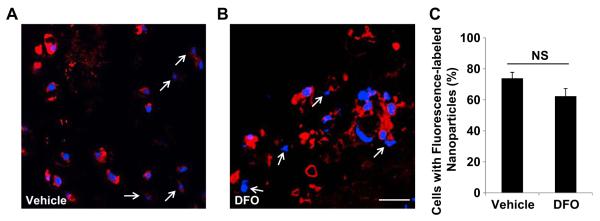
Subepithelial cellular localization of nanoparticle formulations. A and B. Confocal microscopy images showing the subepithelial cellular distribution of vehicle alone nanoparticles (A) and DFO nanoparticles (B). C. Quantification of cellular nanoparticle localization by percentage of cells with cytoplasmic fluorescence. Red: Rhodamine-labeled DSPE-PEG identifies the lipid vehicles of the nanoparticles; blue: nuclear staining by DAPI. White arrows: cells with no cytoplasmic fluorescent signal. Data are shown as means ± S.E.M. NS, not significant, Student’s t test (C). Scale Bar: 20 μm (A, B). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Effects of DFO nanoparticle on microvascular anastomosis formation and airway microvascular perfusion
The mouse OTT model has been shown to faithfully replicate lymphocytic bronchitis observed in lung transplant recipients [28], and is useful for studying phenomena associated with clinical airway complications. We have previously shown that the airway microvascular circulation can be easily studied in this model [26] and that the perfusion of the airway allograft can be used to assess the regeneration of the injured airway microvasculature, particularly at the anastomosis [18]. The airway allograft is transplanted en bloc, and there is no vascular perfusion prior to the formation of the microvascular anastomosis between the graft donor and the recipient. Therefore, earlier (i.e. day (d) 3 following transplantation) appearance of graft perfusion indicates an accelerated vascular anastomosis formation. In this model, airway perfusion loss around d10 is consistently observed and is primarily caused by alloimmune-mediated endothelial cells injury as previously described [26]. Thus, persistent airway microvascular perfusion at d10 indicates more efficient repair of damaged vessels. FITC-lectin microvascular perfusion images showed that DFO treatment significantly increased airway perfusion at both d3 and d10 following transplantation (Fig. 5A), and the microvascular perfusion of vehicle treated allografts was not significantly differently from non-treated control transplants (Fig. 5A). Percentages of perfused areas of trachea allografts treated with DFO were >90% in contrast to <20% in control and vehicle treated airways at both d3 and d10 (Fig. 5B). The use of LDF for transplanted tracheal tissue blood perfusion was recently developed by our laboratory and has been previously used to assess airway perfusion [19,27]. LDF showed that perfusion of the allograft treated with DFO was significantly higher at both d3 and d10 compared to control and vehicle treated grafts (Fig. 5C). These studies suggest that DFO nanoparticles accelerated airway microvascular anastomosis formation and promoted the repair of damaged vasculature.
Fig. 5.
Effects of DFO nanoparticle formulation on airway microvascular perfusion. A. Confocal microscopic imaging showing microvascular perfusion of non-, vehicle- and DFO nanoparticle-treated airway allografts at d3 and d10 following transplantation. B. Quantification of perfused airway microvasculature following transplantation. C. Airway blood perfusion measured by laser Doppler flowmetry at d3 and d10 following transplantation. Scale bar: 100 μm (A). Data are shown as means ± SEM. *P < 0.05, Student’s t test (B, C).
3.7. Effects of DFO nanoparticle on angiogenic factor expression in ischemic airways
We next asked how DFO promotes airway microvascular perfusion. Expression of angiogenic factors and cytokines are closely associated with neovascularization. Based on the observation that the promotion of vascular perfusion by DFO was most significant at d3 following transplantation, we isolated mRNA from d3 allografts and analyzed the expression of angiogenic factors and cytokines (PLGF, SDF-1, VEGF, ANGPT1 and ANGPT2) and the angiogenic receptor, Tie2 by quantitative real time RT-PCR. Expression of PLGF and SDF-1 was significantly increased (Fig. 6A, B), but there was no significant difference observed in the expression of angiogenic factors, VEGF, ANGPT1 and ANGPT2 or the Tie2 receptor (Fig. 6C–F). Consistent with the results of the mRNA study, immunofluorescent staining showed that the levels of PLGF and SDF proteins were also increased (Fig. S2A, B). These data suggest that DFO likely promotes early microvascular anastomosis formation through the upregulation of angiogenic growth factors.
