Abstract
Background:
Dysphagia and aspiration pneumonia are two causes of morbidity in Parkinson disease (PD). In PD, impaired airway clearance can lead to penetration of foreign material, resulting in a high prevalence of aspiration pneumonia and death. This study examines three different devices for measurement of peak airflow during voluntary cough in healthy control subjects and those with PD. Two simple and low-cost devices for measuring peak cough airflow were compared with the “gold standard” pneumotachograph.
Methods:
Thirty-five healthy control subjects and 35 individuals with PD produced voluntary cough at three perceived strengths (weak, moderate, and strong cough) for each of the three devices.
Results:
A significant difference in mean peak cough airflow was demonstrated for disease (F[1,56] = 4.0, P < .05) and sex (F[1,56] = 9.59, P < .003) across devices. The digital and analog meters were comparable to the gold standard demonstrating no significant difference (statistical) by device (digital vs analog) in receiver operating characteristic curve analysis. Both devices were discriminative of the presence of PD.
Conclusions:
The analog and digital peak airflow meters are suitable alternatives to the gold standard pneumotachograph due to their low cost, portability, ease of use, and high sensitivity relative to normative peak cough airflows. Voluntary cough airflow measures may serve as a noninvasive means of screening for aspiration risk in target populations. Additionally, quantification of cough strength through use of predetermined limens for weak, moderate, and strong cough may assist clinicians in better describing and tracking cough strength as a contributing factor to aspiration risk.
Swallow and cough, respectively, serve as preventative and corrective airway processes protecting the lower airways through presumed reconfiguration of integrated neural pathways important for respiratory control. For example, intricate coordination of breathing and swallowing is required to safely transfer a bolus from the oral cavity into the lower digestive tract. Should material be aspirated into the lower airways, a separate series of events also involving cough and swallow will be elicited as an initial attempt to clear the airway. When the coordination of these processes fails, there is increased potential for penetration of foreign material, which can seed the subglottic airways resulting in a high prevalence of aspiration pneumonia.1 Mortality rates for aspiration pneumonia in those with Parkinson disease (PD) can approach 40%.2 Aspiration is of particular concern for individuals with neurodegenerative disease processes, where breathing and swallowing are frequently impaired.3‐10 There is a compelling need for strategies to screen and treat weak or inadequate airway protective behaviors in those with PD and others with neurodegenerative disease.
The addition of voluntary peak cough airflow measures to existing swallow-based clinical assessments may improve existing procedures for determining aspiration risk. We seek to facilitate this transition by empirically evaluating a simple, cost-effective means of measuring voluntary peak cough airflow in comparison with the current “gold standard” pneumotachograph. This project intends to reveal a device-driven, cost-effective tool for the noninvasive evaluation of voluntary cough function. Ultimately, this tool, in combination with swallow-based clinical assessments, may assist in efficiently assessing patients’ ability to produce both preventative (swallow) and corrective (voluntary cough) airway protective functions. Additionally, quick quantitative assessment of cough strength using consensus limens for weak, moderate, or strong cough can assist clinicians in the reporting of cough function for the purposes of describing aspiration risk in individuals with PD.
Materials and Methods
Participants
Seventy participants completed all study tasks (34 women, 36 men) of which 35 were healthy control (HC) subjects (24 women, mean age, 67.75 years, SD = 9.4 years; 11 men, mean age, 60.4 years, SD = 16.2 years), and 35 were diagnosed with PD (10 women, mean age, 73.4 years, SD = 4.6 years; 25 men, mean age, 72.0 years, SD = 5.1 years). The Hoehn and Yahr (HY) ratings of participants with PD ranged from 2 to 5 with a mean HY of 3.31 (SD = 0.96). Mean years since diagnosis of PD was 6.8 years (SD = 5.8 years). Participants with PD were considered for inclusion based on the following criteria: (1) diagnosis of PD by a neurologist; (2) between 30 and 80 years of age; (3) nonsmoking or no smoking within the previous 5 years; (4) no history of head and neck cancer, asthma, COPD, or untreated hypertension; (5) sufficient facial muscle strength so as to achieve and maintain adequate lip closure around a circular mouthpiece; (6) cognition within normal limits as determined by the Mini Mental Status Examination; and (7) no neurologic (other than PD) condition that adversely affects respiratory muscle or gas exchange system. All participants with PD were tested while in the on-medication response curve, defined as 1 h postmedication intake. At the time of testing, no participants showed signs of dyskinesia.
