Abstract
Background:
Smoking cessation is of major importance for all smokers; however, in patients with COPD, little information exists on how smoking cessation influences lung function and high-resolution CT (HRCT) scan appearances.
Methods:
In this single-center study, we performed screening spirometry in a group of heavy smokers aged 40 to 80 years (N = 358). We then studied the effects of smoking cessation in two groups of selected subjects: smokers with COPD (n = 38) and smokers with normal spirometry (n = 55). In parallel to subjects undergoing smoking cessation, we studied a control group of nonsmokers (n = 19).
Results:
Subjects with COPD who quit smoking had a marked, but transient improvement in FEV1 at 6 weeks (184 mL, n = 17, P < .01) that was still present at 12 weeks (81 mL, n = 17, P < .05) and only partially maintained at 1 year. In contrast, we saw improvement in the transfer factor of lung for carbon monoxide at 6 weeks in both subjects with COPD who quit smoking (0.47 mmol/min/kPa, n = 17, P < .01) and subjects who quit smoking with normal spirometry (0.40 mmol/min/kPa, n = 35, P < .01). An upper-zone single HRCT image slice reliably identified emphysema at baseline in 74% of smokers with COPD (28 of 38) and 29% of healthy smokers (16 of 55). Smoking cessation had no significant effect on the appearances of emphysema but decreased the presence of micronodules on HRCT imaging.
Conclusions:
Cigarette smoking causes extensive lung function and HRCT image abnormalities, even in patients with normal spirometry. Smoking cessation has differential effects on lung function (FEV1 and gas transfer) and features on HRCT images (emphysema and micronodules). Cessation of smoking in patients with COPD causes a transient improvement in FEV1 and decreases the presence of micronodules, offering an opportunity for concomitant therapy during smoking cessation to augment these effects. Smoking cessation at the earliest possible opportunity is vital to minimize permanent damage to the lungs.
COPD is a major cause of ill health and mortality worldwide, and the Burden of Obstructive Lung Disease (BOLD) study has found a high international prevalence of COPD as defined by spirometry.1 Cigarette smoking is the major cause of COPD in industrialized countries, and important studies have been reported on the effects of smoking cessation.2 Smoking cessation remains the single most important step in the management of cigarette smokers with and without lung disease.3
The landmark British study of Fletcher and Peto4 demonstrated that the hallmark of COPD is an accelerated rate of annual decline in FEV1 in susceptible smokers. The North American Lung Health Study5 showed that slight improvement in FEV1 can be seen in the first year after smoking cessation, with more relative benefit in patients with early COPD followed by a slower rate of decline in sustained quitters.5 Data from the Framingham Offspring cohort confirm that smoking cessation has more benefit in earlier quitters,6 and a more recent study found that smoking cessation in patients with COPD results in improved FEV1 at 12 weeks, but this is not sustained to 1 year.7 Finally, the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study found that the rate of postbronchodilator FEV1 decline among patients with COPD is highly variable.8,9
High-resolution CT (HRCT) imaging is a sensitive and quantitative technique for the detection of low-attenuation areas corresponding to parenchymal destruction due to emphysema. The ECLIPSE study showed that patients with increasing GOLD (Global Initiative of Chronic Obstructive Lung Disease) severity tend to have a greater amount of emphysema.8 Emphysematous lesions are well described in symptom-free smokers with normal lung function10 and help to identify nonobstructed male smokers in whom airway obstruction is likely to develop.11
We carried out a single-center, prospective, 1-year study of the effects of smoking cessation in a group of asymptomatic smokers, selected on the basis of moderate to severe COPD on spirometry, and in a group with normal spirometry. We then longitudinally assessed the effects of smoking cessation by measuring spirometric lung volume and gas transfer criteria. These lung function parameters were monitored in relation to the appearance of emphysema, micronodules, and ground-glass opacification on HRCT scan.
Materials and Methods
Ethics and Consent
This study was given assessment and authorization by the Royal Brompton, Harefield, and National Heart and Lung Institute Research Ethics Committee (Reference 01-222) and was carried out exclusively at the Royal Brompton Hospital. This study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All subjects were fully advised of the need to quit smoking.
Study Design
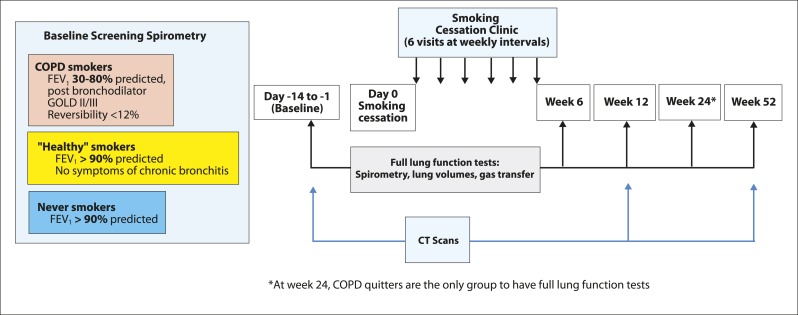
Subjects were recruited through smoking cessation clinics, newspaper advertising, local primary care physicians, and a telephone helpline. We performed spirometric evaluation of a group of heavy cigarette smokers aged 40 to 80 years. Having identified those smokers with and without spirometric features of COPD, we then charted the progress of those who managed to give up cigarettes (smoking cessation phase) (Fig 1).