Fig. 6.
Analysis of angiogenic growth factors and associated angiogenic cytokines. A–E. Real time (RT)-PCR analysis of mRNA expression of angiogenic growth factors in d3 airway allografts treated with vehicle or DFO nanoparticles (n = 3–5). F. RT-PCR analysis of Tie2 mRNA expression in d3 allografts treated with vehicle or DFO nanoparticles (n = 3–5). Data are shown as means ± SEM. NS, not significant; *P < 0.05, Student’s t test.
3.8. Effects of DFO nanoparticle on tracheal endothelial cells
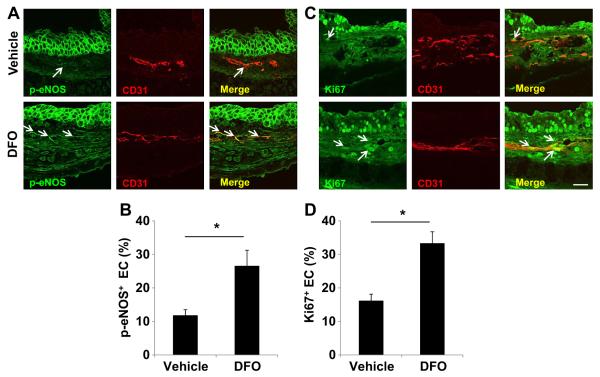
Endothelial nitric oxide synthase (eNOS) phosphorylation is associated with endothelial cell survival and angiogenesis. We hypothesized that DFO may increase eNOS phosphorylation in this transplantation model system. Examination of endothelial phosphorylated eNOS (p-eNOS) expression in d3 allograft showed that DFO treatment increased p-eNOS expression by about 2 fold (Fig. 7A, B). EC proliferation, measured by Ki67 staining, in DFO treated allografts was much higher than that of the vehicle treated samples (about 30% vs 15%) (Fig. 7C, D). Production of ROS in ischemic tissue is associated with EC death. Dihydroethidium (DHE) staining showed that DFO treated allograft exhibited much lower levels of perivascular ROS production (Fig. 8A, B). Lastly, the TUNEL assay showed that DFO treatment significantly decreased EC apoptosis (Fig. 8C, D). These data together suggested that DFO may also improve airway microvascular perfusion by augmenting angiogenesis through the promotion of EC proliferation and prevention of EC apoptosis.
Fig. 7.
Increased levels of p-eNOS and Ki67 in the endothelial cells of tracheas treated with DFO nanoparticles. A, C. Confocal microscopic images showing increased p-eNOS (green, white arrows) and Ki67 (green, white arrows) in ECs of DFO treated airways. B, D. Quantification of p-eNOS+ cells and Ki67+ cells (n = 3–5). Scale bars: 20 μm (A, C). Data are shown as means ± SEM. *P < 0.05, Student’s t test (B, D). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 8.
Decreased levels of perivascular ROS production and endothelial cell apoptosis in DFO treated tracheas. A, C. Confocal microscopic images showing decreased perivascular ROS production by DHE staining (red, white arrows) and EC apoptosis by TUNEL staining (green, white arrows). B, D. Quantification of perivascular DHE staining and EC TUNEL staining (n = 3–5). Scale bars: 20 μm (A, C). Data are shown as means ± SEM. *P < 0.05, Student’s t test (B, D). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Possibly because current practice omits bronchial artery revascularization at the time of surgery, large airway tissue ischemia is a common finding post-operatively and creates risk for developing anastomotic complications in human lung transplantation [2]. We have previously shown that augmenting HIF-1α expression in donor grafts by either adenovirus-mediated gene therapy or knockdown of the VHL expression in recipient-derived Tie2 expressing cells was able to promote airway microvascular regeneration and diminish airway ischemia [18,19]. In the current study, we sought to develop a nanoparticle formulation of DFO, an FDA-approved drug to augment the local expression levels of HIF-1α and ameliorate airway ischemia.