Inclusion and exclusion criteria for HC participants were identical to those of participants with PD with the exception of diagnosis of PD. This study was conducted in accordance with the amended Declaration of Helsinki. The University of Florida Institutional Review Board (UFIRB01, project 367-2010) approved the protocol, and written informed consent was obtained from all participants.
Cough Production
Following informed consent, all participants underwent assessment of voluntary peak cough airflows. Participation in the study required approximately 30 min and involved the production of voluntary coughs at three perceived strengths (weak, moderate, and strong) into three separate sensing devices (an analog peak airflow meter, a digital peak airflow meter, and a pneumotachograph). For each device, the weak, moderate, and strong productions were repeated three times, in a random sequence. Therefore, each participant produced a total of 27 cough samples (three productions of three perceived strengths on each of three devices).
Devices
The analog peak flow meter used in this investigation was the Mini Wright Peak Flow Meter (Mini Wright) featuring a high-visibility scale ranging from 60 to 850 L per minute. The meters used in this investigation were standard range with a larger diameter for use by adults. Per the manufacturer’s website, the meter is lightweight, compact, latex free, and able to be sterilized.11 The analog peak flow meters retail for approximately 30 US dollars per unit.
The digital peak flow meter used in this investigation is also manufactured by Mini Wright. The meter is powered by a lithium coin 3V battery (included and unable to be replaced) and features a range of 60 to 850 L per minute. For all participants, this meter was used in conjunction with a disposable pulmonary function test filter (CBI 1501U small, 30.15 mm outer diameter, 26.40 mm inner diameter) with 99.99% viral and bacterial filtration efficiency (Creative Biotech, Inc). The pneumotachograph used in this investigation consisted of a PowerLab Multi Channel Data Acquisition System used in conjunction with a Spirometer Pod and MLT300L Respiratory Flow Head 300L and disposable pulmonary function test filter with viral and bacteriologic filtration efficiency (all from ADInstruments). Signals were collected and analyzed using LabChart software (ADInstruments). Prior to cough data collection, the integrated pneumotachograph signal was calibrated directly for volume and (via formula) for flow by injecting a known 3-L volume of air through the experimental setup. Flow (F) was then calculated from the slope (rate of change) of the volume (V): F = dV/dt.
The production of voluntary coughs for each participant followed a minimum of three cycles of tidal volume breathing, and each cough trial was separated by approximately 1 min of rest to minimize fatigue. Prior to testing, participants were given verbal instruction to assist them in producing cough responses at the desired strengths. This instruction involved first verbally cueing each participant to produce a very soft cough. Productions that were not reflective of a “true cough” (eg, no glottal closure, just exhaled air) were corrected by the investigator through repeat cueing. After demonstrating adequate production of a soft cough, participants were cued to “cough as hard as you can.” For moderate cough, participants were cued to produce a cough “right between the soft cough and hard cough.” No apparent fatigue was observed in any of the participants during the experiment nor did any participant request to discontinue the trials. The order of the cough productions and device types was computer randomized with the exception of the first three coughs, which participants were always instructed to perform in the following order (so as to mimic the instruction given prior to testing): (1) soft, (2) hard, and (3) medium. No adverse events were reported at the time of the experiment or during the 6-month period following consent of the final participants.12
Analysis
The analysis portion of the project was automated using LabChart 7.0 software (ADInstruments) and its associated algorithms, thereby eliminating the potential for measurement bias. Analysis of the airflow waveform captured from the pneumotachograph was completed following low pass filtering of the airflow signal at 60 Hz. A “window” containing the peak cough airflow was created by placing cursors on the airflow signal immediately prior to the end of the compression phase (where cough airflow equals zero due to vocal fold closure prior to cough release) and immediately following the peak of the cough airflow signal. This window allowed for the automated identification and extraction of the peak cough airflow, which was then exported to a data pad. The voluntary peak cough airflow values for the analog and digital meters were visually extracted by the investigator directly from the devices and the values compiled, with the data from the pneumotachograph system, into a Microsoft Excel dataset (Microsoft Corp). Performance, reliability, and comparability of cough airflow production by the three devices for both participant groups were reviewed using distributions and correlational analysis. Accuracy of measurement by device, including quantification of systematic variation, was evaluated using the Youden plot, Bland-Altman plot, and Grubbs test.13,14 Here, “measurement accuracy” was determined by comparing results obtained from the analog and peak flow meters with those obtained via calibrated pneumotachograph. Identification and comparison of cough strength thresholds by device for the PD participants was completed using the receiver operator characteristic curve.15 Statistical analysis was completed using SPSS 18.0 (IBM), MedCalc (MedCalc Software), and SAS 9.0 (SAS Institute Inc).
