Abstract
Background
Aberrant epithelial repair is a crucial event in the airway remodeling that characterizes obliterative bronchiolitis (OB) in transplanted lungs. Recent data from experiments using epithelial cell lines and human airway tissues from lung transplant recipients suggest that epithelial to mesenchymal transition (EMT) plays an important role in OB. The aim of this study was to clarify whether EMT is involved in airway remodeling in an animal model.
Methods
We performed orthotopic tracheal transplantation from BALB/c to C57BL/6 mice with from BALC/c to BALB/c mouse grafts as controls. Five allogeneic and 3 syngeneic recipients were humanely killed at predetermined postoperative days 2–12 as well as 14 and 21. Histology was evaluated using hematoxylin-eosin (H&E) staining. We studied the expression of specific markers, including E-cadherin, an epithelial marker; α-smooth muscle actin (SMA), and S100A4, mesenchymal markers, and zinc finger E-box-binding homeobox 1 (ZEB1), an EMT-related transcription factor.
Results
Histologic assessment of serial H&E stains of allogeneic grafts showed remarkable pseudostratified respiratory epithelium with subepithelial inflammatory cell infiltration, as well as denuded and flattened epithelium and subepithelial fibrosis. The dynamic epithelial changes occurred earlier than the subepithelial fibrosis. Immunohistochemical evaluation indicated the emergence of α-SMA-positive epithelial cells that were most prominent on day 7. The expression of E-cadherin was attenuated in α-SMA–positive epithelial cells. S100A4 was also expressed in epithelial cells. A few days before the intraepithelial expression of α-SMA, ZEB1 emerged in the nuclei of epithelial cells.
Conclusions
We observed expression of an EMT-related transcription factor and mesenchymal markers along with the attenuation of epithelial marker expression in epithelial cells, several days before prominent subepithelial fibrosis formation, results that suggest epithelial cells to play an important fibrosis role in airway remodeling during epithelial to mesenchymal transition.
INTRODUCTION
Obliterative bronchiolitis (OB) limits long-term survival after lung transplantation. Fibroblasts and myofibroblasts, considered to be key players in OB-related fibrosis, proliferate in response to the local environment, producing extracellular matrix components such as various collagens and proteoglycans. The source of these fibroblasts is still in question. The 2 major possible sources are recipient bone marrow and transitioned epithelial cells. Several reports have implicated epithelial to mesenchymal transition (EMT) in OB pathogenesis. During EMT, cells lose their epithelial properties gaining those of mesenchymal elements. Epithelial proteins, such as E-cadherin, are downregulated and mesenchymal proteins, such as α-smooth muscle actin (SMA), are up-regulated. EMT occurs naturally during gastrulation, wound repair, and cancer metastasis. Recent data from experiments using epithelial cell lines and human airway tissue from lung transplant recipients have strongly suggested that EMT plays an important role in OB.1 A few studies have demonstrated EMT to be part of the process of airway remodeling in animal models. The aim of this study was to clarify whether EMT is involved in airway remodeling in murine orthotopic tracheal transplantation, a model of lymphocytic bronchitis not OB, albeit that LB is a precursor to a large airway correlate of OB.
MATERIALS AND METHODS
Mice
Animal care measures were taken in compliance with our “Guide for Animal Experimentation, University of Tokyo, revised 2007” and the “Act on Welfare and Management of Animals” of Japan. All mice, including C57BL/6 (B6; H-2b) and BALB/c (H-2d), were purchased from the Japan SLC, Inc.
Orthotopic Tracheal Transplantation
BALB/c and B6 female mice were used as either donors or recipients. Orthotopic tracheal transplantation was performed as previously reported.2 Briefly, both donors and recipients were anesthetized by intraperitoneal injection pentobarbital sodium (90 mg/kg). With the aid of an operating microscope, we removed 7-ring tracheal segments from the donors who were matched to recipients by age. After a short incision in the midline of the recipient’s neck, we bluntly divided and pulled aside with 5-0 sutures the strap muscles, providing clear exposure of the laryngotracheal complex. After the recipient trachea was transected, the donor tissue was sewn with 10-0 nylon sutures and the skin closed with 5-0 silk sutures.
Histologic Evaluation
On postoperative days 2–12, 14, and 21 we harvested grafts from 5 mice in the allogeneic group and 3 in the syngeneic group for histologic evaluation. The grafts were frozen on dry ice in OCT (optimal cutting temperature) compound (Sakura Finetek, Japan) without prior tissue fixation.
