Abstract
Expectancies are a class of psychological and neurobiological processes that may be responsible for part of the improvement observed with psychiatric treatments. Patients' expectations can substantially affect the results of clinical trials, and managing them is an important part of clinical care. This review describes the history of research on expectancy effects in Major Depressive Disorder (MDD), the relationship between expectancies and placebo effects, and what is currently known about the mechanisms of action of expectancy. Meta-analyses of antidepressant trials show that placebo response rates are high (typically ~30%) and often larger than the difference in response rates between drug and placebo (typically ~10%). Some of the response to placebo is due to natural history, but a growing literature suggests that much of the improvement on placebo treatment is due to active neurobiological processes related to expectancy. Several studies have shown that higher expectation of therapeutic improvement leads to greater improvement in psychiatric symptoms, particularly in MDD. New research on the mechanisms of action of expectancy is therefore a priority that could lead to improved interventions. This review discusses the evidence to date and methodological considerations in the design of new studies.
Keywords: expectancy, patient expectation, placebo effect, depression, randomized controlled trial, methodology
INTRODUCTION
Major Depressive Disorder (MDD) affects approximately 120 million people worldwide (including nearly 15 million American adults each year) and is a leading cause of disability due to illness.1,2,3 Even with maximal treatment, many patients will not experience sustained remission of their depression. The cumulative percentage of patients achieving remission after four sequential antidepressant trials in the National Institute of Mental Health (NIMH) sponsored Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study was only 51%.4 Residual depressive symptoms place patients at increased risk of suicide, cardiovascular morbidity and mortality, and depression recurrence.5,6,7 Until novel treatments with proven efficacy for depression can be developed, methods of optimizing response to currently available antidepressant treatments are urgently needed.
The placebo effect, rather than the specific effect of antidepressant medication, appears to be responsible for most of the change observed in patients receiving antidepressants for MDD. A meta-analysis of placebo-controlled RCTs of antidepressants submitted to the Food and Drug Administration (FDA) reported that the placebo groups in these trials averaged 1.5 standard deviation units of improvement, which was 75% of the improvement shown in the antidepressant groups.8 Similar results were reported in another meta-analysis of 75 placebo-controlled antidepressant RCTs published between 1981 and 2000, which found a mean medication response rate of 50%, compared to a mean placebo response rate of 30%.9
Given that treatment with a placebo can have beneficial effects on depression for a number of patients, it is important to understand the mechanisms by which it works. Knowledge of the mechanisms of action of effective treatments can be informative about the pathophysiology of MDD as well as suggest means of improving clinical outcome for patients. In the case of placebo effects in antidepressant clinical trials, patient expectancy is hypothesized to be their major mechanism of action.10,11 It is therefore critical to understand how patients' beliefs and expectations are affected by treatment contexts, how expectancies influence clinical outcome, and the neural mechanisms by which they affect MDD. The importance of studying placebo and expectancy effects was explicitly recognized at a conference held at the National Institutes of Health (NIH) in 2000, titled “The Science of the Placebo: Towards an Interdisciplinary Research Agenda”.12 The published report of the conference stated that “the placebo has been considered a `nuisance factor' in clinical trials, when in fact it represents a powerful therapeutic ally in health care… studies should be conducted to determine how modifications in expectation… affect treatment outcome (p.293).”
Expectancy definition and terminology
`Expectation' and `expectancy' are interchangeable words referring to an individual's beliefs about future events. In the context of psychiatric treatment, expectations can be divided into “prognostic expectations” and “participant role expectations.”13 Prognostic expectations are expectations about outcome and refer to how a patient estimates the probabilities associated with various future scenarios, including anticipated positive or negative effects of treatment. Role expectations refer to anticipated behaviors on the part of doctor and patient during treatment. Expectations can have positive or negative valences, depending on whether a patient believes a treatment will help or harm, and they can vary in strength depending on the reasons supporting the patient's expectation.
Prognostic expectations are the focus of this review given their applicability to both pharmacotherapy and psychotherapy, their extensive history of research, and their well described links with treatment outcome.14 Outcome expectations can be divided into pre-treatment, during treatment, and post-treatment phases. Patients entering treatment have an outcome expectation based on their understanding of the treatment offered, their own illness, and experiences with past treatments. Studies have indicated that the perceived prestige, credibility, and sophistication of a proffered treatment can significantly increase patients' expectations of improvement.15 Once a treatment begins, this expectation may change in a complex process based on the patient's therapeutic alliance with the clinician, initial treatment benefits or side effects, and the severity of the patient's illness. Following a course of treatment, patients may have positive or negative expectations about their likelihood of staying well.
Although patient expectancy is the focus of this review, clinicians and outcome raters in research studies may have expectations regarding patients' likelihood of improvement. The expectations of study physicians and raters have been shown to bias their evaluations of patients in research studies.16,17 For example, clinical response rates in single-blind placebo-controlled studies are higher than in double-blind studies, possibly because clinicians and raters in single-blind studies know the patients' treatment assignment.18 This bias may result from investigators' investment in their research and desire to prove or disprove the hypothesis being studied.19 For these reasons, some methodologists have suggested new technologies such as telephone interviews by off-site raters and computer-administered rating scales be used to assess outcome in clinical trials.20
PATIENT EXPECTANCY IN PSYCHOTHERAPY AND PHARMACOTHERAPY RESEARCH
Psychotherapy researchers have studied expectancy as a treatment factor common to many psychotherapies, distinct from factors specifically related to the theory underlying a particular therapy. Isolating the “active” components of psychotherapy became important in the 1950s, when Eysenck (1952) and others critiqued psychotherapy for failing to bring about recovery beyond what was “obtained through ordinary life experiences and non-specific treatment.”21,22 Jerome Frank suggested that patient improvement in response to psychotherapy was due to the patient's conviction that therapy would help him.23 Frank wrote that “a patient's expectancy of benefit from treatment in itself may have enduring and profound effects upon his mental state (p.36).” He suggested that to determine whether a specific psychotherapy was effective beyond its general anti-demoralizing effects, it must be compared to a placebo therapy in which patients had equal faith but lacked the therapeutic ingredients posited by the theory of therapy being studied.24
While psychotherapy outcome researchers have attempted to differentiate specific and nonspecific treatment factors and determine the influence of patient expectancy effects, pharmacotherapy researchers have believed they were insulated from these issues by the use of double-blind, placebo-controlled trials. However, even in studies involving medication only, the informed consent procedure for a trial can serve as a means of manipulating patient outcome expectations, which can then influence patient outcome.25 Informed consent is essentially a process of setting prognostic expectations. Patients become aware of the study design, the history and past effectiveness of the drugs and placebos used in the study, and the investigator's opinions of the treatment options.
Several studies have now documented that the procedures used during this process can have both harmful and beneficial effects. For example, inclusion of specific expected side effects in consent forms has been shown to dramatically increase the reported incidence of those side effects.26 In addition, the knowledge that one may receive a placebo may introduce uncertainty and decrease patients' expectations for improvement.27 In several studies involving the deceptive administration of placebo, the certainty of receiving a drug maximized observed placebo response rates, while the introduction of doubt about whether the medication is active diminished its effect.28,29
Conversely, being told that one will definitely receive an active drug appears to increase placebo response rates. Response to placebo increases with the number of treatment arms in a trial, which may be explained by the probability of receiving active medication as opposed to placebo increasing with the number of treatment arms in a study.30 Additionally, antidepressant response rates are generally higher in open-label studies compared to placebo-controlled RCTs.31 A number of studies have reported that antidepressant response is higher in comparator (i.e., medication vs. medication) antidepressant trials than in placebo-controlled trials. Patients in comparator trials know they are receiving a medication without knowing the exact agent, while patients in placebo-controlled trials do not know whether they are receiving an active medication. An initial study found an average medication response rate of 49% in placebo-controlled versus 59% in comparator trials for late life depression.32 However, they did not conduct a formal literature search, provide inclusion and exclusion criteria for the studies they examined, or test whether the observed difference was statistically significant.