Results
Comparison of all three devices using a repeated measures analysis of variance demonstrated a significant difference in mean peak cough airflow (L/s) by device type for both groups (F[1,56] = 4.0, P < .05) and sex (F[1,56] = 9.59, P < .003). No significant relationship was demonstrated between groups (PD vs HC) by age. Therefore, the differences found for peak cough airflow only occurred between the sexes for HC participants.
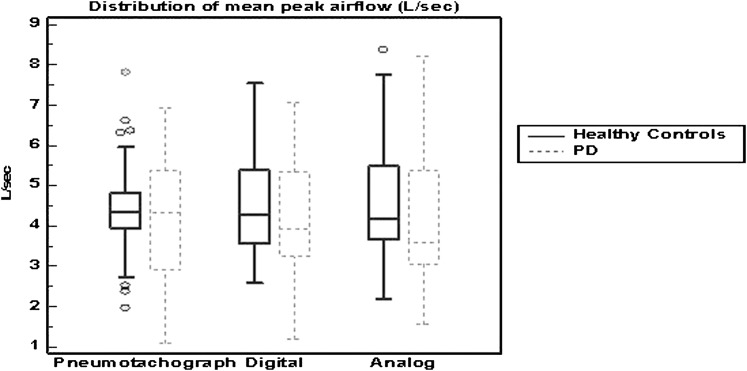
The distribution and comparability of cough airflow measurements produced across devices was reviewed using correlation analysis. The pneumotachograph demonstrated a strong correlation to the analog and digital peak airflow devices, with a slightly stronger relationship with the digital peak airflow device. However, the distribution of mean voluntary peak cough airflow values from the HC participants demonstrated that the digital peak airflow device produced a ± 5% difference from the pneumotachograph vs the analog peak airflow device, which demonstrated a ± 2% difference from the pneumotachograph (Fig 1).
Figure 1.
Distribution of mean peak airflow (L/s) by device and disease state. PD = Parkinson disease.
Differences in the performance of the devices were also reviewed across age strata (10-year bands) and sex to evaluate concurrent validity (Table 1). The digital and analog peak cough airflow devices were most strongly correlated to the pneumotachograph for the measurement of voluntary peak cough airflow in male HC participants, with the digital correlating particularly well among both younger (age 60-70 years) and older (age 71-81 years) healthy male participants. Both peak airflow devices were most strongly correlated to the gold standard pneumotachograph for the measurement of peak cough airflow produced by older women with PD.
Table 1.
—Device Performance Differences
| Device | HCs (n = 35) | PD (n = 35) | ||
| 60-70 y | 71-81 y | 60-70 y | 71-81 y | |
| Pneumotachograph to digital | ||||
| Male | 0.97 | 0.99 | 0.77 | 0.72 |
| Female | 0.60 | 0.56 | 0.81 | 0.85 |
| Pneumotachograph to analog | ||||
| Male | 0.64 | 0.99 | 0.80 | 0.76 |
| Female | 0.81 | 0.56 | 0.81 | 0.85 |
| Digital to analog | ||||
| Male | 0.58 | 0.99 | 0.88 | 0.78 |
| Female | 0.60 | 0.69 | 0.82 | 0.82 |
HC = healthy control; PD = Parkinson disease.