Sections (5 μm) were used for hematoxylin and eosin staining (H&E) and immunofluorescence assays. H&E stains were performed after formalin fixation. Anti–E-cadherin antibodies (Takara, M108) were utilized to detect cells with epithelial properties; anti-α-SMA Cy3-conjugated (Sigma-Aldrich, St Louis, Mo; C6198) and anti-S100A4 antibodies (Abcam, Cambridge, Mass; ab27957), with mesenchymal properties. S100A4 is equivalent to fibroblast-specific protein 1, and zinc finger E-box-binding homeobox 1 (ZEB1) (Sigma-Aldrich; HPA027524) is an EMT-related transcription factor. All sections used for immunofluorescence assays were fixed with 4% paraformaldehyde, blocked with BROCKACE (DS Pharma Biomedical, Osaka, Japan) and then incubated with primary antibodies against α-SMA, S100A4, E-cadherin, or ZEB1 for 40 minutes at 37°C. After careful washing with Tris-buffered saline E-cadherin, S100A4, and ZEB1 were detected using appropriate fluorescein-isothiocyanatelabeled secondary antibodies. We used 4, 6-diamidino-2-phenylindole as a nuclear counterstain. Images of both the stained sections and immunofluorescent sections were acquired using a microscope (Keyence, Osaka, Japan; BIOREVO-9000).
RESULTS
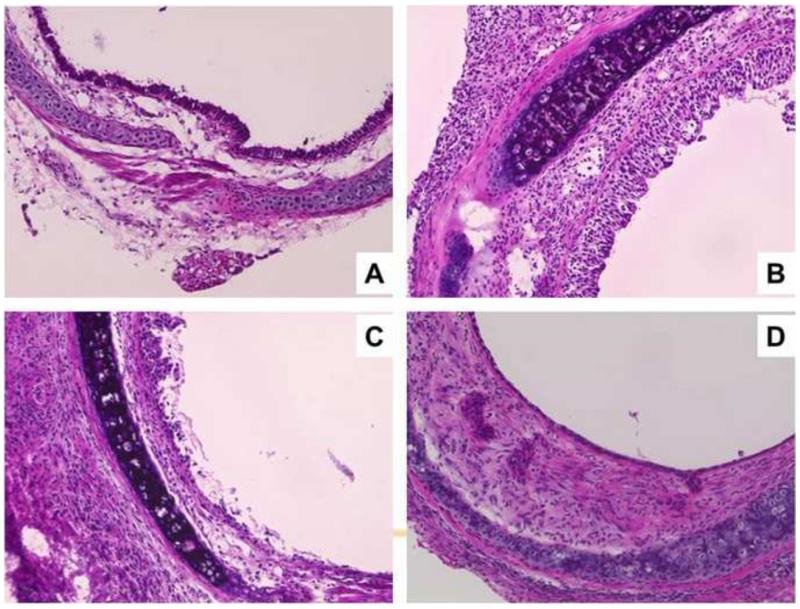
Serial H&E staining of allotransplantations, indicated massive inflammation and pseudostratified epithelia on day 7; that was partially denuded on day 10 and flattened on day 21 with thickened subepithelial spaces (Fig 1), as reported previously.2 The epithelial changes were dynamic appearing before the onset of subepithelial fibrosis. In contrast with the allografts the isografts maintained a normal epithelimic.
Fig 1.
Sequential change after allogransplantation was detected after H&E staining (A) Original trachea of a BALB/c mouse that did not undergo the operation. (B) Massive inflammation and highly pseudostratified epithelium on day 7. (C) partially denuded epithelium on day 10, (D) and flattened epithelium and subepithelial fibrosis on day 21 post-allotransplantation. (Original magnification, ×200).
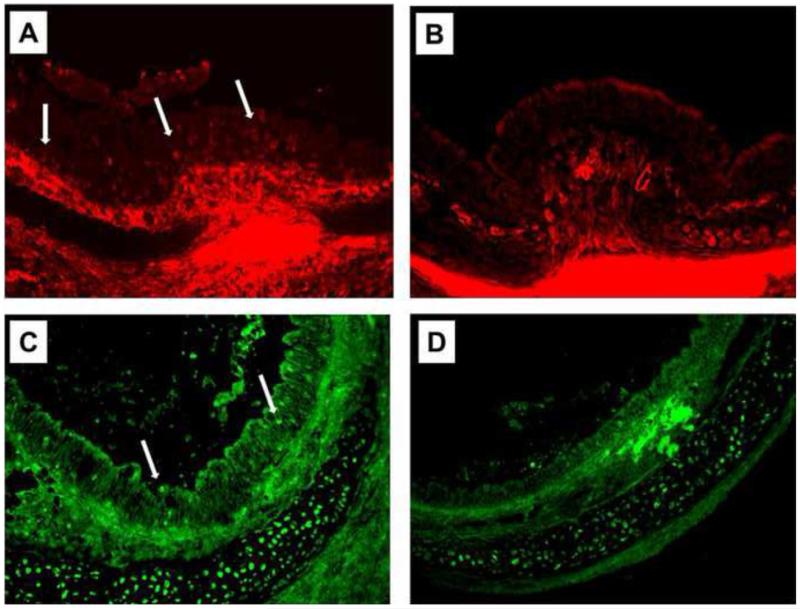
α–SMA-positive epithelial cells appeared on day 6 with expression peaking on day 7 (Fig 2A, B). α-SMA–positive epithelial cells were occasionally detected just above the basement membrane.