Figure 1.
Design of smoking cessation study. Screening spirometry: 387 volunteers (358 smokers and 29 never smokers). Baseline (d −14 to −1): 38 smokers with COPD, 55 healthy smokers, and 19 never smokers. Wk 12 per protocol for lung function: 17 COPD quitters, 33 healthy quitters, 14 healthy continuing smokers, and 19 never smokers. Wk 12 per protocol for high-resolution CT scanning and lung function: 15 COPD quitters, 26 healthy quitters, and 12 healthy continuing smokers. Wk 52 per protocol for lung function: 11 COPD quitters, 21 healthy quitters, 13 healthy smokers, and 19 never smokers. Wk 52 per protocol for high-resolution CT scanning and lung function: 10 COPD quitters, 12 healthy quitters, and eight healthy continuing smokers. GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Inclusion Criteria at Screening
Subjects were current smokers of at least five cigarettes per day, with a > 10-pack-year history. These individuals had no history of atopy, asthma, or other respiratory disease. Individuals with a malignancy or any potentially confounding systemic disease were excluded, including those with cardiovascular disease. Use of inhaled or oral drugs with any potential antiinflammatory action was not permitted; hence, nonsteroidal antiinflammatory drugs and statins were not allowed.
Smokers with COPD (GOLD groups II [moderate] and III [severe]) were defined by spirometry (volumetric spirometer; Vitalograph) as having a postbronchodilator FEV1 of 30% to 80% predicted following the inhalation of salbutamol 400 μg through a metered dose inhaler.12 The prebronchodilator forced expiratory ratio (FER) was < 70%, and bronchodilator reversibility was < 12% or 200 mL for FEV1, FVC, or both. Healthy smokers were defined by spirometry as having an FEV1 > 90% predicted and FER > 70%. They were matched with the subjects with COPD in terms of age, sex, and smoking history. These smokers did not have a productive cough or chronic bronchitis. Never smokers had an FEV1 > 90% predicted, FER > 70%, and no history of respiratory disease or respiratory symptoms.
Smoking Cessation Phase:
All smokers were offered a 6-week course of either group or one-to-one smoking cessation counseling and the option of additional nicotine replacement therapy. Ongoing support and advice to aid cessation was offered throughout the duration of the study.
Definition of Quitters:
Subjects completed smoking diaries to monitor cigarette consumption. Reported smoking of fewer than six cigarettes was allowed over the first 6 weeks, but no cigarettes were permitted after 6 weeks. In addition, quitters had to have negative exhaled breath carbon monoxide (CO) readings at < 6 parts per million (Micro Smokerlyzer; Bedfont Scientific Ltd) for all clinic and hospital visits.
Healthy Continuing Smokers:
A group of healthy smokers wished to carry on cigarette smoking. They maintained levels of at least 10 cigarettes per day despite advice on the health dangers of cigarettes.
Lung Function Tests and HRCT Imaging
Details of lung function and HRCT imaging procedures are described in e-Appendix 1 (359.2KB, pdf) .
Statistical Analysis
The effects of smoking cessation were studied in quitters who had complete lung function tests and smoking diaries from baseline (0) to each of the 6-, 12-, and 52-week time points, comprising a per-protocol lung function group up to each time point. In addition, some of these subjects had full lung function and CT scans to 52 weeks, forming the per-protocol lung function and HRCT scan group. The Shapiro-Wilks test demonstrated that > 90% of the lung function parameters and changes were normally distributed, and changes in lung function were tested using the paired t test. For nonparametric CT scan data, we used the Wilcoxon rank sum test to assess differences between groups at baseline. Longitudinal changes on smoking cessation were calculated using the McNemar χ2 test.
Results
Subject Disposition
A total of 387 volunteers underwent screening spirometry; 358 were smokers, and 29 had never smoked. The baseline cohort comprised 112 subjects, of whom 38 met postbronchodilator spirometric criteria for being smokers with COPD, 55 were healthy smokers with normal spirometry, and 19 never smoked. The number of subjects having quit to 12 and 52 weeks is shown in Figure 1.