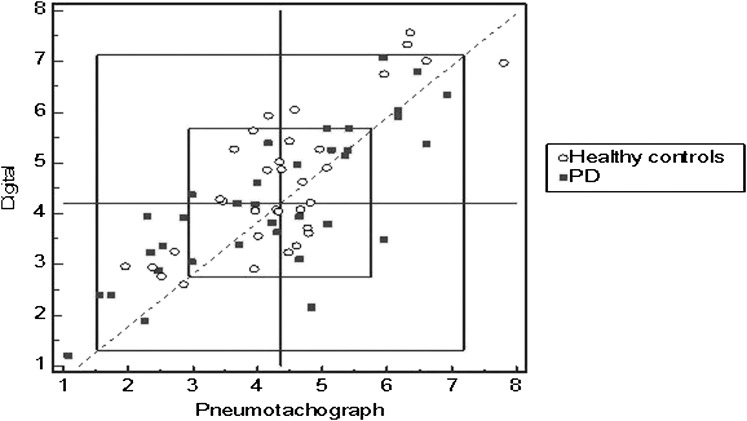
To evaluate the measurement accuracy of the devices, Youden and Bland-Altman plots were generated. Youden plots demonstrate between (reproducibility) and within (repeatability) measurement accuracy. The Youden plot displayed a graphical method to analyze interdevice data, where devices have produced multiple repetitions of the same data on two group samples (ie, cough attempts for HCs and patients with PD). The plot visualizes within-device variability as well as between-device variability for the two samples. The axes in this plot are drawn on the same scale: One unit on the x-axis has the same length as one unit on the y-axis. Each point in the plot corresponds to the results of one device, defined by a first response variable on the horizontal axis and a second response variable on the vertical axis. The proximity of each plotted point to the diagonal line with slope 1 and intercept zero shows agreement of replicate measures. The horizontal median line is drawn parallel to the x-axis, and a second median line is drawn parallel to the y-axis. The intersection of the two median lines is the Manhattan median. The rectangle drawn includes 95% of the devices, if individual constant errors could be eliminated. Rectangles shown represent 1 and 2 SD in variability. The 45° reference line drawn through the Manhattan median identifies points that indicate systematic error. Points that lie far from the 45° line indicate large random error. Points outside the inner rectangle indicate large total error. Alternatively, this can be interpreted as follows: When the points lie closely along the 45° line, the conclusion may be drawn that each measurement is similar (less error). But when points lie outside the inner rectangle, these points almost certainly somehow have substantial systematic errors incorporated in their output. These plots detected a small amount of systematic error surrounding the measurement of peak cough airflow. Only 8.5% (from HC participants) and 5.7% of the data (from PD participants) were considered in error by this method (Fig 2). This finding indicates an acceptable overall error in peak cough airflow measurement.
Figure 2.
Youden plot of measurement error (digital cough device vs pneumotachograph). See Figure 1 legend for expansion of abbreviation.
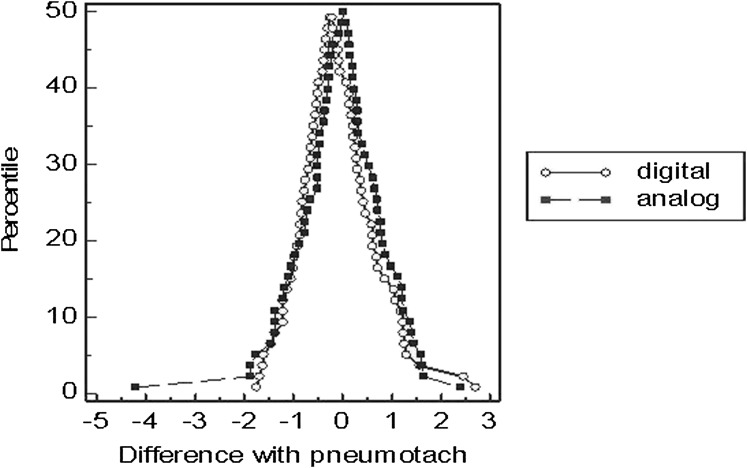
Comparison of the analog and digital peak airflow devices against the gold standard pneumotachograph revealed no significant outliers using the Grubbs test. Bland-Altman difference plots were used to estimate bias and precision of measures for the two peak airflow devices in comparison with the gold standard pneumotachograph. The Bland-Altman comparison revealed a mean peak airflow difference between devices of 0.0012 (SD, 0.016) (digital: x = 4.42 [SD, 1.41; 95% CI, 4.08-4.76]; analog: x = 4.35 [SD, 1.5; 95% CI, 3.99-4.71]). The Bland-Altman plot (Fig 3) demonstrated slightly greater precision in peak cough airflow measurement by the analog peak airflow device (0.7% bias) vs the digital peak airflow device (3.3% bias). Test-retest reliability of the devices across repeated measures for 20% of all data demonstrated strong reliability for the digital (r = 0.956) and analog devices (0.973) at P < .0001.
Figure 3.