Rutherford et al (2009) conducted a systematic analysis of clinical trials of antidepressants for major depression in adults aged 18–65.33 In the 48 placebo-controlled and 42 comparator trials examined, the odds of being classified as a responder to medication in comparator trials were 1.8 times the odds of being classified as a responder in placebo-controlled trials (95% CI = 1.45 – 2.17, p < 0.001), and the odds of being classified as a remitter to medication in comparator trials were 1.5 times the odds of being classified as a remitter in placebo-controlled trials (95% CI = 1.11 – 2.11, p < 0.001). The estimated probability of response to medication in placebo-controlled trials was 0.52 as compared to 0.65 in comparator trials. In a sample of patients with late life depression, Sneed et al (2008) found the odds of being classified a responder in comparator trials were nearly two times the odds of responding in the placebo-controlled trials.34 The estimated probability of response in placebo-controlled trials was 0.46 compared to 0.63 in comparator trials.
Therefore, while treatment randomization, a placebo comparison group, and blinding of both patients and investigators to the treatment assignment ensure that potential confounders and nonspecific factors are equivalent between groups in pharmacotherapy trials, these meta-analyses suggest that patient expectancy remains an important variable to consider when interpreting study results. Expectancy may influence comparisons of results between studies having different designs (e.g., placebo-controlled vs. comparator antidepressant RCTs) or even between treatment arms within a single study if the arms induce different expectancies (e.g., as in studies comparing psychotherapy to medication, where psychotherapy is administered in an open fashion whereas medication treatment is blinded). While most researchers have focused on minimizing or eliminating expectancy effects to improve the signal of efficacy for the treatment being studied, understanding the mechanisms of expectancy may represent an opportunity to safely and effectively improve clinical outcomes. Advances in clinical trials methodology and neuroimaging techniques have made it possible to begin probing the mechanisms of expectancy, as detailed in the following section.
METHODOLOGICAL CONSIDERATIONS IN THE STUDY OF EXPECTANCY EFFECTS
Experimental manipulation of expectancy
Expectancy must be experimentally manipulated in order to determine whether it has a causal relationship with depression outcome, but methods of modifying expectancy available to date have been ineffective or unethical in subjects with psychiatric disorders.35 An early method developed to study patient expectations and treatment outcome is the `balanced placebo' design.36,37 This method involves a placebo-controlled trial of medication in which patients are randomized to one of 4 conditions: receiving medication/expecting medication, receiving medication/expecting placebo, receiving placebo/expecting medication, and receiving placebo/expecting placebo. The balanced placebo design has proven itself useful for studying the effects of many psychoactive substances, from alcohol to caffeine.38,39 However, while this design permits direct comparisons across expectation states, many do not consider the deception involved to be ethical for patients with depression. These ethical difficulties have limited the application of this design to study depression.
Some investigators have attempted to induce different levels of patient outcome expectations by varying the clinician's behavior in a standardized way (e.g., optimistic, neutral, pessimistic). Unfortunately, different clinician styles have frequently failed to induce differential patient expectations. One older study investigated whether an enthusiastic vs. skeptical attitude on the part of the clinician influenced response to meprobamate and placebo for the treatment of anxiety.40 Of the three experimental sites where the study was conducted, one demonstrated better outcomes for patients with enthusiastic doctors, whereas the other two sites did not demonstrate significant differences.
More recently, Kemeny and colleagues (2007) randomized patients with asthma to a 2 × 2 study design examining the effect of salmeterol vs. placebo and “enhanced” vs. “efficient” physician style.41 “Enhanced” encounters were intended to transmit a positive expectation for improvement to the patient—physicians were trained to evince authority and conviction as well as provide a more empathic and supportive environment. Physicians providing the “efficient” care were trained to convey equivocal expectations while being less authoritative and supportive. While robust placebo responses were achieved in the study (as measured by objective physiologic change in forced expiratory volumes), patients did not differ in treatment outcomes or treatment expectations between the enhanced and efficient physician conditions. This finding indicates that the investigators were unsuccessful in inducing differential expectations using different physician styles.
A related way of studying expectancy effects is to compare open pharmacological treatments, where subjects know a treatment is being administered and expect it to have a therapeutic effect, with hidden treatments given without the subject's knowledge. Studies of pain-relieving drugs have shown hidden treatments are substantially less effective.42 These data suggest that clinical trial designs that randomize subjects to open vs. concealed administration of medication may be an attractive strategy for manipulating subject expectancy and antidepressant outcome.
Deceiving patients with depression about the treatment they are receiving is ethically problematic, so our group has opted to study expectancy by randomizing adult outpatients with MDD to a placebo-controlled track, in which subjects are randomly assigned to escitalopram or placebo, or a comparator track, in which patients are randomly assigned to treatment with either escitalopram or citalopram. The primary comparisons of interest are patient expectancy and depression remission rates to escitalopram in each of the two conditions. Preliminary data indicate that subjects who know they are receiving active treatment (i.e., those in the comparator track) have higher expectations of improvement than subjects in the placebo-controlled track.43
Measurement of expectancy
Regardless of the method used to manipulate patient expectations, some assessment of the impact of these methods should be performed. A comprehensive review of research studies that attempted to manipulate patient expectations found that only 6 of 24 studies reviewed reported measuring expectations to confirm they had changed post-manipulation, and in only 4 of these did the check confirm expectations did in fact change.44 Some would argue that without determining whether the experimental manipulation indeed altered participant expectations, it is much more difficult to interpret a study's findings. For example, patients may forget what they are told, talk to another subject given different instructions, be apathetic or hopeless despite positive instructions, or else improved despite a negative expectancy.
Experimenters who fail to measure expectations after attempting to manipulate them are often guilty of circular reasoning, as pointed out by Wilkins (1973).45 If positive outcome expectations are instilled in a group of subjects who then demonstrate greater improvement than subjects given lower expectations, it is concluded that the subject indeed had different expectation states and that these caused the disparate outcomes. Had a difference in outcomes not been observed, then either the instructions failed to generate the planned difference in expectations or these expectations failed to affect outcome. In either case, the presence of high or low expectation states in the research subjects is identified indirectly by the treatment outcome which expectation is said to produce. Subjects were known to have high expectations since they improved, and they improved since they had high expectations. In order for expectations to be validly studied, they must be measured independently of the outcome they are purported to influence.