Lung Function
Longitudinal Spirometry (FEV1):
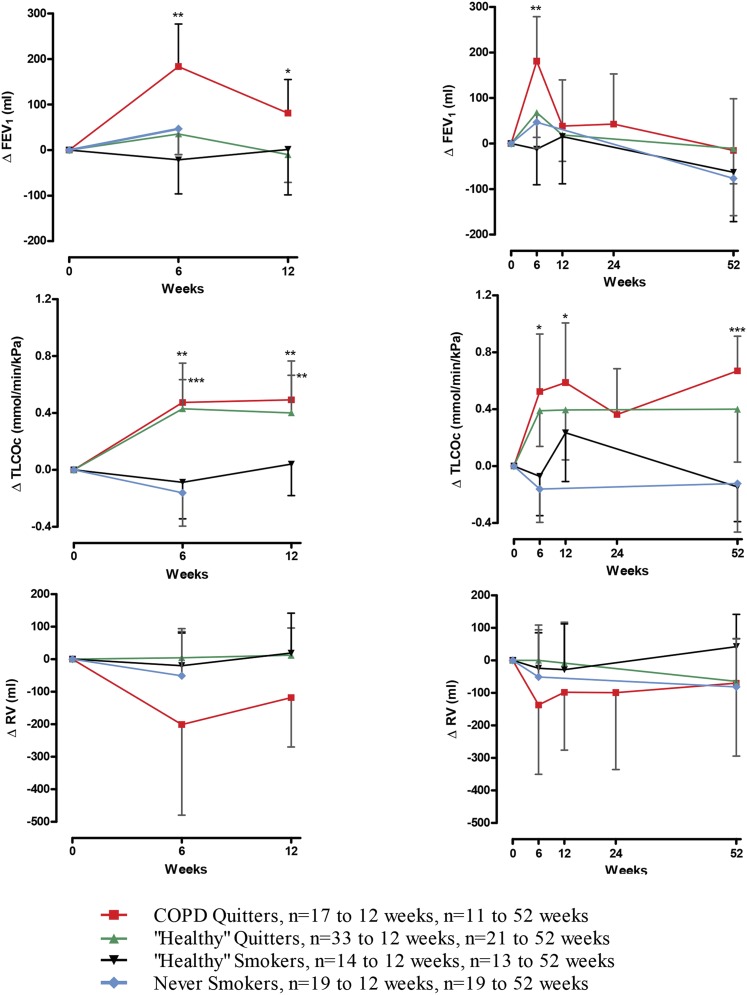
In subjects with complete data to 12 weeks, those with COPD showed a significant increase from baseline in FEV1 of 184 mL at 6 weeks (n = 17, P < .01), which represented an increase in FEV1 % predicted of 7.2% (P < .001). At 12 weeks, the improvement in FEV1 from baseline in the COPD quitter group was 81 mL (n = 17, P < .05), with an increase in FEV1 % predicted of 3.3% (n = 17, P < .05). In the COPD quitter group, there was a small fall in FEV1 from baseline at 12 months, although the number of smokers with COPD having quit to 1 year was small (n = 11, change from baseline to 52 week nonsignificant at P = .80). At 6 weeks, 12 weeks, and 1 year, there was no significant difference in FEV1 from baseline within the healthy quitter and never smoker groups (Fig 2).
Figure 2.
Changes in FEV1, TLCOc, and RV after smoking cessation in per-protocol subjects. The left side shows per-protocol subjects to 12 wk, with the equal numbers of subjects at baseline, 6 wk, and 12 wk (COPD quitters, 17; healthy quitters, 33; healthy smokers, 14; never smokers, 19). The right side shows per-protocol subjects to 1 y, with equal numbers of subjects at each time point (COPD quitters, 11; healthy quitters, 21; healthy smokers, 13; never smokers, 19). Data are presented as means with 95% CIs. Significant changes from baseline are shown as *P < .05, **P < .01, and ***P < .0001. RV = residual volume; TLCOc = transfer factor of lung for carbon monoxide corrected for hemoglobin concentration.
Residual Volume:
There was a trend toward a decrease in residual volume at all time points in the COPD quitter group, but this did not reach significance.
Gas Transfer:
Both smokers with COPD and healthy smokers showed abnormalities in gas transfer tests at baseline (Table 1). Improvements in the transfer factor of lung for carbon monoxide (Tlco) and transfer coefficient were seen in both groups at 6, 12, and 52 weeks. The COPD quitter group showed an increase in Tlco of 0.47 mmol/min/kPa at 6 weeks (n = 17, P < .01) for an increase of 5.5% predicted. There was an increase of 0.49 mmol/min/kPa at 12 weeks (n = 17, P < .01), for an increase of 5.6% predicted. At 52 weeks, there was a change in Tlco corrected for hemoglobin concentration (Tlcoc) of 0.67 mmol/min/kPa (n = 11, P < .01), for an increase of 8.2% predicted. The healthy quitter group showed an increase in Tlcoc of 0.40 mmol/min/kPa (6.2% predicted) at 6 weeks (n = 35, P < .01), 0.40 mmol/min/kPa (4.6% predicted) at 12 weeks (n = 33, P < .01), and 0.40 mmol/min/kPa (4.5% predicted) at 1 year (n = 21, P < .05).
Table 1.