Bland-Altman measurement difference plot of digital vs analog devices compared with gold standard pneumotachograph device (all cough attempts).
An additional objective of this investigation was the establishment of voluntary cough airflow function ranges for HC participants and those with PD. The analysis of cough strength by device was completed using the area under the receiver operating characteristic curve (AUROC).16 The ability of each device to discriminate cough strength among HC individuals at the various strength ranges was reviewed by the AUROC and Youden index. The measures obtained via pneumotachograph for HC participants functioned as the standard referent for this analysis. Results revealed no significant difference by device type (digital vs analog) For this analysis, pneumotachograph cough response was categorized according to the following cut points: weak denoted by peak airflow rates of ≤ 3.5 L/min, moderate cough ≤ 4.0 L/min, and strong cough ≤ 5.5 L/min. For the weak cough strength, the analog device demonstrated superior values in comparison with the digital peak airflow device (area under the curve, 0.80 [95% CI, 0.65-0.86] analog vs 0.77 [95% CI, 0.68-0.88] digital). For the moderate and strong cough strengths, the digital device demonstrated superior values (area under the curve, 0.83 [95% CI, 0.72-0.91] digital vs 0.79 [95% CI, 0.68-0.88] analog for moderate, and 0.83 [95% CI, 0.72-0.91] digital vs 0.81 [95% CI, 0.69-0.89] analog for strong) in comparison with the analog peak airflow device. The ability of each device to discriminate between disease state (HC vs PD) and PD severity based upon HY score > 3 for total mean cough production was also compared. Results demonstrated the analog device produced marginally larger AUROC values for both disease state (Table 2) and PD severity discrimination (Table 3). The likelihood value of the analog device (which is not affected by prevalence) was superior (at 10.26) to the digital device (at 2.74).
Table 2.
—AUROC Values for Disease State
| Device | AUROC | Se | Sp | 95% CI |
| Pneumotachograph | 0.483 | 40.1 | 77.1 | 0.36-0.60 |
| Digital peak airflow meter | 0.571 | 51.4 | 68.6 | 0.45-0.68 |
| Analog peak airflow meter | 0.584 | 51.4 | 76.5 | 0.46-0.70 |
AUROC = area under the receiver operating characteristic curve; Se = sensitivity; Sp = specificity.
Table 3.
—AUROC Values for PD Severity Discrimination
| Device | AUROC | Se | Sp | 95% CI |
| Pneumotachograph | 0.70 | 64.7 | 77.1 | 0.55-0.82 |
| Digital peak flow meter | 0.72 | 70.6 | 68.6 | 0.58-0.84 |
| Analog peak flow meter | 0.74 | 70.6 | 76.5 | 0.59-0.85 |
Discussion
Although diagnostic tools currently exist within clinical settings to assess aspiration risk, the focus of these assessments is on defining swallow function. Though these tools are widely used in medical settings, there is poor consensus among health-care professionals and little outcome data regarding which swallow assessment protocol is sensitive for detecting aspiration. Research continues to seek a rapid measure for determining aspiration risk. The lack of consensus has resulted in elimination of a Joint Commission on Accreditation of Healthcare Organizations (JCAHO) mandated brief swallow assessment for all patients with a suspected diagnosis of stroke in stroke-certified centers.17,18 Additionally, swallow screening measures are unable to assess corrective airway protection modalities, and cannot assess a patient’s ability to defend his or her airway, when and if aspiration occurs.
Evaluation of voluntary cough function serves to characterize one mechanism of corrective airway protection. Such assessments have been largely limited to subjective measures that are highly subject to error. Previous investigations, along with the European Respiratory Society (ERS) and American College of Chest Physicians (ACCP), have outlined the need for diagnostic tools to objectively assess cough.19‐22 Assessing voluntary cough function using airflow waveform analysis has been used with individuals with PD as well as those poststroke to detect penetration and aspiration during oropharyngeal swallowing23‐28 (T. E. Pitts, PhD, unpublished data, June 2009). These studies have shown that airflow measures from voluntary cough production can identify individuals at risk for penetration or aspiration. Voluntary peak cough airflow characteristics could be used to model airway compromise in people with PD or those poststroke. And while these datasets have been published, the methodology used in past investigations was a pneumotachograph.