We started with the characterization of the biophysical properties of DFO nanoparticles by utilizing various techniques. Raman spectroscopy structure analysis and imaging showed that DFO encapsulation by lecithin was very efficient. Next, the SEM morphological study of the dry nanoparticle powder showed that the DFO formulation was also homogeneous. Lastly, confocal microscopy showed a very high percentage of drug-positive cells in tracheas treated with the DFO nanoparticles. Consistent with these, in vitro identified superior biophysical properties, the DFO nanoparticles were highly effective in promoting airway microvascular perfusion at both d3 and d10 following transplantation. Our data suggest that combination of the usage of Raman spectroscopy, SEM imaging, AFM imaging, confocal microscopy and HPLC–MS analysis can efficiently characterize biophysical and biological properties of the lecithin nanoparticle formulations. Nanoparticles with more efficient encapsulation, better tissue penetration and retention are likely to display higher bioactivity in vivo.
Prior to the formation of the microvascular anastomosis between the graft donor and the recipient, the airway allograft is not perfused. Therefore, improved d3 microvascular perfusion is a result of enhanced donor-recipient microvascular anastomosis formation. In clinical lung transplantation, early post-operative airway ischemia is observed as a result of delayed microvascular anastomosis formation and sacrifice of the bronchial circulation [29]. Thus, the effect of DFO nanoparticles on promoting airway microvascular anastomosis formation may have clinical relevance in terms of alleviating tissue ischemia with the potential to diminish airway complications. Airway ischemia has also been shown to be a risk factor for anastomotic bacterial and fungal overgrowth, which often further increases the risk of the development of airway complications [2,30,31]. We recently demonstrated that Aspergillus fumigatus airway invasion could be attenuated in transplant recipients with genetically-upregulated HIF-1α levels that resulted in better airway allograft perfusion [19]. These data together suggest that DFO nanoparticles may limit airway complications through alleviating tissue ischemia and diminishing relevant microbial infection.
DFO is a bacterial siderophore produced by the Actinobacteria Streptomyces pilosus. Because DFO depletes iron, it is generally used as an iron-chelating drug to treat iron overload conditions [8,9]. Recent studies suggest that, DFO also promotes angiogenesis and alleviates tissue ischemia in animal models [32,33]. This property of DFO is generally thought to be due to its ability to stabilize HIF-1α through the inhibition of prolyl 4-hydroxylase by chelation of iron from enzyme’s catalytic center [34,35]. In this study, we found that DFO treated airway grafts expressed significantly higher levels of PLGF and SDF-1, but no significant difference was noted in the expression of VEGF, ANGPT1 and ANGPT2. The increase in expression levels of PLGF and SDF-1 with DFO is consistent with our previous study utilizing adenovirus-mediated HIF-1α gene therapy [18]. HIF-1 activates transcription of the gene encoding SDF-1, and increased SDF-1 expression promotes vascular regeneration by enhancing recruitment of CXCR4-expressing angiogenic cells [7]. While other studies have shown that VEGF is often upregulated following DFO treatment [16,36], the DFO nanoparticles in this study did not increase VEGF expression in d3 allografts. This suggests that DFO may promote airway anastomotic microvascular formation mainly through PLGF-mediated signaling. It is likely that PLGF, like SDF1, serves as a chemotactic factor for the recruitment of bone marrow-derived angiogenic cells. PLGF is a member of the VEGF family of growth factors, but unlike VEGF, PLGF is not required for vascular development and homeostasis; PLGF has diverse nonredundant roles in various physiological or pathological status such as tissue ischemia, inflammation and malignancy [37]. PLGF is also considered a protective paracrine effector in the heart [38] and was recently shown to promote myocardial blood flow and contractile function in chronic myocardial ischemia by increasing neovascularization [39]. PLGF has also been shown to enhance endothelial cell proliferation, migration and survival [40–42]. Consistent with these studies, we observed increased expression of Ki67 in DFO nanoparticles treated tracheal endothelial cells, supporting the notion that, in this airway transplantation model, PLGF may promote airway anastomotic microvascular formation through stimulating endothelial cell proliferation and subsequent angiogenesis. However, we cannot rule out alternative mechanisms by which PLGF may promote angiogenesis, such as recruiting myeloid progenitor cells which facilitate the growth of vascular sprouts has been suggested [37].