—Baseline Demographics, Spirometry, and Lung Function
| Characteristic | Smokers With COPD (n = 38) | Healthy Smokers (n = 55) | Never Smokers (n = 19) |
| Age, y | 58.9 (42-80) | 54.6 (40-79) | 54.1 (40-77) |
| Male (female) sex | 19 (19) | 26 (29) | 9 (10) |
| Smoking history, pack-y | 66 (25-150) | 53 (10-160) | (0-0) |
| Prebronchodilator FEV1,a L | 1.54 (1.36-1.71) | 3.05 (2.85-3.24) | 3.11 (2.80-3.43) |
| Prebronchodilator FEV1,a % predicted | 55.4 (50.6-60.3) | 106.0 (102.9-109.0) | 113.4 (107.1-119.7) |
| Postbronchodilator FEV1,a L | 1.62 (1.45-1.79)b | …c | …c |
| Postbronchodilator FEV1,a % predicted | 58.4 (53.7-63.2)b | …c | …c |
| Postbronchodilator FEV1 absolute change,a mL | 71 (32-110)b | …c | …c |
| Postbronchodilator reversibility,a % {[(post − pre)/pre] × 100} | 5.6 (3.3-7.8)b | …c | …c |
| Prebronchodilator FERa | 0.53 (0.50-0.56) | 0.77 (0.76-0.79) | 0.78 (0.76-0.81) |
| FEV1, L | 1.59 (1.40-1.77) | 3.05 (2.84-3.25) | 3.16 (2.85-3.47) |
| FVC, L | 3.19 (2.93-3.46) | 4.00 (3.74-4.26) | 4.03 (3.58-4.48) |
| MEF25, L/s | 0.23 (0.19-0.27) | 0.91 (0.77-1.04) | 1.01 (0.86-1.17)d |
| Tlcoc, mmol/min/kPa | 5.08 (4.56-5.61) | 6.96 (6.53-7.40) | 7.85 (6.93-8.77) |
| Kcoc, mmol/min/kPa/L | 1.00 (0.91-1.09) | 1.31 (1.25-1.37) | 1.50 (1.39-1.61) |
| RV, L | 3.65 (3.36-3.94) | 2.34 (2.21-2.47) | 2.27 (2.02-2.52) |
| TLC, L | 6.92 (6.49-7.35) | 6.34 (6.02-6.66) | 6.22 (5.71-6.93) |
| IVC, L | 3.30 (3.03-3.56) | 4.01 (3.75-4.27) | 4.13 (3.66-4.59) |
Data are presented as mean (minimum-maximum) for age and smoking history and as mean (95% CI) for lung function tests. FER = forced expiratory ratio; HRCT = high-resolution CT; IVC = inspiratory vital capacity; Kcoc = corrected transfer coefficient; MEF25 = forced expiratory flow at 25% FVC; RV = residual volume; TLC = total lung capacity; Tlcoc = transfer factor of lung for carbon monoxide corrected for hemoglobin concentration.
Spirometry performed on Vitalograph volumetric spirometer on populations with full lung function and HRCT scans at baseline.
n = 37 for postbronchodilator spirometry.
Bronchodilator not given.
n = 18.
HRCT Scans at Baseline
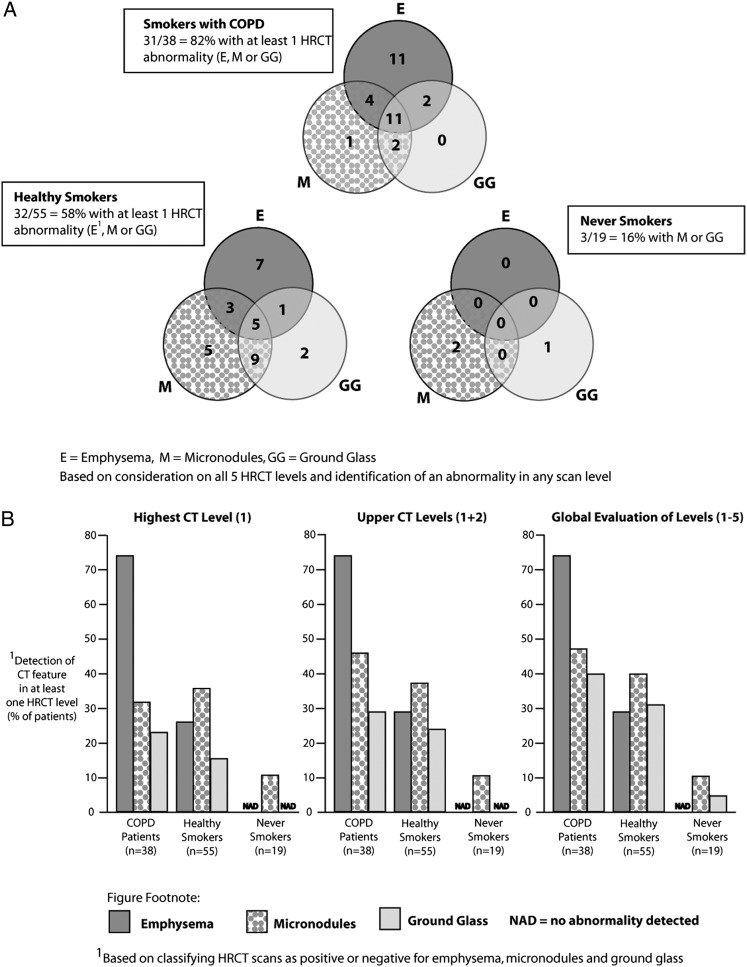
HRCT scans from 112 subjects were analyzed (Fig 3). Thirty-one of 38 subjects with COPD (82%) had at least one abnormality (emphysema, micronodules, or ground-glass opacification) at baseline imaging (Fig 3A). Thirty-two of 55 healthy smokers (58%) but only three of 19 never smokers (16%) had at least one baseline HRCT scan abnormality. If emphysema was present anywhere on the five HRCT image levels, it was invariably detected in level 1 (Fig 3B). There was a significantly greater extent of emphysema in subjects with COPD than in either of the other groups (P < .0001) and in healthy smokers compared with never smokers (P < .01).