In spite of this evidence, measures of voluntary cough airflow have failed to translate easily into clinical practice. We believe this translational difficulty has occurred due to technical demands and the considerable cost of the pneumotachograph device, inherent to gold standard methodologies for assessing cough. At minimum, the gold standard methodology can be cost prohibitive, requiring a spirometer, pneumotachograph, filters, and commercial software for charting and analysis. Although Pitts et al24 provided detailed analysis of the voluntary cough airflow pattern, cough airflow signals can be highly variable and subject to numerous technical considerations. These include the behavior of transducers used to collect the raw signal, as well as characteristics of post hoc filtering of signals (for example, to remove noise) prior to analysis.29 The possibility of an inexpensive, portable, and readily interpreted screening test for deficiencies in airway protection holds high potential clinical utility. Establishment of validated normative values, both for patient and healthy populations, will allow clinicians who treat those with potential for deficient airway protection the ability to quickly assess the risk that such a deficiency exists, recommend appropriate diagnostic test(s), and implement a treatment plan.
This investigation is original in that it provides measurement of voluntary cough peak airflow via a simple and inexpensive portable device and relates the peak airflow measure to PD disease severity, a need previously outlined within the literature.30,31 The use of a portable and noninvasive tool for measurement of peak airflow during voluntary cough provides important functional information that can be collected at bedside or in the home environment for diagnosis and discrimination of disease severity, as well as a means to track the outcomes of rehabilitations aimed at improving cough strength. These results also establish and validate normative cough strength ranges in HC participants across three methods for airflow data collection (analog peak meter, digital peak airflow meter, and pneumotachograph), providing a dataset that can be used for clinical pathology comparison.32 Continued work is needed to establish normative ranges within larger cohorts of patient and nonpatient groups, across all available age groups.
Although the experimental and control groups were somewhat disparate in their average age, one past investigation has shown no difference in laryngopharyngeal mechanosensitivity relative to age.33 Although all participants with PD were tested while on their medication response curve, by collecting data approximately 1 h after intake of PD medications, it is possible that medication effects may have exerted an influence on the observed results. All peak cough airflows obtained during this investigation were volitional in nature (eg, participants were instructed to repeatedly produce coughs at various strength levels) and arguments can be made regarding the comparability of volitional to reflexive peak cough airflows. Current understanding of the neurophysiology of cough supports an integrated model of cough generation involving both cortical and subcortical structures in most physiologic conditions with cortical involvement in detection of sensory stimuli as well as the configuration of the cough response itself.34 Aside from nonawake conditions (deep anesthesia, coma, and so forth) the influence of cortical processing on cough motor outputs is evident. These findings strengthen the argument that volitional and reflexive or evoked cough are inextricably linked.
It is known that age, height, weight, race, and sex can directly affect the results, which one would predict for a given individual from respiratory testing. In this study, sex was included as an independent variable in the repeated measures analysis. Post hoc comparisons revealed that sex differences in mean peak cough airflow (L/s) only occurred in male HC participants across all devices. Our study did not control height or race, however; in this study, we aimed to evaluate the relative comparability of measurement accuracy across measurement devices. As such, controlling variables of height and race may have strongly impacted recruitment duration and sample size, but would not have directly affected results as each subject served as his or her own control across the three different device conditions.
Portability of the peak airflow meters offers expediency in clinical care and while the intent of this investigation was not to present peak airflow monitoring as a replacement for swallow screening measures of airway protection, evidence exists to support the inclusion of such measures as supplementary to those already in widespread clinical use. The major findings of this study indicate that the analog peak airflow device is superior in the identification and discrimination of voluntary cough strength in both healthy adults and individuals with PD. Consequently, use of the analog peak airflow device may not only save valuable clinical time and reduce the cost of instrumentation needed for assessment of cough airflow, but also reduce the complexity and cost-prohibitive nature of cough assessment. In phase 2 of this project (presently underway), we are relating measures of peak airflow during voluntary cough to the presence of penetration or aspiration during videofluoroscopy in participants with PD to determine the relationship between the cough peak airflow measure and penetration aspiration score for those with PD. It is our intent to evaluate the contribution of voluntary cough function to the overarching mechanism of airway protection by comparing data obtained during peak cough airflow sampling to observations of penetration and aspiration during videofluoroscopy. From this, we hope to develop a predictive model for the categorization of PD participants with impairment of airway protective mechanisms based on a twofold (preventative-corrective) distinction obtained by merging findings from assessments of both swallow and cough function. These findings, once published, will serve as a crucial link between voluntary peak cough airflows and aspiration risk for individuals with PD.