Unfortunately, many expectancy scales were created for a single study and did not result in wider use. Among these is the Patient Prognostic Expectancy Inventory (PPEI), which comprises 15 items rated on a 4 point scale reflecting potential improvement in psychopathology and behavioral disturbances observed in psychiatric populations. The PPEI failed to predict the outcome of inpatient hospitalization for 150 patients with mostly psychotic disorders that were evaluated.46 The Expectancies for Change Inventory (ECI) asks patients to rate their expectation that treatment will eliminate their problem, improve their social and work adjustment, be better than letting the problem go untreated, promote new adaptive behaviors, make the patient worse, improve self-understanding, and lead to lasting change.47
A more widely used scale to measure patient expectations is the Credibility and Expectancy Scale (CES).48 Development of this scale began with Borkovec and Nau's (1972) method of measuring treatment credibility for treatments of anxiety, but it is easily modified to assess expectancy in treatments for other conditions, such as depression.49 The CES comprises two parts, the first containing 3 credibility questions (pertaining to logicalness, success in reducing symptoms, confidence in recommending therapy to a friend) and 1 expectation question (by the end of treatment, how much improvement in your symptoms do you think will occur?). In the second part, the participant is asked to imagine the treatment, identify how he feels about it, and then answer 1 credibility question (success in reducing symptoms) and the same expectation question. Psychometric study of the CES has demonstrated that it derives two factors (credibility and expectancy) that are stable across different populations.50 It has been shown to have high internal consistency, with a Cronbach's α of 0.79–0.90 for the expectancy factor, 0.81–0.86 for the credibility factor, and a standardized α of 0.84 for the CES composite score. Test-retest reliability over a one-week period was also found to be good at 0.82 for expectancy and 0.75 for credibility. Versions of the CES have been used to measure treatment credibility and patient expectation in several psychotherapy and pharmacotherapy studies.51
The CES was modified to develop the Reaction to Treatment Questionnaire (RTQ) by Holt, Heimberg, and colleagues (1990).52 The RTQ includes the 4 questions developed by Borkovec et al to assess treatment credibility, 9 questions asking patients to rate how confident they are that treatment will result in improvement in various areas, and 4 questions assessing the patient's current symptom severity and their expected severity at the end of treatment, 1 year later, and 5 years later. Items are scored on a scale from 1 to 10 (not at all to very confident) producing a total score with a possible range of 13–130. This scale has been used to measure expectations in a limited number of psychotherapy outcome studies but has not been subjected to psychometric studies documenting its reliability.
More recently, Westra and colleagues have developed a promising scale to measure patient expectations called the Anxiety Change Expectancy Scale (ACES).53 This scale differs from other measures of expectation by attempting to capture patients' specific expectations about their abilities to produce the desired change in addition to the change they expect will be brought about with treatment. The ACES was constructed by generating items from other existing scales, patients' verbalizations, and expert opinions, then winnowing these items based on expert ratings. It comprises 20 questions that are rated 1 to 5 on a Likert scale, resulting in total scores from 20 to 100. Examples of questions are “My problems with anxiety are too severe to benefit from treatment,” “There is no solution to my anxiety problems,” and “I believe it's quite possible for me to feel less worried and more relaxed.” In samples of normals, patients, and patients receiving treatment, the ACES has been shown to have excellent internal consistency and appears to tap a single dimension.54
Whatever scale is used, the timing of expectation assessment is a difficult problem. At the beginning of treatment, patients may be asked to rate their expectation of improvement after exposure to a small or unrepresentative portion of the treatment. Expectations vary considerably over the course of a treatment, and in the middle or near the end of treatment, expectation will likely be confounded with treatment efficacy.55 In general, it appears simplest to attempt to assess patients' expectations independently of the treatment outcome it is intended to improve, which would mean sampling at the beginning of a treatment.
Data linking patient expectancy and clinical outcome
Several studies of psychiatric patients show a positive correlation between patient outcome expectations and therapeutic improvement, but most of the available data come from patients with diagnoses other than depression.56 One review found significant associations between patient expectation and outcome in a Scandinavian study of inpatients with borderline personality disorder,57 outpatients with panic and agoraphobia,58 two community mental health samples,59,60 and two inpatient populations with mixed diagnoses.61,62 More recently, two studies of cognitive-behavioral group therapy for anxiety disorders reported that positive outcome expectations predicted greater and longer lasting symptomatic improvements, even after controlling for pre-treatment symptom severity and comorbid depression.63,64
Two studies of expectancy and clinical outcome are available in patients with MDD. First, the National Institute of Mental Health's Treatment of Depression Collaborative Research Program (TDCRP) found that higher patient expectation of improvement predicted greater likelihood of depression response and lower final depression scores in all four treatment conditions (cognitive behavior therapy, interpersonal therapy, imipramine, and placebo-clinical management).65 Among the 156 subjects completing a trial of psychotherapy or medication, 48% with expectation scores above the median exhibited a complete response to treatment compared to 33% with scores below the median. Subjects' expectancy was significantly correlated with their final depressive symptoms score (composite Beck Depression Inventory (BDI)66 and Hamilton Rating Scale for Depression (HRSD)67 (r=0.22, p<0.01).68 Second, in a single-blind trial of reboxetine for 25 subjects with MDD, subjects with a higher pre-treatment expectation of medication effectiveness had a greater likelihood of response: 90% of patients with high expectations of improvement responded compared to 33% of patients with lower expectations (X2= 7.819, p<0.005).69
A substantial literature also exists to support the link between patient expectations of improvement and outcome in medical illness and treatment. While a complete review of these studies is beyond the scope of the present paper, published studies show that expectations predict outcome in pain,70,71,72 post-operative recovery,73 asthma,74 neurodegenerative diseases,75 and onset of HIV-related symptoms.76 Meta-analyses by Mondloch, Cole, and Frank (2001)77 and Di Blasi and colleagues (2001)78 also demonstrated links between pre-treatment expectations and outcome.
NEURAL MECHANISMS OF EXPECTANCY
Neuroimaging studies of expectancy
An important conceptual question regarding placebo and expectancy effects has been whether they actually affect patients' experience (i.e., depressive symptoms) or whether they predominantly affect patients' reports of their symptoms. In other words, does expectancy diminish the severity of depressive illness or simply predispose patients to say they are feeling better when in fact they remain just as depressed? Neuroimaging studies, predominantly positron emission tomography (PET) and functional Magnetic Resonance Imaging (fMRI), have provided compelling evidence in favor of the former interpretation by demonstrating that expectancy alters neural activity in brain areas associated with subjective experience.79
First, it has been shown in patients with Parkinson's Disease that expectation of clinical benefit from brain stimulation has a powerful effect on clinical symptoms. In one study, clinical benefit increased when patients were advised stimulation was on and clinical worsening resulted when patients were told stimulation was off.80 A separate PET study of patients with Parkinson's Disease found release of endogenous dopamine in the striatum when patients expected improvement in motor performance after administration of a placebo.81 Strikingly, the best predictor of neural and behavioral outcomes in this study was the patient's belief that the stimulator was on rather than the actual delivery of stimulation.
Second, in a study of the mechanisms of placebo analgesia using functional Magnetic Resonance Imaging (fMRI) techniques by Wager et al (2004),82 expectancy was manipulated by applying an inert cream to subjects' forearms and informing them it was either an analgesic or a control agent. Identical painful heat and shock stimuli were given on skin patches treated with both placebo analgesic and control creams. In each of two studies, placebo-induced pain reduction was demonstrated. In addition, the neural mechanisms of placebo analgesia were investigated using concurrent fMRI neuroimaging. Decreased responsiveness to painful stimulation was found in regions related to the affective experience of pain, while increased activity occurred in orbitofrontal (OFC), dorsolateral prefrontal (DLPFC), parietal, and pre-genual anterior cingulate cortices during the anticipation of pain. Wager and colleagues interpreted these results to mean that anticipatory placebo-induced activations reflected the expectation of pain relief, which was then transformed into a change in sensitivity to input and/or processing activity of pain centers.
In a follow up PET study, Wager et al (2007) found that placebo treatment had widespread effects on endogenous opioid activity in cortical and subcortical regions critical for the determination of affective value and context based control of pain.83 Specifically, placebo-induced increases in opioid activity specific to heat in OFC, right amygdala, and rACC suggested that placebo potentiates pain-related opioid release. Opioid activity in many of these regions was correlated with reported placebo analgesia.
These results were replicated and extended by Scott et al (2007), who studied the neural correlates of placebo analgesia based on the hypothesis that placebo-related expectancy modulates the activity of the NAC during reward anticipation.84 Healthy controls were informed they would receive either a novel analgesic agent or placebo, then underwent an fMRI study using the Monetary Incentive Delay task, which activates NAC during the anticipation of a reward. The investigators found that the anticipation of analgesia was positively correlated with pain reduction, and increased NAC activity on the MID task predicted greater placebo analgesic response. These results demonstrate a link between placebo response and reward processing, and they implicate the NAC as an underlying mechanism for placebo-associated expectations.