Figure 3.
HRCT scan abnormalities at baseline. A, Venn diagram of E, M, and GG opacification (based on consideration of all five HRCT scan levels and identification of an abnormality in any scan level). B, Consideration of one, two, or five levels. HRCT = high-resolution CT.
Although micronodules were found most often in the upper levels of the HCRT image, the extent was underestimated if only level 1 was reviewed (Fig 3B). Using the maximum scores, there was no significant difference in the extent of micronodules between subjects with COPD and healthy smokers. Nonsmokers, however, had significantly fewer micronodules than subjects with COPD (P < .01) and healthy smokers (P < .05). Consideration of just the upper level of the HCRT image is sufficient to detect emphysema, but for detection of micronodules and ground-glass opacification, it is better to study all five levels (Fig 3B).
Longitudinal Changes in HRCT Appearances
The number of subjects included for longitudinal analysis of HRCT image appearances are shown in Figure 1 and Table 2. There was no significant change in the presence of emphysema on smoking cessation in the COPD quitter group, healthy quitter group, or in a combined group of COPD and healthy quitters (Table 2). Although a trend toward a decrease in the number of micronodules present in both COPD quitter and healthy quitter groups was observed, this did not reach significance. When looking at the combined group of those who quit smoking, however, there was a significant decrease in the presence of micronodules at both 12 weeks (n = 41, P < .05) and 52 weeks (n = 22, P < .05). Ground-glass opacification appearance was generally maintained at 12 and 52 weeks.
Table 2.
—Longitudinal Changes in HRCT Scan Appearances of Emphysema, Micronodules, and Ground-Glass Opacification
| 12 Wk | 52 Wk | |||||||||||
| HRCT Scan Feature | Subject Group | Present at Baseline,a % | More,b,c % | Same | Less | P Value | Subject Group | Present at Baseline,a % | More,b,c % | Same | Less | P Value |
| Emphysemad | CQ (n = 15) | 13 (87) | 2 (15) | 11 (85) | 0 (0) | .29 | CQ (n = 10) | 8 (80) | 0 (0) | 5 (63) | 3 (38) | .15 |
| HQ (n = 26) | 11 (42) | 1 (9) | 8 (73) | 2 (18) | .77 | HQ (n = 12) | 6 (50) | 1 (17) | 4 (67) | 1 (17) | .72 | |
| CQ + HQ (n = 41) | 24 (56) | 3 (13) | 19 (79) | 2 (8) | .82 | CQ + HQ (n = 22) | 14 (64) | 1 (7) | 9 (64) | 4 (29) | .26 | |
| HCS (n = 12) | 2 (17) | 1 (50) | 1 (50) | 0 (0) | .62 | CQ (n = 10) | 8 (80) | 0 (0) | 5 (63) | 3 (38) | .62 | |
| Micronodulese | CQ (n = 15) | 9 (60) | 1 (11) | 3 (33) | 5 (56) | .15 | CQ (n = 10) | 6 (60) | 0 (0) | 1 (17) | 5 (83) | .04 |
| HQ (n = 26) | 10 (38) | 1 (10) | 3 (30) | 6 (60) | .09 | HQ (n = 12) | 6 (50) | 1 (17) | 0 (0) | 5 (83) | .15 | |
| CQ + HQ (n = 41) | 19 (46) | 2 (11) | 6 (32) | 11 (58) | .02 | CQ + HQ (n = 22) | 12 (55) | 1 (8) | 1 (8) | 10 (83) | .01 | |
| HCS (n = 12) | 8 (67) | 3 (38) | 1 (13) | 4 (50) | .85 | HCS (n = 8) | 6 (75) | 1 (17) | 2 (33) | 3 (50) | .45 | |
| Ground-glass opacificatione | CQ (n = 15) | 6 (40) | 0 (0) | 5 (83) | 1 (17) | .62 | CQ (n = 10) | 4 (40) | 0 (0) | 2 (50) | 2 (50) | .29 |
| HQ (n = 26) | 8 (31) | 2 (25) | 4 (50) | 2 (25) | .80 | HQ (n = 12) | 4 (33) | 0 (0) | 2 (50) | 2 (50) | .29 | |
| HCS (n = 12) | 6 (50) | 2 (33) | 1 (17) | 3 (50) | .82 | HCS (n = 8) | 6 (75) | 1 (17) | 2 (33) | 3 (50) | .45 | |
For longitudinal analysis, all scans with an HRCT image abnormality at baseline were identified. The baseline scans and both 12- and 52-wk scans were compared blindly side by side for each subject. Scans were documented as more, same, or less for the extent of emphysema, micronodules, or ground-glass opacification compared with baseline. Significance was tested with McNemar χ2 test. CQ = COPD quitter; HCS = healthy continuing smoker; HQ = healthy quitter. See Table 1 legend for expansion of other abbreviation.