Acknowledgments
Author contributions: Drs Silverman, Carnaby-Mann, and Sapienza had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Additionally, all authors confirm that the study objectives and procedures are honestly disclosed. Dr Silverman was primarily responsible for data collection, and she has reviewed study execution data and confirms that procedures were followed to an extent that the results are valid and generalizable to a population similar to that enrolled in this study. Dr Silverman assumes responsibility for this submission as a whole.
Dr Silverman: contributed to the conception, organization, and execution of the research project; reviewed and critiqued the statistical analysis; wrote the first draft of the manuscript; and reviewed and critiqued the manuscript.
Dr Carnaby-Mann: contributed to the conception of the research project; designed, executed, reviewed, and critiqued the statistical analysis; reviewed and critiqued the manuscript; and served as a principal investigator.
Dr Pitts: contributed to the conception and organization of the research project and reviewed and critiqued the manuscript.
Dr Davenport: contributed to the conception of the research project, reviewed and critiqued the statistical analysis, and reviewed and critiqued the manuscript.
Dr Okun: contributed to the review and critique of the statistical analysis and reviewed and critiqued the manuscript.
Dr Sapienza: contributed to the conception, organization, and execution of the research project; reviewed and critiqued the statistical analysis; wrote the first draft of the manuscript; reviewed and critiqued the manuscript and served as a principal investigator.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Pitts has received grant monies from National Institutes of Health (NIH) and the University of Florida’s Opportunity Fund and also makes public statements at scientific conferences nationally and internationally on the subject of this manuscript. Dr Okun serves as a consultant for the National Parkinson Foundation (NPF), and has received research grants from the NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, and the University of Florida Foundation; has previously received honoraria, but in the past > 36 months has received no support from industry including travel; has received royalties for publications with Demos, CRC Press (Taylor & Francis Group, LLC), and Cambridge University Press (movement disorders books); has participated in continuing medical education (CME) activities on movement disorders sponsored by the University of South Florida CME office, PeerView, and by Vanderbilt University; and has participated as a site principal investigator (PI) and/or coinvestigator for several NIH, foundation, and industry-sponsored trials over the years but has not received honoraria. The institution, and not Dr Okun, receives grants from Medtronic, Inc and Advanced Neuromodulation Systems, Inc/St Jude Medical, Inc and the PI has no financial interest in these grants. Dr Sapienza has an organized interest in Aspire Products, LLC; is an avid invited speaker-receiving reimbursement and honoraria; is a book author with Plural Publishing, Inc; and is a grant awardee with the NIH. Drs Silverman, Carnaby-Mann, and Davenport have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Abbreviations
- AUROC
area under the receiver operating characteristic curve
- HC
healthy control
- HY
Hoehn and Yahr
- PD
Parkinson disease
Footnotes
Funding/Support: This study was supported by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders [Grants R21/R33 and 7R33DC011131-04].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70(7):793-802 [DOI] [PubMed] [Google Scholar]
- 2.Smith Hammond CA, Goldstein LB. Cough and aspiration of food and liquids due to oral-pharyngeal dysphagia: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1_ suppl):154S-168S [DOI] [PubMed] [Google Scholar]
- 3.Altman KW. Oropharyngeal dysphagia pathophysiology, complications and science-based interventions. Nestle Nutr Inst Workshop Ser. 2012;72:119-126 [DOI] [PubMed] [Google Scholar]
- 4.Ebihara S, Saito H, Kanda A, et al. Impaired efficacy of cough in patients with Parkinson disease. Chest. 2003;124(3):1009-1015 [DOI] [PubMed] [Google Scholar]
- 5.Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8(4):CR241-CR246 [PubMed] [Google Scholar]