Demonstration of such neural placebo effects provide evidence that expectancy and placebo effects can combine with and modulate subjective experience. These studies also begin to provide converging evidence suggesting that the brain areas involved with generating and maintaining expectancies comprise PFC subregions, OFC, and rACC, which has been born out by subsequent investigation. For example, Petrovic et al (2005) studied the effects of expectation on 15 healthy subjects' anxiety response to viewing unpleasant visual images.85 First, subjects were shown unpleasant and neutral pictures and asked to rate the perceived unpleasantness of the unpleasant pictures under three conditions: (1) no drug, (2) following infusion of the benzodiazepine midazolam, and (3) following infusion of the benzodiazepine antagonist flumazenil. This procedure instilled the expectation of decreased and increased anxiety during the second and third blocks, respectively, so when the procedures were repeated with the subjects receiving saline infusions rather than active drugs, a strong placebo anxiolytic effect was observed. When brain activity was compared in the placebo and control conditions, increased activity was observed in brain areas associated with expectations in prior studies (rACC and OFC) and decreased activity in the networks relevant to the emotional response produced by unpleasant pictures.
Keltner et al (2006) examined the brain mechanisms underlying the modulating effect of pain expectation on pain perception in 27 healthy subjects.86 The investigators conditioned subjects to expect high temperature stimulations when presented with high pain visual cues and low temperature stimulations when presented with low pain visual cues. Subjects rated a given temperature more painful when they expected a painful stimulus, and high expectations of pain intensity were associated with increased activity in the cACC, cerebellum, and nCF. Conversely, low expectations of pain intensity resulted in decreased activity in peri-limbic regions associated with affective valuation processes (OFC, amygdala, ventral striatum).
Nitschke et al (2006) examined the mechanism by which expectations attenuate insula and amygdala activation to a highly aversive bitter taste in 43 healthy adults.87 Subjects were trained to expect highly aversive tastes following highly aversive cues, mildly aversive taste following mildly aversive cues, and neutral tastes following neutral cues. Presented with the combination of a mildly aversive cue and a highly aversive taste, subjects demonstrated increased activations in the rACC, OFC, and DLPFC to the misleading cues that predicted less aversive ratings of the taste and decreased activations in amygdala and insula activation to the highly aversive taste.
Given that these studies have been conducted largely in normal controls, it is unknown whether the results can be generalized to patients with depression. However, intriguing recent reports suggest that this may be the case. Bermpohl et al (2009) conducted a study in which depressed subjects and normal controls were shown affectively charged photographs preceded by an expectancy cue that informed subjects whether the picture to follow was emotionally salient or neutral.88 The investigators found that normal controls displayed the expected neural activation changes in medial and DLPFC during cued emotional picture perception, these modulatory effects were significantly attenuated in depressed patients. Pizzagalli and colleagues (2009) recently reported data from a study comparing neural activation on the MID in depressed patients vs. controls.89 They found that patients with depression had decreased striatal responses to anticipated monetary gains and decreased activation in the NAcc bilaterally to monetary rewards. These results are consistent with those of Scott et al (2007) described above.
Regulation of emotional experience by expectancy
Neuroimaging investigations of individuals' emotional self-regulation have provided additional information about the cognitive processes involved with expectancy as well as their neural substrates. The cognitive processes underlying treatment expectations likely involve appraisals of the significance or meaning of treatment to the patient. Appraisals facilitate an individual's emotional response by supplying meaning to perceptions and organizing cognitions and behavior appropriate to the situation.90 Personality traits such as optimism and pessimism as well as the presence of anxiety and mood disorders affect the probability, endurance, and consistency of appraisals about the world.91,92,93,94,95
The neural correlates of emotional appraisal systems have been studied by investigating how cognitive change affects emotional response. For example, Ochsner et al (2002) showed participants photographs that induced a negative emotional response and neutral photographs.96 Participants were trained to reinterpret (i.e., reappraise) the negative pictures so that they no longer elicited a negative emotion or else attend to the photographs without attempting to modify their emotional reaction. The investigators found that reappraisal reduced participant ratings of negative emotion, and it was associated with increased activation in DLPFC and ACC and decreased activation in brain areas associated with negative emotion (i.e., amygdala, insula, and OFC). Furthermore, increases in neural activation occurring during reappraisal were correlated with decreased behavioral ratings of negative emotion.
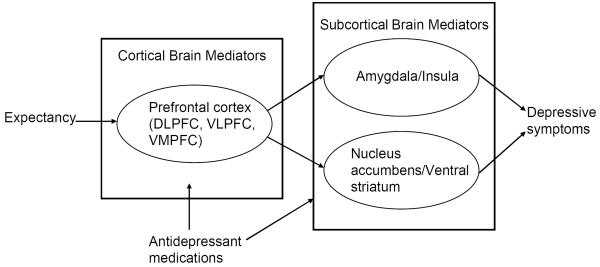
Such studies have now established the prefrontal cortex (PFC) as critical to the cognitive regulation of emotion, particularly the dorsolateral, ventrolateral, and ventromedial prefrontal cortices (DLPFC, VLPFC, and VMPFC).97 Together with other neuroimaging studies of expectancy, they provide the basis of a model for how expectancy might lead to changes in depressive symptoms (see Figure 1). PFC regions reciprocally connect with subcortical areas important for appraising the aversive or rewarding properties of stimuli, such as the amygdala, nucleus accumbens (NAcc), and insula.98,99 The amygdala has been implicated in detecting, attending to, and recording in memory affectively arousing and threatening stimuli,100,101 while the NAcc and ventral striatum (VS) have been shown to be important in reward learning and approach motivation in general.102,103 There is now evidence to suggest a PFC-amygdala pathway underlies a negative appraisal process leading to the generation of negative emotional responses to stimuli and a PFC-NAcc/VS pathway underlies a positive appraisal process involved with positive affective experience.104
Figure 1.
Model of expectancy effects in the treatment of depression.
Mechanisms of expectancy in Major Depression
For the most part, studies of the brain mechanisms of expectancy in psychiatry have been limited to demonstrations of objective differences in brain activity between responders and nonresponders to placebo antidepressants. Mayberg et al (2002) showed that placebo responses in a PET study of hospitalized patients with depression were associated with regional metabolic increases in cortical areas (prefrontal, anterior and posterior cingulated, posterior insula) and decreases in limbic and paralimbic areas (thalamus, parahippocampus, subgenual cingulate).105 Leuchter et al (2002) found that placebo responders in an antidepressant clinical trial had unique prefrontal changes on quantitative EEG compared to nonresponders and medication responders.106,107 These studies share the common problem that expectations of improvement were not measured, so it cannot be definitively determined that higher expectations in fact caused placebo response. However, these studies stand out as important initial data to be confirmed by later studies.
Our group is currently conducting one of the first studies of the neural mechanisms of expectancy in patients with depression. Subjects in a clinical trial who know they are receiving active medication as opposed to possibly receiving placebo undergo fMRI neuroimaging before and after antidepressant treatment. We hypothesize that higher expectancy of therapeutic improvement in subjects who know they are receiving active medication will be associated with greater pre- and post-treatment activation increases in higher level cortical appraisal systems, activation decreases in the amygdala, and activation increases in the NAcc.