Fig 2.
Immunofluorescent staining for mesenchymal markers at 7 days post-tracheal transplantation. (A) α-SMA (red) expression was readily detected in many epithelial cells of the allografts (arrows). (B), There was no detectable α-SMA expression in the epithelia of the isografts. (C) The expression of S100A4 (green) was also deteced in the allografts on day 7. (D) No significant S100A4 expression was noted in the epithelia of the isografts. (Original magnification, ×200).
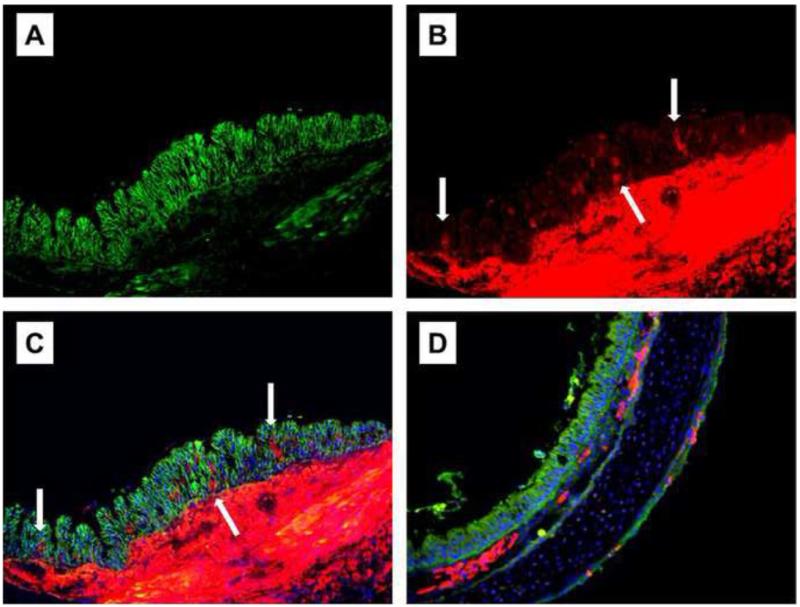
After double staining of the epithelium, with α-SMA and E-cadherin α-SMA–positive cells presented reduced E-cadherin expression on the day 7th, (Fig 3).
Fig 3.
(A–C) Sections from an allograft on day 7 post-transplantation were double-stained for E-cadherin (green) and α-SMA (red) using isograft sections (D). E-cadherin was expressed in most epithelial cells (A). Numerous α-SMA–positive cells (arrows) were detected in the epithelia (B). The attenuation of E-cadherin expression accompanying α-SMA–positive epithelial cell expression (arrows) is apparent with a merged image (C). No epithelial α-SMA expression was detected in the isograft sections (D) blue, DAPI nuclear counterstain). (Original magnification, ×200).
S100A4, another mesenchymal marker, was also expressed in the epithelium (Fig 2C, D). The expression of S100A4 did not present a clear peak and was not parallel to α-SMA expression.