Percentage of all analyzable scans.
Percentage of scans with abnormality present at baseline.
More, same, or less on reading paired CT scans in a blinded manner.
Level 1 alone was assessed for emphysema.
Levels 1 to 5 were combined for detection of micronodules and ground-glass opacification.
Discussion
This study illustrates that spirometry only detects a fraction of patients with cigarette-associated lung disease. Indeed, if a heavy cigarette smoker has normal spirometry, he or she may still have profound abnormalities of lung function and defects on lung HRCT scan. Hence, for the individual cigarette smoker, a normal FEV1 on spirometry should not provide reassurance because abnormal spirometry identifies just the tip of the iceberg. In almost 30% of smokers with normal spirometry in this study, some degree of emphysema was found on HRCT scan, indicating that all smokers should quit smoking.
In this single-center study of a large screened group, we selected small groups of highly defined subjects. These subjects met strict criteria in relation to FEV1 and did not have concomitant medications and comorbidities. We then studied subjects who had completely given up smoking and an ever-decreasing cohort up to 12 months. In contrast to large-scale natural history studies of COPD, the effects of smoking cessation in the present study are so marked that the findings are significant in small numbers. A positive feature of smoking cessation studies is that participation can help the smoker to quit.13 Indeed, approximately one-third of the subjects in the present study had given up smoking to 1 year.
The daily smoking of cigarettes for many years contributes to the cause of COPD, and smoking cessation provides a clinical research model of the effects of abrupt withdrawal of the known noxious respiratory stimulus. On smoking cessation in patients with COPD, there is an acute and transient improvement in FEV1 6 weeks after smoking cessation, with some of this benefit being lost by 12 weeks, and FEV1 returning to near baseline by 1 year. This response was described at 12 weeks in a smoking cessation study in patients with COPD, and the authors found that the benefit diminished to nonsignificant by 1 year.7 However, we found that the improvement in FEV1 at 6 weeks is greater than that of 12 weeks and of a magnitude equivalent to a bronchodilator response of 13%.
For the design of clinical therapeutic trials in COPD, the effect of smoking cessation on FEV1 suggests the importance of monitoring the number of cigarettes that subjects are smoking because there may also be marked effects from smoking fewer cigarettes. In other words, the effect of smoking cessation or decreased smoking intake on increasing FEV1 could confound clinical studies. The transient improvement in FEV1 after smoking cessation is analogous to the initial increases in FEV1 seen in clinical studies in patients with COPD treated with an inhaled corticosteroid14 and another phosphodiesterase 4 inhibitor (roflumilast).15
The marked and transient effects of smoking cessation on FEV1 in patients with COPD has important implications for therapy because they demonstrate some element of reversibility in the complex range of lung pathology caused by smoking. There is potential for therapies to be given at the time of smoking cessation to conserve or even augment these effects. It would be of interest to study the mechanism of this FEV1 improvement7 in an effort to design drug therapy that uses this mechanism. Of note, transient improvements in acinar small airway function studied with the multiple breath washout technique have been reported after smoking cessation in smokers without airway obstruction.16 In the present study, we noted extensive small airways disease in the cigarette smokers, but measurement of maximal expiratory flow is too variable to be useful on an individual patient basis. Gas transfer of CO was decreased at baseline in healthy smokers with normal spirometry mainly as a result of increased CO in the circulation from cigarette smoking. However, complete restoration of gas transfer function at 1 year after smoking cessation was not observed, suggesting some residual defect in the lung interstitium despite a normal FEV1. Other studies have noted diffusion defects in healthy current smokers17 and found that smoking cessation caused restoration within 1 month.18 Furthermore, a longitudinal investigation over 10 years found that if healthy smokers stop smoking, Tlco returns to normal19; however, the reversibility of effects on gas transfer is likely to depend on the prior smoking exposure and the extent of preexisting lung damage.
The present study confirms that single-slice HRCT scans of the upper lung field are effective at detecting centrilobular emphysema in smokers with COPD and normal spirometry.20 Hence, in asymptomatic heavy smokers, emphysema can be detected through a modified HRCT scanning protocol that uses low doses of radiation. A disparity between presence of emphysema and spirometry has previously been found,21 and there are greater rates of lung function decline in smokers with severe emphysema as detected on CT scan.11 In the current study, no improvement was found, as expected, in the presence of emphysema on HRCT scan 12 months after smoking cessation.