We selected these brain regions of interest based on the above model of expectancy effects in the treatment of MDD. Pathological decreases in PFC function, increases in limbic activity, and disordered connectivity between these regions all have been observed in depression.108,109,110 Available evidence suggests that antidepressant medications may function by normalizing these pathological changes and increasing functional connectivity between PFC and subcortical regions.111 For example, decreased amygdala activation is observed with effective antidepressant treatment112 and predicts the extent of attenuation of negative affect following reappraisal.113 We predict that higher expectancy will be associated with greater therapeutic improvements in these brain areas, leading to improvement in depressive symptoms by reversing depressed patients' excessive sensitivity to negative environmental cues and reduced responsivity to positive cues.114,115
CONCLUSIONS AND IMPLICATIONS FOR FUTURE RESEARCH
In summary, patients in all clinical contexts have expectations about whether and how much they will improve based on the information made available to them at the beginning of treatment. These cognitions have a neurobiological basis and demonstrably modify patients' subjective experience and neural activation in response to a given stimulus or treatment. Expectancy appears to be a primary mechanism of the placebo effect and causes the majority of change observed in treatments for depression and other psychiatric disorders. Given these powerful effects, it is surprising that expectancy has not been a greater focus of study in the treatment of MDD. Ongoing and future studies of expectancy offer the potential to improve patient care, clarify the interpretation of clinical trials for depression, and elucidate the mechanisms of action of effective treatments for depression.
Modifying expectancy to affect clinical outcome
Available pharmacologic interventions for MDD are limited in efficacy, and while research on antidepressant compounds with novel mechanisms of action is ongoing, no new medications currently appear imminent. In this setting psychosocial interventions could be a valuable means of optimizing patient response to currently available medications and minimizing treatment attrition. Toward this end, developing a standardized means of increasing patient expectations so as to optimize clinical response to antidepressants would offer a valuable addition to clinical care. While the standard clinical management protocol for patients receiving antidepressant medication developed by Fawcett et al (1987) refers numerous times to enhancing patient expectations of improvement, little specific information about how to do that is offered.116 Research on patient expectations may lead to specific techniques to improve expectations on outcome, essentially resulting in an update of the pharmacotherapy clinical management manual.
Conversely, there are contexts in which it would be useful to minimize patient expectations. Placebo-controlled antidepressant medication studies “fail” approximately 50% of the time, meaning they do not demonstrate a statistically significant difference between drug and placebo. A portion of these failures may be caused by high placebo response rates, which make it more difficult to distinguish a signal of efficacy for new drugs. Research on expectancy effects in the treatment of depression has the potential to provide insights about the factors affecting their magnitude, which could then be manipulated in pharmacotherapy trials. As has been previously remarked, while high placebo response rates may be a challenge to researchers, they are potentially a boon to clinicians and their patients.117
Interpreting the results of clinical trials for MDD
Clinicians aim to practice Evidence Based Medicine (EBM) by selecting treatments based on research studies testing the proposed medication for depression.118 However, there are many studies to choose between when gathering evidence about the anticipated effectiveness of antidepressants, and differences in expectancies between studies or treatment arms being evaluated can bias comparisons. For example, in the Treatment for Adolescents with Depression Study (TADS), adolescents with Major Depression were randomized to CBT alone, fluoxetine alone, combined CBT and fluoxetine, and pill placebo.119 The benefit of this design is that the study can be “internally calibrated,” meaning differences between the psychotherapy and medication conditions can be more easily interpreted when it is known whether or not the medication treatment condition was effective compared to placebo. However, the study compares blinded treatments (fluoxetine or pill placebo) to unblinded treatments (CBT and combined CBT and fluoxetine). In other words, patients in the CBT alone condition knew they were receiving psychotherapy (active treatment), while patients taking pills did not know whether they were fluoxetine (active) or placebo (inactive). Similarly, patients in the combined cell knew they were receiving two active treatments rather than one (CBT alone) or possibly none (fluoxetine and pill placebo).
Since antidepressant response and remission rates are lower when patients do not know they are receiving active treatment (i.e., placebo-controlled trials) versus when they know they are receiving medication without knowing the exact agent (i.e., comparator trials), clinical trials comparing open to blinded treatments are significantly biased against the blinded treatment (i.e., placebo-controlled medication). This illustrates the fact that every trial is designed to answer some questions but not others, since no single trial can validly answer all of the questions clinicians might have. The point made here is that non-specific treatment factors such as patient expectancy must be taken into account when evaluating research studies, just as one might check other design features, such as randomization, blinding, and the validity of statistical analyses.
Understanding mechanisms of treatments for depression
Finally, gaining insight into the cognitive and neural mechanisms of expectancy will be helpful in determining mechanisms of psychopharmacologic and psychotherapeutic action in patients with MDD. Neuroimaging studies have begun to elucidate the brain regions contributing to general symptomatic improvement in depression and those contributing to specific treatment mechanisms.120,121,122,123 Goldapple et al (2004) found that depressed patients treated with cognitive behavior therapy showed some common and some different changes in brain metabolic activity compared to patients treated with paroxetine.124 Similarly, determining the neural correlates of expectancy would provide information about the brain changes associated with successful depression improvement. It may also help distinguish placebo responders from patients who improve due to medication or psychotherapy, since current evidence suggests there are differences in brain activity between responders and nonresponders to placebo in antidepressant clinical trials.125,126,127 Continuing neuroimaging investigation is aimed at evaluating the mechanisms of depression pathogenesis, following the success of therapeutic interventions for depression, and predicting outcome before treatment.128 One problem with these studies is that they ascribe the brain changes observed during treatment with medications or psychotherapy to those specific treatments, but this is not the case. It is critical to differentiate the brain changes associated with treatment specific factors and those due to expectancy, which will not be possible until the neural mechanisms of expectancy are defined.
References
- 1.The World Health Organization . The World Health Report 2004: Changing History, Annex Table 3: Burden of disease in DALYs by cause, sex, and mortality stratum in WHO regions, estimates for 2002. WHO; Geneva: 2004. [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steos: A STAR*D Report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 5.Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- 6.Jiang W, Krishnan RR, O'Connor CM. Depression and heart disease: evidence of a link, and its therapeutic implications. CNS Drugs. 2002;16:111–127. doi: 10.2165/00023210-200216020-00004. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80:135–144. doi: 10.1016/S0165-0327(03)00054-5. [DOI] [PubMed] [Google Scholar]
- 8.Kirsch I, Sapirstein G. Listening to prozac but hearing placebo: A meta-analysis of antidepressant medication. Prev Treat. 1998 posted at http://journals.apa.org/prevention/volumeI/pre0010002a.html.