DFO treatment significantly increased the levels of the p-eNOS. eNOS is activated/phosphorylated by the PI3K-Akt pathway [43,44]. Interestingly, PLGF has been shown to enhance Akt activation in endothelial cells to promote their proliferation and migration [45] and has also been shown to activate Akt in monocytes [46]. Recent studies showed that PLGF is a direct HIF target gene [47] and that it dilates mesenteric arteries through NO production [48]. It is therefore likely that, in this airway transplantation model, DFO increased p-eNOS through PLGF activated PI3K-Akt pathway.
ROS are known to cause endothelial cell dysfunction [49,50], and increased ROS production promotes eNOS uncoupling, which is a significant contributor to oxidative stress [51]. Iron participates in the redox reactions that lead to the production of ROS [52], and the reduction of ROS by iron chelation has been shown to be an effective therapy for atherosclerosis [53,54]. These studies suggest that endothelial cell damage may be promoted by a feed-forward cycle of eNOS dysfunction leading to ROS production which leads to further eNOS dysfunction. In airways treated with DFO, we observed a reduction in ROS production concomitantly with increased levels of p-eNOS; this finding suggests that through its iron-chelating activity, DFO may prevent or at least ameliorate endothelial cell injury through reducing oxidative stress by enhancing the function of eNOS. In summary, DFO augmented airway anastomotic microvascular regeneration through the production of angiogenic factors as well as reduction of ROS, which improved overall endothelial cell health and decreased airway ischemia.
5. Conclusion
We have successfully developed a lipid-based nanoparticle formulation that can effectively improve tracheal anastomotic microvascular formation and airway microvascular repair following initial surgical injury as well as alloimmune injury. The DFO nanoparticle formulation improves airway blood flow through the production of angiogenic growth factors as well as reduction of the production of ROS. As airway anastomotic complications continue to be a cause of morbidity and mortality in lung transplants patients, our DFO nanoparticle formulation may be a promising therapy for diminishing airway ischemia and thereby preventing airway complications. The current study also provided a proof-of-concept result which suggests that airway ischemia and complications may be limited through augmenting anastomotic HIF-1α expression by using iron chelators.
Supplementary Material
Acknowledgments
We thank the members of the BioADD lab for their support, especially Marina Fridlib and Si Wan Kim who helped us with the mass spectrometer measurements. This study was supported by NIH grant HL095686, Veterans Affairs Merit Award BX000509 and by the Ranzotti Philanthropic Support Fund to M.R. Nicolls. These funds provided salary support as well as paying for all services, supplies and equipment described in this proposal.
Footnotes
Appendix A. Supplementary data Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biomaterials.2013.09.092.
References
- [1].Weigt SS, Wallace WD, Derhovanessian A, Saggar R, Lynch JP, Belperio JA. Chronic allograft rejection: epidemiology, diagnosis, pathogenesis, and treatment. Semin Respir Crit Care Med. 2010;31:189–207. doi: 10.1055/s-0030-1249116. [DOI] [PubMed] [Google Scholar]
- [2].Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009;6:79–93. doi: 10.1513/pats.200808-094GO. [DOI] [PubMed] [Google Scholar]
- [3].Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- [4].Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bader HL, Hsu T. Systemic VHL gene functions and the VHL disease. FEBS Lett. 2012;586:1562–9. doi: 10.1016/j.febslet.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marce M, Semenza GL. Adenoviral transfer of HIF-1alpha enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci U S A. 2009;106:18769–74. doi: 10.1073/pnas.0910561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- [8].Cianciulli P, Sorrentino F, Maffei L, Amadori S. Continuous low-dose subcutaneous desferrioxamine (DFO) to prevent allergic manifestations in patients with iron overload. Ann Hematol. 1996;73:279–81. doi: 10.1007/s002770050241. [DOI] [PubMed] [Google Scholar]