Parenchymal micronodules and ground-glass opacification were found in smokers with and without spirometric abnormalities and occasionally in never smokers. Parenchymal micronodules are a feature of respiratory bronchiolitis-interstitial lung disease that may evolve to become areas of emphysema.22 An analysis of multidetector CT and microCT scans in patients with various stages of COPD demonstrated that narrowing and loss of terminal bronchioles occurs prior to the development of both centrilobular and panlobular emphysema.23 However, a study reviewing HRCT scans from > 2,000 cigarette smokers found that approximately one in 12 had respiratory bronchiolitis-interstitial lung disease abnormalities, but these abnormalities were associated with a lesser amount of emphysema.24 Another study found that centrilobular nodules and ground-glass opacification detected on HRCT scan can be improved by smoking cessation.25 However, the present study demonstrates that smoking cessation causes a decrease in the number of micronodules, with no significant changes in ground-glass opacification. Hence, the association of micronodules, ground-glass opacification, and emphysema remains controversial.
We performed a careful visual analysis of HRCT scans by trained operators working with agreed definitions and did not use densitometry. Another study used low-dose CT scanning to detect lung density in cigarette smokers in conjunction with densitometry and found that smokers can have a higher lung density due to contamination of the lung with tar and inflammation, which decreases on smoking cessation.26 However, a study looking at sputum and bronchial biopsy specimens taken 12 months after smoking cessation in patients with COPD showed that inflammation may persist and even increase.27
A limitation of the present study is the small sample size in relation to studies on the natural history of lung function in COPD.4 However, from a large screened population, we performed serial observation of four groups of highly defined subjects, using full lung function analysis in a single specialized laboratory and a strict per-protocol approach in individual subjects to assess longitudinal changes in lung function. Another limitation is that the CT scans were assessed by trained observers, but we did not use density masks or other quantitative image assessment procedures.
The public health message is that heavy cigarette smoking causes widespread damage throughout the bronchial tree of many asymptomatic cigarette smokers, despite normal spirometry. Lung physiology defects can be identified by spirometric assessment of FEV1 in conjunction with measurement of gas transfer after cessation, and these two functions are improved with different kinetics. In particular, the mechanism of the transient improvement in FEV1 could provide the basis for rational therapy, and therapy could be used concomitantly with smoking cessation to consolidate these effects. Furthermore, a single-slice upper-lobe HRCT scan will reliably identify the low-attenuation areas of centrilobular emphysema. The assessment of patients before and after smoking cessation forms a useful clinical research model that identifies diverse early COPD phenotypes as well as provides clinical benefit for participants.
Conclusions
• Spirometry only detects a fraction of patients with cigarette-associated lung disease.
• Cigarette smokers with normal spirometry have abnormalities on lung HRCT scan in almost 30% of cases.
• Patients with COPD who quit smoking have a transient improvement in FEV1 at 6 weeks after smoking cessation; this improvement is only partially maintained at 1 year. However, all smokers who quit have improvements in transfer factor.
• Smoking cessation does not improve emphysema but decreases the presence of micronodules detectable on HRCT scan.
• Smoking cessation at the earliest opportunity remains vital for lung health, and new therapies could be used during the cessation process to augment the beneficial effects of cessation.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Hansel had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Dhariwal: contributed to the statistical analysis, data interpretation, drafting of manuscript, and review and approval of the final manuscript.
Dr Tennant: contributed to the recruitment and clinical procedures, HRCT scan assessments, data interpretation, drafting of manuscript, and review and approval of the final manuscript.
Dr Hansell: contributed to the HRCT scan assessments, data interpretation, and review and approval of the final manuscript.
Dr Westwick: contributed to the data interpretation and review and approval of the final manuscript.
Dr Walker: contributed to the study concept and design, data interpretation, and review and approval of the final manuscript.
Mr Ward: contributed to the lung function analyses, data interpretation, and review and approval of the final manuscript.
Dr Pride: contributed to the lung function analyses, data interpretation, and review and approval of the final manuscript.
Dr Barnes: contributed to the study concept and design, data interpretation, and review and approval of the final manuscript.
Dr Kon: contributed to the study concept and design, HRCT scan assessments, data interpretation, and review and approval of the final manuscript.
Dr Hansel: contributed to the study concept and design, data interpretation, drafting of manuscript, and review and approval of the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Kon is involved in clinical studies in airway diseases with pharmaceutical funding and has grants from the National Institute for Health Research. Dr Hansel has been the principal investigator for respiratory clinical studies carried out at the Imperial College London that have been sponsored by the following pharmaceutical companies: Novartis AG; Merck Sharp & Dohme Corp; GlaxoSmithKline plc; and Dainippon Sumitomo Pharma Co, Ltd. He has received lecture fees from Novartis AG; Merck Sharp & Dohme Corp; GlaxoSmithKline plc; and Dainippon Sumitomo Pharma Co, Ltd. He is involved in a spin-off company with Imperial Innovations called Mucosal Diagnostics Limited and has received support to attend conferences from Boehringer Ingelheim GmbH. Drs Dhariwal, Tennant, Hansell, Westwick, Walker, Pride, and Barnes and Mr Ward have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: This manuscript is dedicated to the memory of the late Christoph Walker, PhD. This study is part of his legacy of research in respiratory disorders. The authors thank Ann Want, BSc, for data management and Jackie Turner, MSc, for statistical analysis.