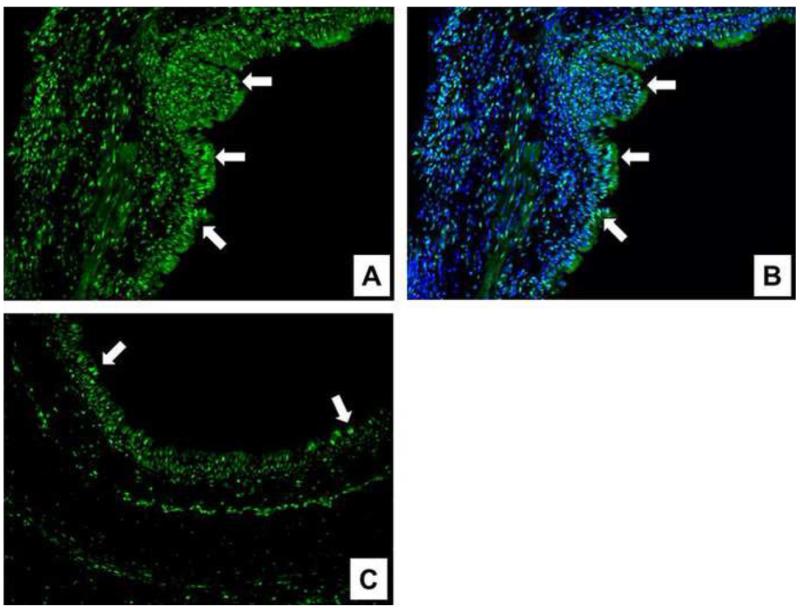
Figure 4 depicts Zeb1 expression in the nuclei of epithelial cells. It was maximal from postoperative days 2 to 4. In allografts, the epithelium presented high levels of nuclear Zeb1 expression. In isografts, the epithelium showed only scant and fewer Zeb1-positive cells.
Fig 4.
Sections stained to detect the EMT-related transcription factor ZEB1. The stained allograft on day 4 presented a great amount of ZEB1 (green) expression in the nucleus of epithelial cells (arrows) (A), in contrast to a stained isograft (C). ZEB1 was localized in the nucleus (B) (blue, DAPI nuclear counterstain). (Original magnification, ×200).
DISCUSSION
Rejected tracheal allografts in orthotopic transplantations demonstrated reproducible subepithelial fibrosis, which represents the deposition of extracellular matrix produced by fibroblasts. The origin of these fibroblasts, especially in transplanted organs, remains unknown. One possible source is recipient bone marrow cells; another, local epithelial cells. Several recent reports3 have indicated that fibrocytes from recipient bone marrow play a role in fibrosis. There is the possibility that both bone marrow cell and EMT participate in post-transplant fibrosis, but it is unclear whether EMT is involved in fibrosis of transplanted organs. Our findings indicated dynamic epithelial morphologic changes before the onset of fibrosis. These results suggested that epithelial cells play an important role. The fact that α-SMA expression correlated with reduced levels of E-cadherin expression in the rejected allografted epithelium suggests that EMT plays a role in fibrosis. S100A4 expression was also observed in rejected epithelial cells, but its expression did not show a clear peak and was not parallel with α-SMA expression. These S100A4-expressing elements were presumably inflammatory cells, such as macrophages.4 The strong expression of Zeb1 in allografted epithelium, which occurred in advance of the increase and decrease in α-SMA and E-cadherin expression, respectively, supports a role for EMT in airway remodeling. There was a low expression of Zeb1 in isografts, which may be due to ischemia; however, this result remains to be elucidated in the future.
In the orthotopic tracheal transplantation model, donor epithelia in the rejected allograft are finally replaced by recipient epithelial cells.2 Another open question that should be investigated is the relationship between local epithelial chimeras and EMT.
In conclusion, this preliminary study demonstrated a possible role for EMT as a part of airway fibrosis in rejected airway tissues. It remains unclear whether local transitioned epithelium is the sole source of airway fibrosis; central bone marrow cells may have a role in airway remodeling.
Acknowledgments
This work was supported by grants from the Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research (KAKENHI) (22591560) (to T.M.).
Footnotes
Presented at the 24th International Congress of the Transplantation Society, Berlin, 15–19 July 2012.
REFERENCES
- 1.Borthwick LA, Parker SM, Brougham KA, et al. Epithelial to mesenchymal transition (EMT) and airway remodeling after human lung transplantation. Thorax. 2009;64:770–777. doi: 10.1136/thx.2008.104133. [DOI] [PubMed] [Google Scholar]
- 2.Murakawa T, Kerklo MM, Zamora MR, et al. Stimultaneous LFA-1 and CD40 ligand antagonism prevents airway remodeling in orthotopic airway transplantation: implications for the role of respiratory epithelium as a modulator of fibrosis. J Immunol. 2005;174:3869–3879. doi: 10.4049/jimmunol.174.7.3869. [DOI] [PubMed] [Google Scholar]
- 3.Gilpin SE, Lung KC, Sato M, et al. Altered progenitor cell and cytokine profiles in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2012;31:222–228. doi: 10.1016/j.healun.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Inoue T, Plieth D, Venkov CD, et al. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 2005;67:2488–2493. doi: 10.1111/j.1523-1755.2005.00358.x. [DOI] [PubMed] [Google Scholar]