- [9].Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–95. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- [10].Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- [11].Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G, Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol. 2008;172:738–47. doi: 10.2353/ajpath.2008.070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–5. [PubMed] [Google Scholar]
- [13].Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, et al. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest. 2010;120:2171–83. doi: 10.1172/JCI35846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sinis N, Di Scipio F, Schonle P, Werdin F, Kraus A, Koopmanns G, et al. Local administration of DFO-loaded lipid particles improves recovery after end-to-end reconstruction of rat median nerve. Restor Neurol Neurosci. 2009;27:651–62. doi: 10.3233/RNN-2009-0517. [DOI] [PubMed] [Google Scholar]
- [15].Najafi R, Sharifi AM. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opin Biol Ther. 2013;13:959–72. doi: 10.1517/14712598.2013.782390. [DOI] [PubMed] [Google Scholar]
- [16].Ikeda Y, Tajima S, Yoshida S, Yamano N, Kihira Y, Ishizawa K, et al. Deferoxamine promotes angiogenesis via the activation of vascular endothelial cell function. Atherosclerosis. 2011;215:339–47. doi: 10.1016/j.atherosclerosis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- [17].Kapoor S. Deferoxamine: emerging, new neuro-protective benefits. Neurol Sci. 2013 doi: 10.1007/s10072-013-1441-6. [DOI] [PubMed] [Google Scholar]
- [18].Jiang X, Khan MA, Tian W, Beilke J, Natarajan R, Kosek J, et al. Adenovirus-mediated HIF-1alpha gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. J Clin Invest. 2011;121:2336–49. doi: 10.1172/JCI46192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jiang X, Hsu JL, Tian W, Yuan K, Olcholski M, de Jesus Perez V, et al. Tie2-dependent VHL knockdown promotes airway microvascular regeneration and attenuates invasive growth of Aspergillus fumigatus. J Mol Med (Berl) 2013;91:1081–93. doi: 10.1007/s00109-013-1063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hu K, Cao S, Hu F, Feng J. Enhanced oral bioavailability of docetaxel by lecithin nanoparticles: preparation, in vitro, and in vivo evaluation. Int J Nanomed. 2012;7:3537–45. doi: 10.2147/IJN.S32880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Delmas T, Couffin AC, Bayle PA, de Crecy F, Neumann E, Vinet F, et al. Preparation and characterization of highly stable lipid nanoparticles with amorphous core of tuneable viscosity. J Colloid Interface Sci. 2011;360:471–81. doi: 10.1016/j.jcis.2011.04.080. [DOI] [PubMed] [Google Scholar]
- [22].Nitta SK, Numata K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 2013;14:1629–54. doi: 10.3390/ijms14011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lanigan RS. Final report on the safety assessment of PPG-11 and PPG-15 stearyl ethers. Int J Toxicol. 2001;20(Suppl. 4):53–9. doi: 10.1080/10915810152902583. [DOI] [PubMed] [Google Scholar]
- [24].Miller RR, Hermann EA, Young JT, Landry TD, Calhoun LL. Ethylene glycol monomethyl ether and propylene glycol monomethyl ether: metabolism, disposition, and subchronic inhalation toxicity studies. Environ Health Perspect. 1984;57:233–9. doi: 10.1289/ehp.8457233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].LaKind JS, McKenna EA, Hubner RP, Tardiff RG. A review of the comparative mammalian toxicity of ethylene glycol and propylene glycol. Crit Rev Toxicol. 1999;29:331–65. doi: 10.1080/10408449991349230. [DOI] [PubMed] [Google Scholar]
- [26].Babu AN, Murakawa T, Thurman JM, Miller EJ, Henson PM, Zamora MR, et al. Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis. J Clin Invest. 2007;117:3774–85. doi: 10.1172/JCI32311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Khan MA, Dhillon G, Jiang X, Lin YC, Nicolls MR. New methods for monitoring dynamic airway tissue oxygenation and perfusion in experimental and clinical transplantation. Am J Physiol Lung Cell Mol Physiol. 2012;303:L861–9. doi: 10.1152/ajplung.00162.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sato M, Keshavjee S. Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling. Semin Thorac Cardiovasc Surg. 2008;20:173–82. doi: 10.1053/j.semtcvs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- [29].Nicolls MR, Zamora MR. Bronchial blood supply after lung transplantation without bronchial artery revascularization. Curr Opin Organ Transplant. 2010;15:563–7. doi: 10.1097/MOT.0b013e32833deca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Herrera JM, McNeil KD, Higgins RS, Coulden RA, Flower CD, Nashef SA, et al. Airway complications after lung transplantation: treatment and long-term outcome. Ann Thorac Surg. 2001;71:989–93. doi: 10.1016/s0003-4975(00)02127-5. Discussion 93-4. [DOI] [PubMed] [Google Scholar]