- 9.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo Response in Studies of Major Depression: Variable, Substantial, and Growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 10.Haour F. Mechanisms of the Placebo Effect and of Conditioning. Neuroimmunomodulation. 2005;12:195–200. doi: 10.1159/000085651. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch I. Specifying Nonspecifics: Psychological Mechanisms of Placebo Effects. In: Harrington A, editor. The placebo effect: An interdisciplinary exploration. Harvard University Press; Cambridge, MA: 1997. [Google Scholar]
- 12.Guess HA, Kleinman A, Kusek JW, Engel LW, editors. The Science of the Placebo: Toward an Interdisciplinary Research Agenda. BMJ Books; London: 2002. [Google Scholar]
- 13.Goldstein A. Therapist and patient expectancies in psychotherapy. Macmillan; New York: 1962. [Google Scholar]
- 14.Noble LM, Douglas BC, Newman SP. What do patients expect of psychiatric services? A systematic and critical review of empirical studies. Soc Sci Med. 2001;52:985–998. doi: 10.1016/s0277-9536(00)00210-0. [DOI] [PubMed] [Google Scholar]
- 15.Horvath P. Treatment Expectancy as a Function of the Amount of Information Presented in Therapeutic Rationales. J Clin Psychology. 1990;46:636–642. doi: 10.1002/1097-4679(199009)46:5<636::aid-jclp2270460516>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Jacob T. Experimenter Bias Effect as a Function of Demand Characteristics and Experimenter Investment. Psych Reports. 1971;28:1003–1010. [Google Scholar]
- 17.Shames ML, Adair JG. Effect of Experimenter's Expectancy in Relation to Type and Structure of the Experimental Task. Psych Reports. 1980;46:487–497. [Google Scholar]
- 18.Glick BS, Margolis R. A Study of the Influence of Experimental Design on Clinical Outcome in Drug Research. J Consult Clin Psychol. 1962:1087–1096. [Google Scholar]
- 19.Jacobson NE, Hollon SD. Cognitive-behavior therapy vs. pharmacotherapy: Now that the jury has returned its verdict, it's time to present the rest of the evidence. J Consult Clin Psychol. 1996;64:74–80. doi: 10.1037//0022-006x.64.1.74. [DOI] [PubMed] [Google Scholar]
- 20.Greist JH, Mundt JC, Kobak K. Factors Contributing to Failed Trials of New Agents: Can Technology Prevent Some Problems? J Clin Psychiatry. 2002;63(Suppl 2):8–13. [PubMed] [Google Scholar]
- 21.Eysenck HJ. The effects of psychotherapy: An evaluation. J Consult Psychology. 1952;16:319–324. doi: 10.1037/h0063633. [DOI] [PubMed] [Google Scholar]
- 22.Eysenck HJ. The effects of psychotheraoy. In: Eysenck HJ, editor. Handbook of abnormal psychology: An experimental approach. Basic Books; New York: 1961. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein AP. Participant Expectancies in Psychotherapy. Psychiatry. 1962;25:72–79. doi: 10.1080/00332747.1962.11023298. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal D, Frank JD. Psychotherapy and the Placebo Effect. Psych Bull. 1956;53:294–302. doi: 10.1037/h0044068. [DOI] [PubMed] [Google Scholar]
- 25.Swartzman LC, Burkell J. Expectations and the placebo effect in clinical drug trials: Why we should not turn a blind eye to unblinding, and other cautionary notes. Clin Pharm Ther. 1998;64:1–7. doi: 10.1016/S0009-9236(98)90016-9. [DOI] [PubMed] [Google Scholar]
- 26.Myers MG, Cairns JA, Singer J. The consent form as a possible cause of side effects. Clin Pharmacol Ther. 1987;42:250–253. doi: 10.1038/clpt.1987.142. [DOI] [PubMed] [Google Scholar]
- 27.Kaptchuk TJ. The double-blind, randomized, placebo-controlled trial: Gold standard or golden calf? J Clin Epi. 2001;54:541–549. doi: 10.1016/s0895-4356(00)00347-4. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann JF, Chassany O, Gandiol J, et al. A randomized clinical trial of the effect of informed consent on the analgesic activity of placebo and naproxen in cancer pain. Clin Trials Meta-Anal. 1994;29:41–47. [PubMed] [Google Scholar]
- 29.Kirsch I, Weixel LJ. Double-blind versus deceptive administration of a placebo. Behav Neurosci. 1988;2:319–323. doi: 10.1037//0735-7044.102.2.319. [DOI] [PubMed] [Google Scholar]
- 30.Khan A, Kolts RL, Thase ME, Krishnan RR, Brown W. Research Design Features and Patient Characteristics Associated with the Outcome of Antidepressant Clinical Trials. Am J Psychiatry. 2004;161:2045–2049. doi: 10.1176/appi.ajp.161.11.2045. [DOI] [PubMed] [Google Scholar]
- 31.Kim SY, Halloway RG. Burdens and Benefits of Placebos in Antidepressant Clinical Trials: A Decision and Cost-Effectiveness Analysis. Am J Psychiatry. 2003;160:1272–1276. doi: 10.1176/appi.ajp.160.7.1272. [DOI] [PubMed] [Google Scholar]
- 32.Roose SP, Schatzberg AF. The Efficacy of Antidepressants in the Treatment of Late-Life Depression. J Clin Psychopharm. 2005;25(Suppl 1):S1–7. doi: 10.1097/01.jcp.0000162807.84570.6b. [DOI] [PubMed] [Google Scholar]
- 33.Rutherford BR, Sneed JR, Roose SP. Does Study Design Affect Outcome? The Effects of Placebo Control and Treatment Duration in Antidepressant Trials. Psychother Psychosom. 2009;78:172–181. doi: 10.1159/000209348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sneed JR, Rutherford BR, Rindskopf D, Roose SP. Design Makes a Difference: Antidepressant Response Rates in Placebo-controlled versus Comparator Trials in Late Life Depression. Am J Geri Psychiatry. 2008;16:65–73. doi: 10.1097/JGP.0b013e3181256b1d. [DOI] [PubMed] [Google Scholar]
- 35.Rubin DB. Statistics and Causal Inference: Comment: Which Ifs Have Answers. J Am Stat Assoc. 1986;81:961–962. [Google Scholar]
- 36.Rohsenow DJ, Marlatt GA. The Balanced Placebo Design: Methodological Considerations. Addict Behav. 1981;6:107–122. doi: 10.1016/0306-4603(81)90003-4. [DOI] [PubMed] [Google Scholar]
- 37.Knight LJ, Barbaree HE, Boland FJ. Alcohol and the Balanced-Placebo Design: The Role of Experimenter Demands in Expectancy. J Abnormal Psych. 1986;4:335–340. doi: 10.1037//0021-843x.95.4.335. [DOI] [PubMed] [Google Scholar]
- 38.Kidorf M, Sherman MF, Johnson AG. Alcohol expectancies and changes in beer consumption of first-year college students. Addict Behav. 1995;22:501–515. doi: 10.1016/0306-4603(94)00067-0. [DOI] [PubMed] [Google Scholar]
- 39.Lotshaw SC, Bradley JR, Brooks LR. Illustrating caffeine's pharmacological and expectancy effects utilizing a balanced placebo design. J Drug Educ. 1996;26:13–24. doi: 10.2190/UUCL-E5V6-XC25-5MC6. [DOI] [PubMed] [Google Scholar]
- 40.Uhlenhuth EH, Rickels K, Fisher S, et al. Drug, Doctor's Verbal Attitude and Clinic Setting in the Symptomatic Response to Pharmacotherapy. Psychopharmacologia. 1967;9:392–418. doi: 10.1007/BF00406450. [DOI] [PubMed] [Google Scholar]
- 41.Kemeny ME, Rosenwasser LJ, Panettieri RA, et al. Placebo Response in Asthma: A Robust and Objective Phenomenon. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Benedetti F, et al. Open versus hidden medical treatments: the patient's knowledge about a therapy affects the therapy outcome. Prev. Treatment. 2003:6. [online] < http://journals.apa.org/prevention/volume6/toc-jun-03.html>.