- 6.Garon BR, Sierzant T, Ormiston C. Silent aspiration: results of 2,000 video fluoroscopic evaluations. J Neurosci Nurs. 2009;41(4):178-185 [PubMed] [Google Scholar]
- 7.Gorell JM, Johnson CC, Rybicki BA. Parkinson’s disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990. Neurology. 1994;44(10):1865-1868 [DOI] [PubMed] [Google Scholar]
- 8.Nakashima K, Maeda M, Tabata M, Adachi Y, Kusumi M, Ohshiro H. Prognosis of Parkinson’s disease in Japan. Tottori University Parkinson’s Disease Epidemiology (TUPDE) Study Group. Eur Neurol. 1997;38(suppl 2):60-63 [DOI] [PubMed] [Google Scholar]
- 9.Schiermeier S, Schäfer D, Schäfer T, Greulich W, Schläfke ME. Breathing and locomotion in patients with Parkinson’s disease. Pflugers Arch. 2001;443(1):67-71 [DOI] [PubMed] [Google Scholar]
- 10.Shill H, Stacy M. Respiratory function in Parkinson’s disease. Clin Neurosci. 1998;5(2):131-135 [PubMed] [Google Scholar]
- 11.Mini Wright peak flow meter. Mini Wright website. http://miniwrightpeakflowmeter.com/collections/peak-flow-meters. Accessed March 7, 2014
- 12.Flow and volume calibration. ADI Instruments website. www.adinstruments.com. Accessed October 24, 2013
- 13.Hemphill JF. Interpreting the magnitudes of correlation coefficients. Am Psychol. 2003;58(1):78-79 [DOI] [PubMed] [Google Scholar]
- 14.Youden WJ. Graphical diagnosis of interlaboratory test results. Industrial Quality Control. 1959;15:24-28 [Google Scholar]
- 15.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160 [DOI] [PubMed] [Google Scholar]
- 16.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29-36 [DOI] [PubMed] [Google Scholar]
- 17.The Stroke Performance Measurement Implementation Guide. 2nd ed Washington, DC; The Joint Commission; 2008 [Google Scholar]
- 18.Carnaby-Mann G, Lenius K, Crary MA. Update on assessment and management of dysphagia post stroke. Northeast Florida Medicine. 2007;58(2):31-34 [Google Scholar]
- 19.Krajnik M, Damps-Konstanska I, Gorska L, Jassem E. A portable automatic cough analyser in the ambulatory assessment of cough. Biomed Eng Online. 2010;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morice AH, Fontana GA, Belvisi MG, et al. ; European Respiratory Society (ERS). ERS guidelines on the assessment of cough. Eur Respir J. 2007;7(6):1256-1276 [DOI] [PubMed] [Google Scholar]
- 21.Irwin RS. Assessing cough severity and efficacy of therapy in clinical research: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1_suppl):232S-237S [DOI] [PubMed] [Google Scholar]
- 22.Smith J, Woodcock A. New developments in the objective assessment of cough. Lung. 2008;186(suppl 1):S48-S54 [DOI] [PubMed] [Google Scholar]
- 23.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56(4):502-506 [DOI] [PubMed] [Google Scholar]
- 24.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23(3):297-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135(5):1301-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith Hammond CA, Goldstein LB, Horner RD, et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769-777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey DJ, Smithard DG, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke. 2003;34(5):1252-1257 [DOI] [PubMed] [Google Scholar]
- 28.Piirilä P, Sovijärvi AR. Differences in acoustic and dynamic characteristics of spontaneous cough in pulmonary diseases. Chest. 1989;96(1):46-53 [DOI] [PubMed] [Google Scholar]
- 29.Knudson RJ, Mead J, Knudson DE. Contribution of airway collapse to supramaximal expiratory flows. J Appl Physiol. 1974;36(6):653-667 [DOI] [PubMed] [Google Scholar]
- 30.Pavesi L, Subburaj S, Porter-Shaw K. Application and validation of a computerized cough acquisition system for objective monitoring of acute cough: a meta-analysis. Chest. 2001;120(4):1121-1128 [DOI] [PubMed] [Google Scholar]
- 31.Chung KF. Measurement of cough. Respir Physiol Neurobiol. 2006;152(3):329-339 [DOI] [PubMed] [Google Scholar]
- 32.Korpás J, Sadlonová J, Vrabec M. Analysis of the cough sound: an overview. Pulm Pharmacol. 1996;9(5-6):261-268 [DOI] [PubMed] [Google Scholar]
- 33.Leow LP, Beckert L, Anderson T, Huckabee ML. Changes in chemosensitivity and mechanosensitivity in aging and Parkinson’s disease. Dysphagia. 2012;27(1):106-114 [DOI] [PubMed] [Google Scholar]
- 34.Hegland KW, Bolser DC, Davenport PW. Volitional control of reflex cough. J Appl Physiol (1985). 2012;113(1):39-46 [DOI] [PMC free article] [PubMed] [Google Scholar]