- [31].FitzSullivan E, Gries CJ, Phelan P, Farjah F, Gilbert E, Keech JC, et al. Reduction in airway complications after lung transplantation with novel anastomotic technique. Ann Thorac Surg. 2011;92:309–15. doi: 10.1016/j.athoracsur.2011.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chekanov VS, Zargarian M, Baibekov I, Karakozov P, Tchekanov G, Hare J, et al. Deferoxamine-fibrin accelerates angiogenesis in a rabbit model of peripheral ischemia. Vasc Med. 2003;8:157–62. doi: 10.1191/1358863x03vm491oa. [DOI] [PubMed] [Google Scholar]
- [33].Chekanov VS, Nikolaychik V, Maternowski MA, Mehran R, Leon MB, Adamian M, et al. Deferoxamine enhances neovascularization and recovery of ischemic skeletal muscle in an experimental sheep model. Ann Thorac Surg. 2003;75:184–9. doi: 10.1016/s0003-4975(02)04122-x. [DOI] [PubMed] [Google Scholar]
- [34].Souchay C, Isingrini M. Age-related differences in the relation between monitoring and control of learning. Exp Aging Res. 2004;30:179–93. doi: 10.1080/03610730490274248. [DOI] [PubMed] [Google Scholar]
- [35].Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, et al. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med. 2005;38:1002–13. doi: 10.1016/j.freeradbiomed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [36].Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A. 2009;106:13505–10. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dewerchin M, Carmeliet P. PlGF: a multitasking cytokine with disease-restricted activity. Cold Spring Harb Perspect Med. 2012;2:a011056. doi: 10.1101/cshperspect.a011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Accornero F, Molkentin JD. Placental growth factor as a protective paracrine effector in the heart. Trends Cardiovasc Med. 2011;21:220–4. doi: 10.1016/j.tcm.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu X, Claus P, Wu M, Reyns G, Verhamme P, Pokreisz P, et al. Placental growth factor increases regional myocardial blood flow and contractile function in chronic myocardial ischemia. Am J Physiol Heart Circ Physiol. 2013;304:H885–94. doi: 10.1152/ajpheart.00587.2012. [DOI] [PubMed] [Google Scholar]
- [40].Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–83. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- [41].Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–75. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- [42].Schmidt T, Kharabi Masouleh B, Loges S, Cauwenberghs S, Fraisl P, Maes C, et al. Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1(+) leukemia. Cancer Cell. 2011;19:740–53. doi: 10.1016/j.ccr.2011.05.007. [DOI] [PubMed] [Google Scholar]
- [43].Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- [45].Cai J, Ahmad S, Jiang WG, Huang J, Kontos CD, Boulton M, et al. Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes. 2003;52:2959–68. doi: 10.2337/diabetes.52.12.2959. [DOI] [PubMed] [Google Scholar]
- [46].Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood. 2003;102:1515–24. doi: 10.1182/blood-2002-11-3423. [DOI] [PubMed] [Google Scholar]
- [47].Chaturvedi P, Gilkes DM, Wong CC, Kshitiz, Luo W, Zhang H, et al. Hypoxiainducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013;123:189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mandala M, Gokina N, Barron C, Osol G. Endothelial-derived hyperpolarization factor (EDHF) contributes to PlGF-induced dilation of mesenteric resistance arteries from pregnant rats. J Vasc Res. 2012;49:43–9. doi: 10.1159/000329821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- [50].Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164:213–23. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Galaris D, Pantopoulos K. Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci. 2008;45:1–23. doi: 10.1080/10408360701713104. [DOI] [PubMed] [Google Scholar]
- [53].Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99:1222–9. doi: 10.1161/01.cir.99.9.1222. [DOI] [PubMed] [Google Scholar]
- [54].Ishizaka N, Saito K, Mori I, Matsuzaki G, Ohno M, Nagai R. Iron chelation suppresses ferritin upregulation and attenuates vascular dysfunction in the aorta of angiotensin II-infused rats. Arterioscler Thromb Vasc Biol. 2005;25:2282–8. doi: 10.1161/01.ATV.0000181763.57495.2b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.