- 43.Rutherford BR, Sneed JS, Eisenstadt R, Roose SP. Psychological Medicine. Antidepressant Study Design Affects Patient Expectations: Initial Results from a Randomized Controlled Trial. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tinsley HEA, Bowman SL, Ray SB. Manipulation of expectancies about counseling and psychotherapy: Review and analysis of expectancy manipulation strategies and results. J Counseling Psychol. 1988;35:99–108. [Google Scholar]
- 45.Wilkins W. Expectancy of Therapeutic Gain: An Empirical and Conceptual Critique. J Consult Clin Psychol. 1973;40:69–77. doi: 10.1037/h0034056. [DOI] [PubMed] [Google Scholar]
- 46.Martin PJ, Sterne AL. Prognostic Expectations and Treatment Outcome. J Consult Clin Psychol. 1975;43:572–576. doi: 10.1037/h0076886. [DOI] [PubMed] [Google Scholar]
- 47.Kazdin AE, Krouse R. The Impact of Variations in Treatment Rationales on Expectancies for Therapeutic Change. Behav Ther. 1983;14:657–671. [Google Scholar]
- 48.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiat. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 49.Borkovec TD, Nau SD. Credibility of Analogue Therapy Rationales. J Behav Ther Exp Psychiat. 1972;3:257–260. [Google Scholar]
- 50.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiat. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 51.Borkovec TD, Costello E. Efficacy of Applied Relaxation and Cognitive-Behavioral Therapy in the Treatment of Generalized Anxiety Disorder. J Consult Clin Psychol. 1993;61:611–619. doi: 10.1037//0022-006x.61.4.611. [DOI] [PubMed] [Google Scholar]
- 52.Holt CS, Heimberg RG. The Reaction to Treatment Questionnaire: Measuring Treatment Credibility and Outcome Expectancies. Behav Ther. 1990;13:213–215. [Google Scholar]
- 53.Dozois DJA, Westra HA. Development of the Anxiety Change Expectancy Scales (ACES) and validation in college, community, and clinical sample. Behav Res Ther. 2005;43:1655–1672. doi: 10.1016/j.brat.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Dozois DJA, Westra HA. Development of the Anxiety Change Expectancy Scales (ACES) and validation in college, community, and clinical sample. Behav Res Ther. 2005;43:1655–1672. doi: 10.1016/j.brat.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Stone DA, Kerr CE, Jacobson E, et al. Patient expectations in placebo-controlled randomized clinical trials. J Eval Clin Pract. 2005;11:77–84. doi: 10.1111/j.1365-2753.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 56.Noble LM, Douglas BC, Newman SP. What do patients expect of psychiatric services? A systematic and critical review of empirical studies. Soc Sci Med. 2001;52:985–998. doi: 10.1016/s0277-9536(00)00210-0. [DOI] [PubMed] [Google Scholar]
- 57.Antikainen R, Kponen HJ, Lehtonen J, et al. Factors predicting outcome of psychiatric hospital treatment in patients with borderline personality organization. Nordic J Psychiatry. 1994;48:177–185. [Google Scholar]
- 58.Basoglu M, Marks IM, Swinson Rp, et al. Pre-treatment predictors of treatment outcome in panic disorder and agoraphobia treated with alprazolam and exposure. J Affect Disord. 1994;30:123–132. doi: 10.1016/0165-0327(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 59.Bowden CL, Schoenfield LS, Adams RL. Mental health attitudes and treatment expectations as treatment variables. J Clin Psychology. 1980;36:653–657. doi: 10.1002/1097-4679(198007)36:3<653::aid-jclp2270360307>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 60.Greer FL. Prognostic expectations and outcome of brief therapy. Psychol Rep. 1980;46:973–974. doi: 10.2466/pr0.1980.46.3.973. [DOI] [PubMed] [Google Scholar]
- 61.Collins J, Hyer L. Treatment expectancy among psychiatric inpatients. J Clin Psychology. 1986;42:562–569. doi: 10.1002/1097-4679(198607)42:4<562::aid-jclp2270420404>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 62.Hansson L, Berglund M. Factors influencing treatment outcome and patient satisfaction in a short-term psychiatric ward: A path analysis study of the importance of patient involvement in treatment planning. Eur Arch Psych Neurol Sci. 1987;236:269–275. doi: 10.1007/BF00380951. [DOI] [PubMed] [Google Scholar]
- 63.Chambless HL, Tran GQ, Glass CR. Predictors of Response to Cognitive-Behavioral Group Therapy for Social Phobia. J Anxiety Disorders. 1997;11:221–240. doi: 10.1016/s0887-6185(97)00008-x. [DOI] [PubMed] [Google Scholar]
- 64.Safren SA, Heimberg RG, Juster HR. Clients' Expectancies and Their Relationship to Pretreatment Symptomatology and Outcome of Cognitive-Behavioral Group Treatment for Social Phobia. J Consult Clin Psychol. 1997;65:694–698. doi: 10.1037//0022-006x.65.4.694. [DOI] [PubMed] [Google Scholar]
- 65.Sotsky AM, Glass DR, Shea MT, et al. Patient Predictors of Response to Psychotherapy and Pharmacotherapy: Findings in the NIMH Treatment of Depression Collaborative Research Program. Am J Psychiatry. 1991;148:997–1008. doi: 10.1176/ajp.148.8.997. [DOI] [PubMed] [Google Scholar]
- 66.Beck AT, Ward CH, Mendelson M, et al. An inventory of measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 67.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer B, Pilkonis PA, Krupnick JL, et al. Treatment Expectancies, Patient Alliance, and Outcome: Further Analyses from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 2002;70:1051–1055. [PubMed] [Google Scholar]
- 69.Krell HV, Leuchter AF, Morgan M, et al. Subject Expectations of Treatment Effectiveness and Outcome of Treatment with an Experimental Antidepressant. J Clin Psychiatry. 2004;65:1174–1179. doi: 10.4088/jcp.v65n0904. [DOI] [PubMed] [Google Scholar]
- 70.Branthwaite A, Cooper P. Analgesic effects of branding in treatment of headaches. Br Med J. 1981;282:1576–1578. doi: 10.1136/bmj.282.6276.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barrios FX, Karoly P. Treatment Expectancy and Therapeutic Change in Treatment of Migraine Headache: Are They Related? Psychol Rep. 1983;52:59–68. doi: 10.2466/pr0.1983.52.1.59. [DOI] [PubMed] [Google Scholar]
- 72.Turner JA, Jensen MP, Warms CA, et al. Blinding effectiveness and association of pretreatment expectations with pain improvement in a double-blind randomized controlled trial. Pain. 2002;99:91–99. doi: 10.1016/s0304-3959(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 73.Flood AB, Lorence DP, Ding J, et al. The Role of Expectations in Patients' Reports of Post-Operative Outcomes and Improvement Following Therapy. Med Care. 1993;31:1043–1046. doi: 10.1097/00005650-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Luparello TJ, Leist N, Lourie CH, et al. The Interaction of Psychologic Stimuli and Pharmacologic Agents on Airway Reactivity in Asthmatic Subjects. Psychosom Med. 1970;32:509–513. doi: 10.1097/00006842-197009000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Pollo A, Torre E, Lopiano L, et al. Expectation modulates the response to subthalamic nucleus stimulation in Parkinsonian patients. NeuroReport. 2002;13:1383–1386. doi: 10.1097/00001756-200208070-00006. [DOI] [PubMed] [Google Scholar]
- 76.Reed GM, Kemeny ME, Taylor SE, et al. Negative HIV-Specific Expectancies and AIDS-Related Bereavement as Predictors of Symptom Onset in Asymptomatic HIV-Positive Gay Men. Health Psychol. 1999;18:354–363. doi: 10.1037//0278-6133.18.4.354. [DOI] [PubMed] [Google Scholar]
- 77.Mondloch MV, Cole DC, Frank JW. Does how you do depend on how you think you'll do? A systematic review of the evidence for a relation between patients' recovery expectations and health outcomes. Can Med Assoc J. 2001;165:174–179. [PMC free article] [PubMed] [Google Scholar]
- 78.Di Blasi Z, Harkness E, Ernst E, et al. Influence of context effects on health outcomes: a systematic review. Lancet. 2001;357:757–762. doi: 10.1016/s0140-6736(00)04169-6. [DOI] [PubMed] [Google Scholar]
- 79.Smith EE. Expectancy and the Perception of Adverse Events [Google Scholar]
- 80.Mercado R, Constantoyannis C, Mandat T, Kumar A, Schulzer M, Stoessl A, Honey CR. Expectation and the Placebo Effect in Parkinson's Disease Patients with Subthalamic Nucleus Deep Brain Stimulation. Movement Disord. 2006;21:1457–1461. doi: 10.1002/mds.20935. [DOI] [PubMed] [Google Scholar]
- 81.Pollo A, Torre E, Lopiano L, Rizzone M, Lanotte M, Cavanna A, Bergamasco B, Benedetti F. Expectation modulates the response to subthalamic nucleus stimulation in Parkinsonian patients. NeuroReport. 2002;13:1383–1386. doi: 10.1097/00001756-200208070-00006. [DOI] [PubMed] [Google Scholar]
- 82.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in fmri in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 83.Wager T, Scott DJ, Zubieta J. Placebo effects on human μ-opioid activity during pain. PNAS. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual Differences in Reward Responding Explain Placebo-Induced Expectations and Effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 85.Petrovic P, Dietrich T, Fransson P, et al. Placebo in Emotional Processing—Induced Expectations of Anxiety Relief Activate a Generalized Modulatory Network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 86.Keltner JR, Furst A, Fan C, et al. Isolating the Modulatory Effect of Expectation on Pain Transmission: A Functional Magnetic Resonance Imaging Study. J Neuroscience. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, Smith EE. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nature Neuroscience. 2006;9:435–442. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- 88.Bermpohl F, Walter M, Sajonz B, Lucke C, Hagele C, Sterzer P, Adli M, Heinz A, Northoff G. Attentional modulation of emotional stimuls processing in patients with major depression—alteration in prefrontal cortical regions. Neuroscience Letters. doi: 10.1016/j.neulet.2009.07.061. doi:10.1016/j.neulet.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 89.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals with Major Depressive Disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roseman IJ, Smith CA. Appraisal theory: Overview, assumptions, varieties, controversies. In: Scherer KR, Schorr A, Johnstone T, editors. Appraisal processes in emotion: Theory, methods, research. Oxford University Press; New York: 2001. pp. 3–19. [Google Scholar]
- 91.Jerusalem M. Personal resources, environmental constraints, and adaptational processes: The predictive power of a theoretical stress model. Personality and Individual Differences. 1993;14:15–24. [Google Scholar]
- 92.Clark DM, Teasdale JD. Diurnal variation in clinical depression and accessibility of memories of positive and negative experiences. Journal of Abnormal Psychology. 1982;91:87–95. doi: 10.1037//0021-843x.91.2.87. [DOI] [PubMed] [Google Scholar]
- 93.Beck AT, Brown G, Steer RA, Eidelson JI, Riskind JH. Differentiating anxiety and depression: A test of the cognitive content specificity hypothesis. Journal of Abnormal Psychology. 1987;96:179–183. doi: 10.1037//0021-843x.96.3.179. [DOI] [PubMed] [Google Scholar]
- 94.Carver CS, Scheier MF. Origins and functions of positive and negative affect: A control-process view. Psychological Review. 1990;97:19–35. [Google Scholar]
- 95.Kuiper NA, Martin RA. Type A behavior: A social cognition motivational perspective. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 24. Academic Press; New York: 1989. pp. 311–341. [Google Scholar]
- 96.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J Cog Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 97.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cog Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 98.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 99.O'Doherty JP, et al. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 100.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 101.Holland PC, Gallagher M. Amygdala circuitry in attentiona land representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 102.Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- 103.Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics, and behavioral ecology. Curr Opin Neurobiol. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 104.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-Subcortical Pathways Mediating Successful Emotion Regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 106.Leuchter AF, Cook IA, Witte EA, et al. Changes in Brain Function of Depressed Subjects during Treatment with Placebo. Am J Psychiatry. 2002;159:122–129. doi: 10.1176/appi.ajp.159.1.122. [DOI] [PubMed] [Google Scholar]
- 107.Hunter AM, Leuchter AF, Morgan ML, et al. Changes in Brain Function (Quantitative EEG Cordance) during Placebo Lead-In and Treatment Outcomes in Clinical Trials for Major Depression. Am J Psychiatry. 2006;163:1426–1432. doi: 10.1176/ajp.2006.163.8.1426. [DOI] [PubMed] [Google Scholar]
- 108.Beck AT. The Evolution of the Cognitive Model of Depression and Its Neurobiological Correlates. AM J Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 109.Johnstone T, van Reekum CM, Urry HL, et al. Failure to regulate: counterproductive recruitment of top-down, prefrontal-subcortical circuitry in major depression. J Neurosc. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siegle GJ, Thompson W, Carter CS, et al. Increased amygdala and decreased dorsolateral prefrontal bold responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 111.Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an fMRI study. Neuropsychopharmacology. 2005;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- 112.Fu CHY, Williams SCR, Cleare AJ, et al. Attenuation of the Neural Response to Sad Faces in Major Depression by Antidepressant Treatment. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 113.Banks SJ, Eddy KT, Angstadt M, et al. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosc. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chiu PH, Deldin PJ. Neural Evidence for Enhanced Error Detection in Major Depressive Disorder. Am J Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- 115.Vallance AK. A systematic review comparing the functional neuroanatomy of patients with depression who respond to placebo to those who recover spontaneously: Is there a biological basis for the placebo effect in depression? J Affect Disord. 2007;98:177–185. doi: 10.1016/j.jad.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 116.Fawcett J, Epstein P, Fiester SJ, et al. Clinical management: imipramine/placebo administration manual. NIMH Treatment of Depression Collaborative Research Program. Psychopharmacol Bull. 1987;23:309–324. [PubMed] [Google Scholar]
- 117.Andrews G. Placebo response in depression: bane of research, boon to therapy. Br J Psychiatry. 2001;178:192–194. doi: 10.1192/bjp.178.3.192. [DOI] [PubMed] [Google Scholar]
- 118.Sackett DL, Richardson WS, Rosenberg W, et al. Evidence-based medicine: how to practice and teach EBM. Churchill-Livingstone; London: 1997. [Google Scholar]
- 119.Treatment for Adolescents with Depression Study (TADS) Team Fluoxetine, Cognitive-Behavioral Therapy, and Their Combination for Adolescents with Depression: Treatment for Adolescents with Depression Study (TADS) Randomized Controlled Trial. JAMA. 2004;292(7):807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 120.Mayberg HS, Brannan SK, Tekell JL, et al. Regional Metabolic Effects of Fluoxetine in Major Depression: Serial Changes and Relationship to Clinical Response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 121.Brody AL, Saxena S, Stoessel P, et al. Regional Brain Metabolic Changes in Patients with Major Depression Treated with Either Paroxetine or Interpersonal Therapy: Preliminary Findings. Arch Gen Psychiatry. 2001;58:631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 122.Brody Al, Saxena S, Silverman DHS, et al. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psych Res Neuroimaging. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 123.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: coverging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 124.Goldapple K, Segal Z, Garson C, et al. Modulation of Cortical-Limbic Pathways in Major Depression: Treatment-Specific Effects of Cognitive Behavior Therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 125.Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 126.Leuchter AF, Cook IA, Witte EA, et al. Changes in Brain Function of Depressed Subjects during Treatment with Placebo. Am J Psychiatry. 2002;159:122–129. doi: 10.1176/appi.ajp.159.1.122. [DOI] [PubMed] [Google Scholar]
- 127.Hunter AM, Leuchter AF, Morgan ML, et al. Changes in Brain Function (Quantitative EEG Cordance) during Placebo Lead-In and Treatment Outcomes in Clinical Trials for Major Depression. Am J Psychiatry. 2006;163:1426–1432. doi: 10.1176/ajp.2006.163.8.1426. [DOI] [PubMed] [Google Scholar]
- 128.Etkin A, Pittenger C, Polan HJ, et al. Toward a Neurobiology of Psychotherapy: Basic Science and Clinical Applications. J Neuropsychiatry Clin Neurosci. 2005;17:145–158. doi: 10.1176/jnp.17.2.145. [DOI] [PubMed] [Google Scholar]