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- CO
carbon monoxide
- FER
forced expiratory ratio
- HRCT
high-resolution CT
- Tlco
transfer factor of lung for carbon monoxide
- Tlcoc
transfer factor of lung for carbon monoxide corrected for hemoglobin
Footnotes
Funding/Support: This study received funding from Novartis Pharmaceuticals UK Ltd.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Buist AS, McBurnie MA, Vollmer WM, et al. ; BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741-750 [DOI] [PubMed] [Google Scholar]
- 2.Willemse BW, Postma DS, Timens W, ten Hacken NH. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23(3):464-476 [DOI] [PubMed] [Google Scholar]
- 3.Tønnesen P, Carrozzi L, Fagerström KO, et al. Smoking cessation in patients with respiratory diseases: a high priority, integral component of therapy. Eur Respir J. 2007;29(2):390-417 [DOI] [PubMed] [Google Scholar]
- 4.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1(6077):1645-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scanlon PD, Connett JE, Waller LA, et al. ; Lung Health Study Research Group. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2):381-390 [DOI] [PubMed] [Google Scholar]
- 6.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180(1):3-10 [DOI] [PubMed] [Google Scholar]
- 7.Tashkin DP, Rennard S, Taylor Hays J, Lawrence D, Marton JP, Lee TC. Lung function and respiratory symptoms in a 1-year randomized smoking cessation trial of varenicline in COPD patients. Respir Med. 2011;105(11):1682-1690 [DOI] [PubMed] [Google Scholar]
- 8.Agusti A, Calverley PM, Celli B, et al. ; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:12220831787 [Google Scholar]
- 9.Vestbo J, Edwards LD, Scanlon PD, et al. ; ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184-1192 [DOI] [PubMed] [Google Scholar]
- 10.Tylén U, Boijsen M, Ekberg-Jansson A, Bake B, Löfdahl CG. Emphysematous lesions and lung function in healthy smokers 60 years of age. Respir Med. 2000;94(1):38-43 [DOI] [PubMed] [Google Scholar]
- 11.Mohamed Hoesein FA, de Hoop B, Zanen P, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66(9):782-787 [DOI] [PubMed] [Google Scholar]
- 12.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365 [DOI] [PubMed] [Google Scholar]
- 13.Willemse B, Lesman-Leegte I, Timens W, Postma D, ten Hacken N. High cessation rates of cigarette smoking in subjects with and without COPD. Chest. 2005;128(5):3685-3687 [DOI] [PubMed] [Google Scholar]
- 14.Pauwels RA, Löfdahl CG, Laitinen LA, et al. ; European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med. 1999;340(25):1948-1953 [DOI] [PubMed] [Google Scholar]
- 15.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 Study Groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685-694 [DOI] [PubMed] [Google Scholar]
- 16.Verbanck S, Schuermans D, Paiva M, Meysman M, Vincken W. Small airway function improvement after smoking cessation in smokers without airway obstruction. Am J Respir Crit Care Med. 2006;174(8):853-857 [DOI] [PubMed] [Google Scholar]
- 17.Knudson RJ, Kaltenborn WT, Burrows B. The effects of cigarette smoking and smoking cessation on the carbon monoxide diffusing capacity of the lung in asymptomatic subjects. Am Rev Respir Dis. 1989;140(3):645-651 [DOI] [PubMed] [Google Scholar]
- 18.Sansores RH, Pare P, Abboud RT. Effect of smoking cessation on pulmonary carbon monoxide diffusing capacity and capillary blood volume. Am Rev Respir Dis. 1992;146(4):959-964 [DOI] [PubMed] [Google Scholar]
- 19.Watson A, Joyce H, Hopper L, Pride NB. Influence of smoking habits on change in carbon monoxide transfer factor over 10 years in middle aged men. Thorax. 1993;48(2):119-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatayama O, Kobayashi T, Fujimoto K, Kubo K. Utility of single-slice high-resolution CT in upper lung field combined with low-dose spiral CT for lung-cancer screening in the detection of emphysema. Intern Med. 2007;46(18):1519-1525 [DOI] [PubMed] [Google Scholar]
- 21.Omori H, Fujimoto K, Katoh T. Computed-tomography findings of emphysema: correlation with spirometric values. Curr Opin Pulm Med. 2008;14(2):110-114 [DOI] [PubMed] [Google Scholar]
- 22.Remy-Jardin M, Edme JL, Boulenguez C, Remy J, Mastora I, Sobaszek A. Longitudinal follow-up study of smoker’s lung with thin-section CT in correlation with pulmonary function tests. Radiology. 2002;222(1):261-270 [DOI] [PubMed] [Google Scholar]
- 23.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567-1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washko GR, Hunninghake GM, Fernandez IE, et al. ; COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakanishi M, Demura Y, Mizuno S, et al. Changes in HRCT findings in patients with respiratory bronchiolitis-associated interstitial lung disease after smoking cessation. Eur Respir J. 2007;29(3):453-461 [DOI] [PubMed] [Google Scholar]
- 26.Ashraf H, Lo P, Shaker SB, et al. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax. 2011;66(1):55-60 [DOI] [PubMed] [Google Scholar]
- 27.Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26(5):835-845 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement