Abstract
A common feature of many neurodegenerative diseases is the deposition of β-sheet-rich amyloid aggregates formed by proteins specific to these diseases. These protein aggregates are thought to cause neuronal dysfunction, directly or indirectly. Recent studies have strongly implicated cell-to-cell transmission of misfolded proteins as a common mechanism for the onset and progression of various neurodegenerative disorders. Emerging evidence also suggests the presence of conformationally diverse ‘strains’ of each type of disease protein, which may be another shared feature of amyloid aggregates, accounting for the tremendous heterogeneity within each type of neurodegenerative disease. Although there are many more questions to be answered, these studies have opened up new avenues for therapeutic interventions in neurodegenerative disorders.
Neurodegenerative diseases encompass a wide variety of age-related pathological conditions caused by progressive dysfunction and deterioration of the central nervous system (CNS). Despite their enormous diversity in clinical phenotypes, most neurodegenerative diseases share a common feature, which is the accumulation of disease- specific proteins into insoluble aggregates. This list includes β-amyloid (Aβ) in senile plaques and tau in neurofibrillary tangles (NFTs) of Alzheimer’s disease1,2, α-synuclein (α-syn) in Lewy bodies and Lewy neurites of Parkinson’s disease3, TDP-43 aggregates in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration4, polyglutamine (polyQ)-rich huntingtin inclusions in Huntington’s disease5 and prion plaques in Creutzfeldt-Jakob disease (CJD)6. When viewed by electron microscopy, most of these protein aggregates consist of 8- to 20-nm wide filaments and are characterized by enriched β-pleated sheet structures (‘amyloid’) that can be stained by dyes such as Congo Red or thioflavin S (ThS)7,8, with the exception of TDP-43 inclusions, in which the aggregates comprise mostly granular non-amyloid fibrils9,10.
In the past few years, a growing number of studies have provided converging evidence for the cell-to-cell transmissibility of the diverse disease proteins that form the hallmark lesions of these neurodegenerative disorders (Table 1). Such lesions were traditionally thought to develop in a cell-autonomous manner in selectively vulnerable brain regions. The newly evolved ‘transmission hypothesis’ for non-prion neurodegenerative diseases not only provides a viable explanation for the stereotypical pathology spreading patterns that have long been observed in multiple diseases, but also offers a fresh perspective on the processes underlying the onset and progression of CNS amyloidosis11–13. In this review, we compare recent findings on the transmission of different amyloidogenic proteins, speculate on how intercellular spreading of misfolded proteins may be related to the pathogenesis of neurodegenerative diseases, present evidence for the existence of conformationally diverse pathological strains to account for the divergence and convergence of various diseases and, finally, discuss the therapeutic implications of these findings.
Table 1.
Summary of studies demonstrating the transmissibility of non-prion protein aggregates
| Protein | Type of seed | Seeded aggregation in different model systems
|
||
|---|---|---|---|---|
| Non-neuronal cells | Neuronal cells | Mice | ||
| Aβ | Synthetic fibrils | Not tested | Not tested | Yes22 |
| Mouse brain lysates | Not tested | Not tested | Yes22,41,72 | |
| Human brain lysates | Not tested | Not tested | Yes41 | |
|
| ||||
| Tau | Synthetic fibrils | Yes17,58 | Yes62,133 | Yes19 |
| Mouse brain lysates | Not tested | Not tested | Yes18 | |
| Human brain lysates | Not tested | Not tested | Yes29,42 | |
|
| ||||
| α-Syn | Synthetic fibrils | Yes134 | Yes15,133 | Yes16,20,21 |
| Mouse brain lysates | Not tested | Not tested | Yes20 | |
| Human brain lysates | Not tested | Not tested | Yes21 | |
|
| ||||
| TDP-43 | Synthetic fibrils | Yes135 | Not tested | Not tested |
| Human brain lysates | Not tested | Yes136 | Not tested | |
|
| ||||
| SOD1 | Synthetic fibrils | Not tested | Yes57 | Not tested |
|
| ||||
| PolyQ | Synthetic fibrils | Yes56 | Not tested | Not tested |
Cell-to-cell transmission of amyloid protein aggregates
Templated recruitment by existing seeds
For many years, prion diseases were thought to be a unique group of neurodegenerative disorders in which the conformationally altered prion protein PrPSc constitutes the infectious agent that corrupts normal cellular PrP (PrPC) through ‘seeded’ fibrillization14. Recently, a collection of studies has provided convincing evidence that a ‘prion-like’ self-propagating mechanism may be applicable to a wide range of disease-associated proteins, including Aβ, tau, α-syn, huntingtin with polyQ repeats, superoxide dismutase 1 (SOD1) and TDP-43 (Table 1 and Fig. 1). For each of these proteins, aggregate-containing lysates and/or synthetic fibrils assembled from recombinant proteins were shown to act as templates or seeds that could efficiently recruit their soluble counterparts into elongating fibrils in cultured cells and/or living animals, sometimes even without overexpression of the protein of interest15,16. A miniscule amount of preformed fibrils was shown to be sufficient to initiate robust conversion of normal proteins, suggesting that templated recruitment is probably a self-perpetuating process that, once started, will progress relentlessly17. Moreover, in vivo administration of inoculums containing aggregated proteins not only led to induction of pathology near the inoculation site(s) but also invariably resulted in time-dependent spreading of pathology to synaptically connected distant brain regions16,18–22. For tau and α-syn, a trans-synaptic spreading pattern was also observed when the trans-gene expression was restricted to specific brain regions23–25. Taken together, these studies strongly support templated self-propagation and intercellular transmissibility as shared properties of protein aggregates involved in CNS amyloidosis.
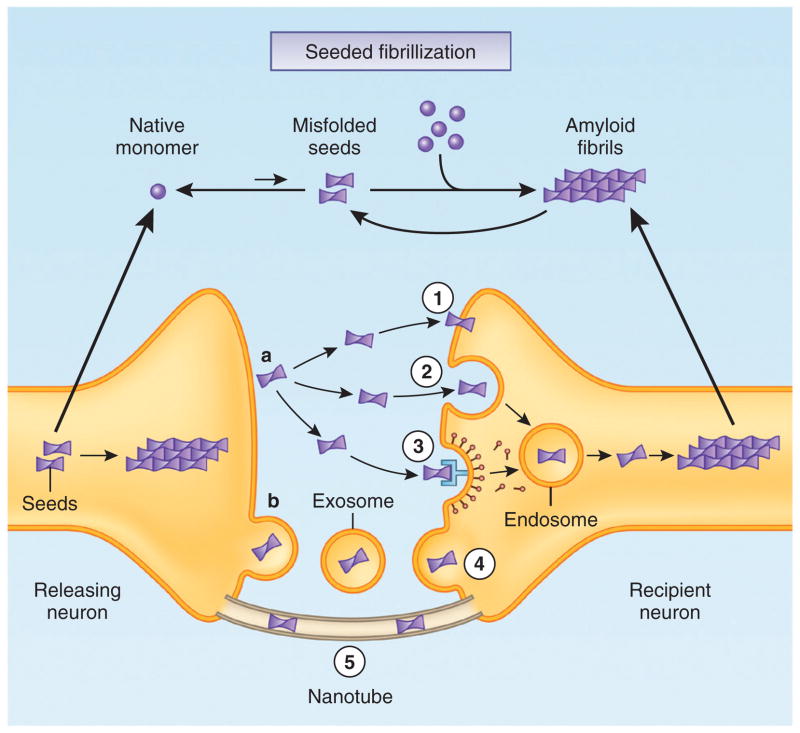
Figure 1.
Potential mechanisms mediating cell-to-cell transmission of cytosolic protein aggregates. (a, b) Misfolded protein seeds (for example, oligomers and protofibrils) first form in the cytoplasm of the releasing neuron (left), where soluble native monomers are recruited into large intracellular aggregates and a positive feedback loop can be initiated by generation of more seeds through fragmentation or secondary nucleation. A small amount of protein aggregates can be released into the extracellular space in the ‘naked’ form (a) or via membrane-bound vesicles such as exosomes (b). Free-floating seeds may directly penetrate the plasma membrane of the recipient neuron (1) or enter by fluid-phase endocytosis (2) or receptor-mediated endocytosis (3), whereas exosomes containing seeds may fuse with the membrane of the recipient neuron (4). Intercellular transfer of seeds may also occur by nanotubes that directly connect the cytoplasm of two cells (5). Internalized seeds then nucleate the fibrillization of native monomers in the cytoplasm of the recipient neuron.
Although fibrillar aggregates were shown to be capable of self-amplification, what triggers the initial conversion of normally soluble proteins into filamentous polymers remains enigmatic. Small misfolded protein species, such as oligomers, have been isolated as intermediates from in vitro fibrillization of multiple disease-associated proteins and have been suggested to be more neurotoxic than mature fibrils26. However, difficulties in observing these prefibrillar species in human brains have prohibited the establishment of a convincing link between these species and disease pathogenesis. Nonetheless, a few studies have reported the presence of tau oligomers in human brains, and oligomeric tau purified from Alzheimer’s disease brains could induce tau pathology in mice through intracerebral injection27–29. Interestingly, oligomeric PrPSc was found to be even more infectious than fibrillar PrPSc (ref. 30). To better understand the roles of soluble misfolded species in the onset and progression of neurodegenerative disorders, future studies should devote more effort to isolating these small protein species from diseased brains, conducting detailed biochemical and biophysical analyses on them and characterizing their transmission properties.
Pathophysiological relevance to human diseases
A highly predictable spatiotemporal progression of protein lesions has been well described for Alzheimer’s disease, Parkinson’s disease and dementia with Lewy bodies31–35 and, very recently, for ALS36. In Alzheimer’s disease brains, for example, NFTs spread from the limbic areas to neocortex with advancing stages of the disease31, whereas Aβ plaques first emerge in the neocortex before arising in the subcortex35. Such a stereotypical involvement of different brain regions was historically attributed to differential vulnerability of distinct brain regions to the misfolding of specific pathogenic proteins. Alternatively, neuronal injury caused by protein aggregation and protein aggregates released from dying neurons may lead to chronic activation of microglia and astrocytes, which secrete proinflammatory cytokines such as tumor necrosis factor-α, interleukin-1β and interleukin-6 and generate reactive oxygen species; these chemical mediators could in turn promote aberrant modifications and misfolding of proteins in neighboring healthy neurons37. This hypothesis is supported by evidence of increased neuroinflammation in various neurodegenerative diseases, although it cannot adequately account for the progression of disease-specific protein lesions over long distances.
The recent surge of studies showing the propagation of misfolded proteins themselves along defined neuronal projections offers yet another possibility (Figs. 1 and 2), which not only could explain parsimoniously the curious anatomical connections between sequentially affected brain areas, but also resolves the paradox that the same type of cells in slightly different anatomical locations (for example, cortical pyramidal neurons in layer Va compared with those in layer IV) have differential tendencies to develop pathology38. Importantly, in vivo studies showed that inoculating aggregated proteins at different sites in the brain elicited distinct patterns of pathology progression, suggesting that the initial areas of insult determine the brain areas to be subsequently affected16,19. Although published in vivo studies have focused on Aβ, tau and α-syn, it is tempting to hypothesize that the same spreading process could underlie the progression of all neurodegenerative proteinopathies. Studies are currently underway to verify how common this pathogenic mechanism is among these diseases.
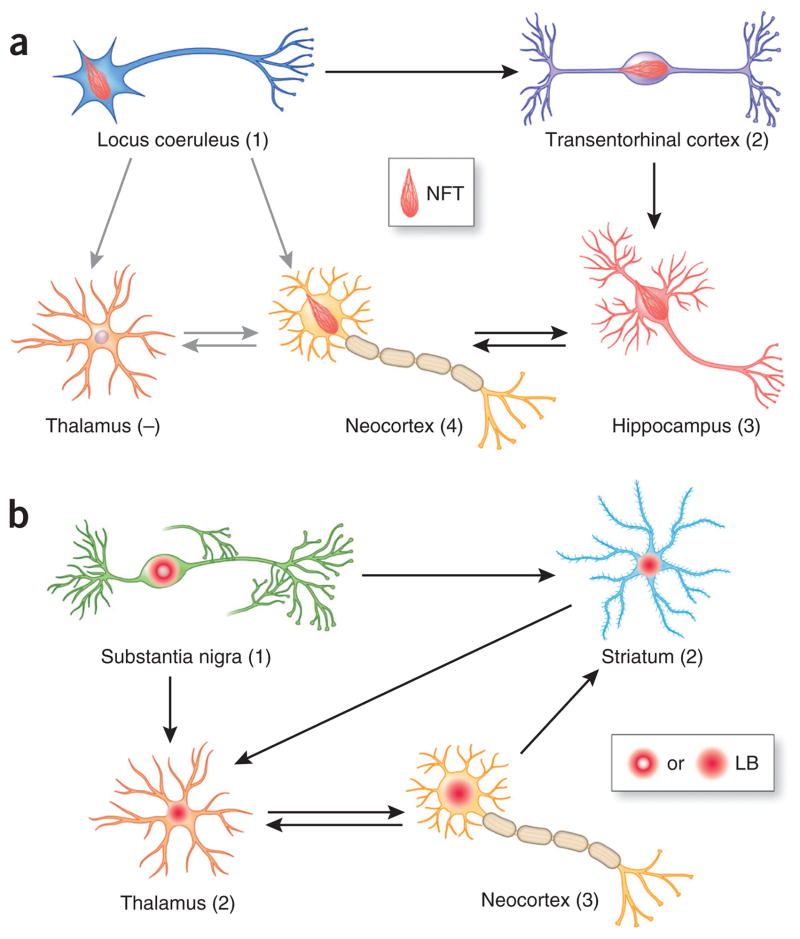
Figure 2.
Hypothetical model accounting for the stereotypical progression of pathologies in Alzheimer’s and Parkinson’s diseases. (a, b) Key brain regions developing NFTs (a) or Lewy bodies (LB) (b) are shown for each disease; numbers in parentheses indicate the relative temporal sequence of pathology progression (−, lack of pathology). Major neuronal projections are indicated by arrows, with black arrows indicating projections that hypothetically contribute to the spreading of pathology and grey arrows showing lack of transmission. Brain areas affected by tau and α-syn inclusions at the early stages of the diseases are different, probably owing to brain region–specific vulnerability to the aggregation of the disease proteins. Subsequently affected brain regions may acquire transmissible protein aggregates along both anterograde and retrograde connections, although not all regions connected with affected areas develop pathology. For example, the thalamus is relatively resistant to the accumulation of NFTs despite direct connections with the locus coeruleus. Therefore, brain region–specific vulnerability combined with network connections gives rise to the characteristic onset and progression patterns of neuropathology for different disease proteins.
One apparent discrepancy between human disease and mouse models of cell-to-cell transmission is the drastically different time course: neurodegenerative proteinopathies in humans typically occur only after decades, but protein aggregation induced by exogenously supplied misfolded proteins in mouse models happens within months, sometimes even weeks. Such inconsistency potentially raises doubts about the validity of these mouse models and the applicability of the transmission hypothesis to explain human diseases. However, a number of factors could explain such temporal discrepancy. First, mouse brains are substantially smaller than human brains, thus the former would require much less time for protein aggregates to propagate from one brain region to the other (assuming a comparable rate of spreading across species). Second, as in other polymerization reactions, the rate-limiting step of protein aggregation is the nucleation phase39,40, whereby small assemblies of misfolded proteins—the seeds—first form. In young, healthy neurons, misfolded proteins are efficiently removed by the cellular protein degradation machinery, whereas in aged neurons, the proteolytic machinery gradually deteriorates, thereby giving rise to the very long incubation time for seed formation. In animal models of transmission, misfolded proteins are directly delivered into the brains of animals, thereby dramatically accelerating the disease process by skipping the nucleation phase. Third, the transmission paradigm has typically been conducted in transgenic animals overexpressing the protein of interest, sometimes even carrying disease-causing mutations that further promote aggregation18–20,22,41,42. Given that seeded fibrillization is a concentration-dependent process, it is not surprising this process takes much longer in human brains that are not engineered to overexpress the pathogenic proteins. In fact, the use of overexpression- based mouse models in demonstrating disease protein transmissibility is unsatisfactory, because the spreading pattern of seeded pathology could be confounded by the transgene expression pattern in a particular mouse model, and—importantly—neurodegenerative diseases in humans do not usually involve upregulation of the disease-associated proteins, except in rare familial cases. Therefore, future studies will need to demonstrate robust propagation of templated pathology in nontransgenic mice to make a stronger case for the transmission hypothesis.
The transcellular movement of cytosolic protein aggregates
Misfolded cytosolic protein aggregates are found in both cell bodies and processes of the asymmetric neurons. To accomplish neuron-to- neuron transmission, they must have means to travel within a neuron to reach potential sites for interneuronal transfer, exit the originating neuron and enter the recipient neuron. Studies using microfluidic chambers have shown that both tau and α-syn aggregates can move anterogradely as well as retrogradely within a neuron, possibly by axonal transport15,43,44. Perhaps the most perplexing aspect of interneuronal transmission lies in the release and uptake of pathological aggregates (Fig. 1).
Recent evidence suggests that tau and α-syn may be released from neurons. The presence of these two proteins in human cerebrospinal fluid has been well documented45–47, and two microdialysis studies in healthy mice detected appreciable concentrations of tau and α-syn in the interstitial fluid, suggesting that these proteins are, for unknown reasons, constitutively released from healthy neurons48,49. Other cell culture studies have shown that non-fibrillar (monomeric or oligomeric) α-syn and tau can be secreted into the cell medium via exosomes50–52, which are cell-derived extracellular vesicles that are also implicated in the propagation of PrPSc (ref. 53), and this process may be modulated by neuronal activity54. Although further studies are needed to determine whether a similar mechanism mediates the efflux of fibrillar protein aggregates, one study suggested that tau aggregates are released into the extracellular space as free-floating fibrils without any membrane association55.
Different mechanisms of internalization have been proposed for various neurodegenerative disease proteins. PolyQ aggregates seem to be able to penetrate cell membranes directly without going through endocytic compartments56. SOD1 aggregates were shown to enter cells through lipid raft–dependent macropinocytosis57, an endocytic pathway mediating nonselective fluid-phase uptake. Tau fibrils were similarly shown to be taken up partially through fluid-phase endo-cytosis or, more specifically, macropinocytosis43,58,59. Furthermore, adsorptive endocytosis (an intermediate between receptor-mediated endocytosis and fluid-phase endocytosis) enhanced the uptake of both tau and α-syn fibrils15,17. Several studies have shown that α-syn fibrils enter cells via receptor-mediated endocytosis that is dynamin dependent60,61. However, considering the size of fibrillar aggregates, receptor-mediated endocytosis, which requires specific interactions between ligands and cell-surface receptors, seems unlikely to be a major mode of fibril internalization. Irrespective of the precise mode of entry, if endocytosis is the predominant pathway for fibril uptake, an additional hurdle for these endocytosed protein aggregates is to exit vesicles to access soluble cytosolic proteins. So far, no study has specifically addressed this issue, but the successful induction of cytoplasmic aggregates that are not bound to vesicular structures, as shown by electron microscopy15,17,62, indirectly demonstrates that internalized misfolded seeds must be able to escape into the cytosol, possibly through physical disruption of the vesicle membrane.
In addition to the mechanisms discussed above, several others could potentially mediate the transcellular movement of cytosolic protein aggregates. First, nanotubes, which are tunnel-like structures connecting two cells, have been demonstrated to be involved in the spreading of PrPSc (ref. 63). These structures would provide a convenient conduit for misfolded proteins, obviating the need to cross plasma membranes. Second, because studies of aggregate inoculation in mice have consistently demonstrated spreading of pathology along neuronal connections, it would be worthwhile to investigate whether synaptic transmission is somehow hijacked by pathological protein aggregates to facilitate interneuronal transfer. Compounds that modulate neuronal activity could be employed to assess the potential roles of synaptic transmission in pathology propagation. Third, a single injection of rabies virus or adeno-associated virus into the mouse brain can result in widespread distribution of viral particles to distant brain regions, and the spreading efficiency depends heavily on sequence variations of the viral capsids64–66. The intriguing similarities between the transmission patterns of viruses and protein aggregates in vivo raise the possibility that similar cellular machineries are being exploited to achieve trans-synaptic movement. Although mechanisms facilitating interneuronal propagation of viruses are also not well understood, the internalization of viruses is known to be mediated by cell-surface receptors, such as membrane-associated heparan sulfate proteoglycans (HSPGs), for adeno-associated virus, and neural cell adhesion molecule, for rabies virus67,68. Intriguingly, HSPGs were recently shown to mediate the uptake of both tau and α-syn fibrils59.
Insights from comparing different amyloidogenic proteins
Transmission, infectiousness and biosafety
Although templated recruitment and cell-to-cell transmission may be shared properties of amyloid protein aggregates, one important distinction between prion diseases and other neurodegenerative diseases is that the former are infectious (as defined by their ability to transmit between individuals via natural routes, such as ingestion), perhaps owing to the exceptional protease resistance of PrPSc (ref. 69). So far, there is no evidence to support the idea that non-prion neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, can spread from one person to another. A retrospective, postmortem study of recipients of cadaver- derived human growth hormone (hGH) found no reported incidence of Alzheimer’s disease or Parkinson’s disease, although the donors of pituitary glands used for hGH preparation (in the United States alone, more than 1.4 million donors between 1963 and 1985) probably included people with Alzheimer’s disease or Parkinson’s disease, and immunohistochemical examination revealed frequent accumulations of pathological Aβ, tau and α-syn in the postmortem pituitary glands of people with Alzheimer’s disease or Parkinson’s disease70. In contrast, hGH administration has resulted in worldwide outbreaks of iatrogenic CJD71 caused, presumably, by PrPSc contamination of the hGH preparation, despite the extremely low rate of CJD incidence in the population. Therefore, existing human data suggest that the potential infectiousness of non-prion protein aggregates is sufficiently low that normal contact with afflicted individuals would not increase one’s lifetime risk for the respective diseases. Nevertheless, in research laboratories where large quantities of aggregated proteins are used experimentally, precautionary measures should be taken to minimize potential occupational risk, such as requiring proper laboratory attire, designating separate work space for handling misfolded proteins and adopting enclosed sonication systems to prevent aerosol transmission.
Spreading propensity of different amyloid aggregates
Peripheral administration of inoculums containing aggregated Aβ or prions was shown to induce pathology in the cerebral cortex of injected animals72–74, suggesting that these extracellularly located protein aggregates can propagate from the peripheral tissues to the brain while preserving their seeding-competent conformation. These experimental results are not surprising for prions, given the transmission routes of acquired prion diseases in humans75, but the pathophysiological relevance of periphery-to-central spreading of Aβ is not yet clear, as Aβ plaques are found only in the brains of patients with Alzheimer’s disease.
Cerebral induction of pathology through peripheral injection has not been demonstrated for intracellular protein aggregates, which conceivably would encounter more obstacles to long-distance spreading than would extracellular plaques. However, recent studies suggest α-syn pathology in Parkinson’s disease may actually initiate from the gut (the enteric nervous system) and progressively ascend to the brain76. Indeed, injection studies in mice showed that pathological α-syn is more transmissible than pathological tau, whereby seeded α-syn pathology spreads to much wider brain regions than seeded tau tangles under the same inoculation paradigm19,20. α-Syn may be privileged in transmission owing to its high local concentration at the presynaptic terminals77, which could be a crucial site for initiating templated recruitment and interneuronal propagation.
Transmissibility of synthetic fibrils and requirement of cofactors
The ‘protein-only’ hypothesis of prion propagation postulates that the infectious agent in prion diseases is composed solely of PrP in the abnormal conformation78. This stringent criterion turns out to be difficult to attain both for prions and for non-prion pathogenic protein aggregates. Synthetic PrPSc fibrils assembled from recombinant protein are not as infectious as brain-derived PrPSc unless RNA and lipid molecules are added as cofactors79–81. Although both Aβ and α-syn synthetic fibrils assembled without any cofactors were shown to be fully capable of inducing substantial pathology in mice16,20,22, the specific seeding activity of synthetic Aβ aggregates is significantly lower than that of Aβ aggregates purified from transgenic mouse brains. Similarly, brain lysates from symptomatic mice containing small amounts of aggregated α-syn propagated pathology with the same potency as high doses of synthetic α-syn fibrils, suggesting that pathological α-syn generated in vivo has enhanced templating efficiency20. No side-by-side comparisons for synthetic and in vivo–generated tau aggregates have yet been reported, but all synthetic tau fibrils shown to transmit in both cells and mice have, so far, been generated in the presence of heparin, a polyanionic cofactor that promotes tau aggregation in vitro82. Thus, it remains unproven whether fibrillar tau is wholly sufficient to template the conversion of normal tau, with heparin merely catalyzing the fibrillization of tau, or whether heparin is intrinsically required to generate a seeding-competent tau conformer. Future studies are needed to explore the exact roles of cofactors in facilitating the transmissibility of disease-associated protein aggregates and their pathological relevance to human diseases.
Transmissible species versus toxic species
Although fibrillar protein aggregates are the most conspicuous hallmarks of various neurodegenerative diseases and were found to be transmissible through self-amplification, they are not necessarily the toxic species that directly cause neurodegeneration. In mouse models of prion diseases, neurodegeneration sometimes occurs without overt accumulation of PrPSc (refs. 83,84). By contrast, some mice with PrPSc titers as high as those of terminally sick mice did not show obvious abnormalities85, and PrPSc itself did not cause toxicity in PrPC knockout mice86. Using a more accurate scrapie cell assay to measure PrPSc titers, a study showed recently that the levels of infective PrPSc reached a plateau long before the onset of symptoms, indicating a clear dissociation between transmissibility and toxicity87. Interestingly, the time of delay between the acquisition of maximal infectivity and the onset of symptoms is inversely proportional to PrPC concentration, suggesting that PrPSc may catalyze the formation of unidentified toxic species— possibly oligomers—in a PrPC-dependent manner.
In patients with Alzheimer’s disease, tau tangles correlate with disease severity, but Aβ plaque burden does not88. It has been suggested that tau aggregation may be the downstream effector of Aβ-induced toxicity and thus a more proximal cause of neuronal dysfunction89,90. Alternatively, smaller assemblies of Aβ, such as oligomers, which are more difficult to detect, may be the real toxic entities. In fact, soluble Aβ oligomers, but not Aβ fibrils, were shown to be toxic to cultured neurons91,92 and to impair long-term potentiation both in cultured hippocampal slices and in vivo93,94. However, Aβ fibrils may not be entirely inert, as a recent study showed that in the presence of Aβ fibrils, the predominant pathway of toxic oligomer formation is actually mediated by a secondary nucleation event catalyzed by mature fibrils rather than by direct nucleation from monomers95.
Similarly, annular or pore-like α-syn oligomers described by in vitro studies have been proposed to be neurotoxic by disrupting membrane integrity96,97, and artificially designed α-syn mutants that have a higher tendency to form oligomers were shown to be more toxic in vivo98,99. Although the existence of oligomeric α-syn species in human diseases remains to be established, transmission studies in cultured cells and mice have consistently demonstrated that substantial toxicity accompanies templated α-syn fibrillization15,16,20, indicating that filamentous α-syn aggregates have deleterious effects. The large Lewy body–like α-syn inclusions induced in cells not only resist clearance by protein degradation machinery but also impair autophagy functions100.
In contrast, induction of NFT-like tau aggregates in cultured cells or tau transgenic mice has not resulted in obvious cellular toxicity17–19,62. One possible reason is that prefibrillar tau, rather than mature tangles, is the truly toxic species. Exogenously supplied misfolded seeds that readily recruit soluble tau into filamentous aggregates may result in a complete bypass of the slow oligomerization phase that normally occurs in uninjected transgenic mice before the onset of overt pathology. This hypothesis could potentially explain the curious paradox in which neuron loss is observed in aged tau transgenic mice bearing ThS-negative tau inclusions but not in fibril-injected transgenic mice that develop more mature tangles. It is also consistent with observations that neuronal dysfunction sometimes precedes tau aggregation or occurs independently of tangle formation in animal models101–103.
Strains: another shared property of amyloid aggregates?
Polymorphism of amyloid protein aggregates
Besides cell-to-cell transmissibility, another property common among amyloidogenic proteins is the ability to form structurally diverse fibrils, the best- studied example being PrPSc (Fig. 3a). Conformationally distinct PrPSc aggregates have been found in different ‘strains’ of prion diseases, which are characterized by specific histopathological lesion profiles (brain region–specific distribution of prion plaques and extent of spongiform changes) and clinical manifestations (incubation time before disease onset and aggressiveness of the disease)104. These phenotypic variations are believed to be caused by distinct PrPSc conformers, which can be propagated in vivo by serial passage and preserve the same phenotypes in subsequently infected animals.
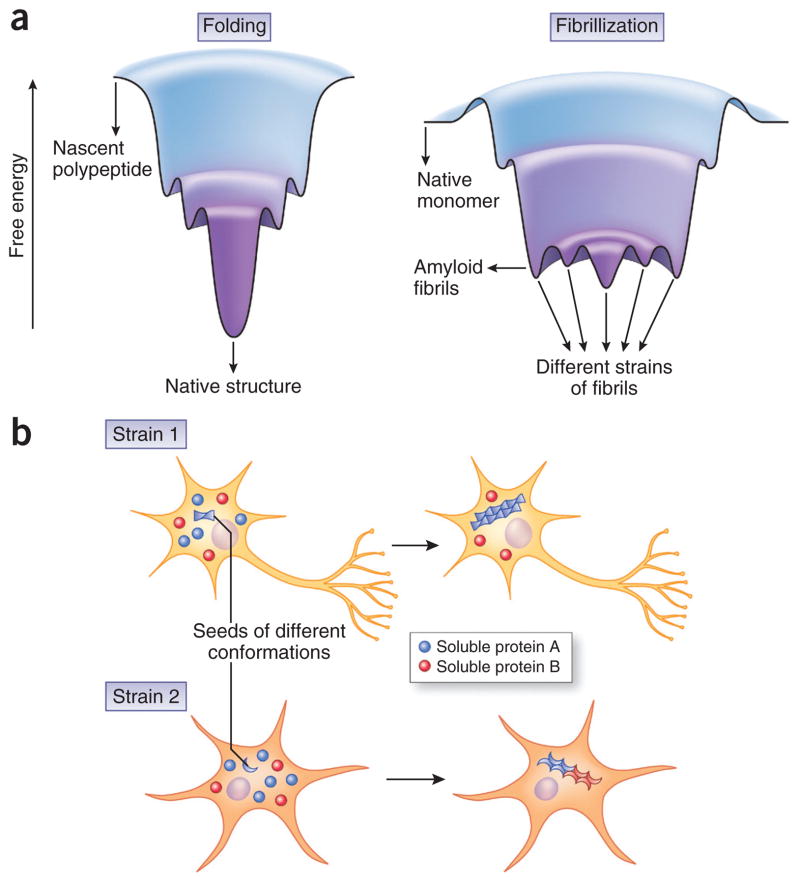
Figure 3.
Pathological strains underlying the divergence and convergence of neurodegenerative proteinopathies. (a) A possible explanation for the existence of multiple fibrillar conformers for a single polypeptide is that the energy landscape of fibrillization is different from that of normal protein folding. Whereas the energy landscape of protein folding resembles a funnel with a global minimum that corresponds to the native structure129,130, the landscape for abnormal protein aggregation may resemble rugged valleys with numerous local minima corresponding to different strains of fibrils. According to this speculative protein-aggregation landscape, multiple strains of fibrillar aggregates could theoretically emerge in one fibrillization event, but the exact environmental conditions may favor the generation and propagation of one strain over the others; one conformational variant could also directly morph into another if the energy barrier between them is overcome. Indeed, this is what was observed for the Darwinian evolution of prion strains in cultured cells and living animals131,132. A similar evolution of strains was recently reported for α-syn fibrils generated from recombinant protein, whereby serial passage in vitro resulted in the conversion of one strain of α-syn fibrils into the other108. (b) Different strains of amyloid fibrils may exhibit distinct cell tropism (indicated by different shapes of affected cells) and differential efficiency in seeding homotypic monomers (blue spheres) as well as in cross-seeding heterotypic monomers (red spheres). These properties could result in distinct neuropathological profiles, including differences in the distribution of pathology and extent of comorbid pathologies, and ultimately lead to heterogeneous clinical manifestations.
An analog to prion strains has been described for other pathogenic protein aggregates. Polymorphic fibrils were shown to assemble from the same proteins with single amino acid substitutions or from proteins with identical primary structures under different fibrillization conditions, and the distinct structures can often be propagated in vitro or even in vivo via seeded fibrillization105–111. Importantly, conformational variants of a single protein can exhibit different biological activities. For example, Aβ fibrils formed with or without agitation display differential toxicity in neurons109, as do polyQ aggregates formed at different temperatures110. Conformational variants of synthetic α-syn fibrils not only cause differential neurotoxicities owing to disparate potencies in seeding α-syn aggregation, but also show striking differences in their ability to act as heterotypic templates to ‘cross-seed’ tau fibrillization108. Along the same line of thought, the lack of obvious neuronal loss in mice inoculated with synthetic tau fibrils19 could well be explained by the possibility that these artificial fibrils represent a variant of fibrillar tau that is different from tau fibrils that naturally develop in brains with tauopathies. In fact, a recent study showed that heparin-induced tau fibrils are indeed structurally distinct from tau fibrils derived from Alzheimer’s disease brain112. Given the many similarities between prion strains and conformational variants of non-prion amyloidogenic proteins, it would be reasonable to extend the scope of strains to include the latter. However, because other neurodegenerative disease–associated proteins seem to be much less transmissible than PrPSc, the operational definition of strains would need to be modified to remove the requirement of propagatability in vivo through serial passage.
Pathophysiological relevance of strains for non-prion proteinopathies
Similarly to prion diseases, each class of neurodegenerative protein-opathies shows tremendous heterogeneity. For example, among the various tauopathies characterized by the accumulation of tau aggregates (for example, Alzheimer’s disease, argyrophilic grain disease, progressive supranuclear palsy, corticobasal degeneration and Pick’s disease), there are substantial variations in clinical symptoms, age of disease onset, rate of progression, types of cells affected (neurons and/or glial cells), brain region–specific distribution of tau inclusions and morphology of tau tangles113,114. At the same time, considerable overlap occurs among different categories of diseases, in both clinical symptoms115,116 and neuropathologies, whereby different protein aggregates such as NFTs and Lewy bodies frequently co-deposit in diseased brains117. Proteostatic stress arising from the aggregation of one protein can elicit a global effect on the structural stability of other unrelated proteins, possibly resulting in misfolding and aggregation of other proteins118. Filamentous aggregates consisting of one protein may also directly seed the fibrillization of another aggregation-prone protein, as supported by histological studies showing that tau and α-syn inclusions are sometimes deposited in close proximity119,120 and also suggested by our recent study in primary neurons and transgenic mice108.
We propose that diverse strains of protein aggregates may offer a potential explanation for both the divergence and the convergence of neurodegenerative proteinopathies (Fig. 3b). Transmissible fibrillar aggregates of different conformations may display highly variable kinetics in templated fibrillization, resulting in different progression rates of various diseases; exhibit distinct cell tropism, leading to pathology development in different cell types and progression to distinct brain areas; and differ in their ability to cross-seed aggregation of other amyloidogenic proteins, causing differential levels of comorbid pathologies. Given these variations in biological activities, conformationally diverse strains can not only potentially account for the tremendous heterogeneity among disorders with the same major protein lesions, but also explain the frequent (though variable) co-deposition of different protein aggregates. Furthermore, during cell-to-cell transmission involving repetitive seeded fibrillization, one strain of pathological aggregates may evolve into another strain, as demonstrated for synthetic α-syn fibrils108. This could lead to brain region–specific pathologies within a single patient, such as the morphologically distinct Lewy bodies found in the substantia nigra and neocortex of patients with Parkinson’s disease3. The variants of synthetic fibrils generated in vitro are not necessarily identical to fibrils formed in human brains, but considering the complexity of human brains relative to test-tube reactions, the chance of developing diverse strains of pathological aggregates in real diseased brains is exceedingly high. In fact, three recent studies have provided preliminary evidence for the presence of structural variants of pathological Aβ, tau and α-syn in human brains42,108,121, but more studies are required to verify the existence of strains in heterogeneous disease groups and their correlation with disease phenotypes.
Therapeutic implications
Studies of prion-like transmissibility of pathogenic protein aggregates have important therapeutic implications. Cell-replacement therapy, which is designed to restore the functions of degenerating brains by replacing dying cells with healthy ones122, may not, if used alone, provide a long-term treatment for neurodegenerative proteinopathies, because the implanted cells may eventually develop pathology owing to transmission from host cells. Indeed, several studies on patients with Parkinson’s disease receiving embryonic nigral transplants showed the emergence of Lewy bodies in the young graft tissues, suggesting a possible host-to-graft transmission of α-syn pathology123,124. One recent study in cultured cells showed that the induction of Lewy body–like α-syn aggregates leads to the futile activation of protein-degradation systems, further stimulation of which is actually more detrimental to cells100. If the same is true for all pathological protein aggregates, we would need to rethink the fervently sought therapeutic approach of boosting autophagy in pathology-laden brains.
On the bright side, recent studies have opened new avenues for therapeutic interventions that could be used in combination with cell-replacement therapy. Small molecules that hinder templated recruitment by existing aggregates would inhibit further corruption of native proteins and thus block self-amplification of misfolded proteins. Because cell-to-cell transmission probably involves release and uptake of misfolded proteins, agents that could block either process would conceivably stop the spreading of pathology, thereby halting disease progression. Compounds that enhance cell membrane integrity might reduce the transcellular movement of amyloid fibrils via direct membrane penetration. As fluid-phase endocytosis seems to be a predominant uptake mechanism of several protein aggregates, agents that suppress this endocytic pathway could potentially be helpful for multiple diseases. If exosomes are truly involved in the release of aggregated proteins, inhibitors of exosome secretion may also hold therapeutic potential. However, because any cellular machinery exploited by pathological protein aggregates to accomplish intercellular transmission is likely to play important parts in many normal cellular functions, drugs affecting these pathways may have substantial side effects.
An alternative approach to inhibit cell-to-cell transmission of misfolded proteins is to sequester them in the extracellular space during transit from one cell to another. Immunotherapy, which was previously thought to be intractable for abrogating intracytoplasmic protein aggregates, would be an attractive strategy to stop the intercellular movement of target protein species. Lessons learned from Aβ immunotherapy would become useful for other pathogenic proteins125. In particular, careful consideration is required to decide whether active or passive immunization is the preferred method, and whether a linear epitope (a continuous stretch of amino acids in a primary structure) or a conformational epitope (discontinuous amino acids that are brought together in tertiary structure) would be more desirable. Moreover, if diverse pathological strains truly exist, they may create additional challenges for effective immunotherapy, because antibodies that exhibit high binding affinity for one strain of protein aggregates may not be equally efficient at forming complexes with another strain. However, antibodies that recognize shared conformations of multiple strains, particularly those that inhibit cross-seeding, may have a broader application than those recognizing highly specific epitopes, but the feasibility of this hypothetical approach awaits empirical testing. A major obstacle for all brain-directed therapies is the blood-brain barrier (BBB), which tightly restricts the entry of both pathogens and therapeutics into the brain. Studies on Aβ passive immunotherapy showed that only about 0.1% of circulating antibodies managed to reach the brain126. Therefore, to maximize therapeutic effects, it would be necessary to employ strategies that promote the uptake of antibodies across the BBB, such as engineering hybrid antibodies—which, besides binding target molecules in the brain, also interact with specific transporters or receptors on the BBB to facilitate shuttling into the brain127.
Conclusions and future directions
A rapidly growing body of literature has provided compelling evidence supporting the intercellular transmission of pathological protein aggregates through templated recruitment as a potential unifying mechanism that underlies the progression of various neurodegenerative proteinopathies. Furthermore, the exact conformation of the template may dictate its recruitment properties, possibly giving rise to heterogeneity among diseases characterized by the same protein lesions. Although these studies have revolutionized our conception of these diseases, more questions remain. For example, it is still unclear what triggers the generation of the misfolded protein seeds that initiate the disease cascade, why only certain proteins aggregate in a disease-specific manner if the main trigger is a global deterioration of protein homeostasis, what determines the selective vulnerability of different brain regions and what roles glial cells have in the transmission of protein aggregates. Numerous studies suggest that prefibrillar species may be more detrimental than fibrillar aggregates26,128, but the exact nature of these early species in the aggregation pathway and their relevance for human diseases require further studies. The biochemical identities of transmissible species also need to be better defined, as most existing studies simply use sonicated synthetic fibrils or crude preparations of brain homogenates, which may contain mixed forms of misfolded proteins. Moreover, although synthetic fibrils have been shown competent as seeds, they may not be identical to the pathological aggregates derived in vivo, which seem to be more potent seeds in transmission. Thus, future research should clarify the differences between these sources of seeds and investigate the potential parts other cellular components play in influencing transmissibility.
From a translational point of view, a detailed molecular dissection of pathways mediating the release and uptake of protein aggregates, which may or may not be the same for different pathogenic proteins, would provide new targets for drug development. Specifically, future studies should determine the exact endocytic pathways involved in the internalization of protein aggregates and verify whether aggregates are released from cells in a free-floating form or enclosed by vesicles, as this will be crucial for the design of effective therapies. Finally, more effort should be directed toward generating animal models of neurodegenerative diseases without overexpression, which would provide better recapitulations of sporadic diseases; so far, this goal has only been partially attained for α-syn16. For other proteins, researchers may need to create strains of aggregates that are more potent than existing ones in propagating pathology, possibly by using specific combinations of cofactors or by amplifying pathological aggregates derived in vivo. With a collaborative effort on the issues mentioned above, we would gain better understanding of the pathogenesis of neurodegenerative proteinopathies and, hopefully, find cures for these devastating diseases.
Acknowledgments
The authors thank J.Q. Trojanowski for critical reading of the manuscript.
This study was supported by NIH/NIA grants AG17586, NS53488 (V.M.Y.L.), the Marian S. Ware Alzheimer Program, the Jeff and Anne Keefer Fund and the Parkinson Council.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 2.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 5.DiFiglia M, et al. Aggregation of hunting tin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 6.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 7.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10 (suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 8.Goedert M. Filamentous nerve cell inclusions in neurodegenerative diseases: tauopathies and α-synucleinopathies. Phil Trans R Soc Lond B. 1999;354:1101–1118. doi: 10.1098/rstb.1999.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorpe JR, Tang H, Atherton J, Cairns NJ. Fine structural analysis of the neuronal inclusions of frontotemporal lobar degeneration with TDP-43 proteinopathy. J Neural Transm. 2008;115:1661–1671. doi: 10.1007/s00702-008-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin WL, Dickson DW. Ultrastructural localization of TDP-43 in filamentous neuronal inclusions in various neurodegenerative diseases. Acta Neuropathol. 2008;116:205–213. doi: 10.1007/s00401-008-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E. Cell-to-cell transmission of non-prion protein aggregates. Nature reviews Neurology. 2010;6:702–706. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 15.Volpicelli-Daley LA, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo JL, Lee VM. Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286:15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iba M, et al. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luk KC, et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda-Suzukake M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stöhr J, et al. Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc Natl Acad Sci USA. 2012;109:11025–11030. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Calignon A, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulusoy A, et al. Caudorostral brain spreading of α-synuclein through vagal connections. EMBO Mol Med. 2013;5:1051–1059. doi: 10.1002/emmm.201302475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid-β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 27.Maeda S, et al. Granular tau oligomers as intermediates of tau filaments. Biochemistry. 2007;46:3856–3861. doi: 10.1021/bi061359o. [DOI] [PubMed] [Google Scholar]
- 28.Patterson KR, et al. Characterization of prefibrillar tau oligomers in vitro and in Alzheimer disease. J Biol Chem. 2011;286:23063–23076. doi: 10.1074/jbc.M111.237974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasagna-Reeves CA, et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep. 2012;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silveira JR, et al. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 33.Braak H, et al. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249:iii1–iii5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 34.Kosaka K, Tsuchiya K, Yoshimura M. Lewy body disease with and without dementia: a clinicopathological study of 35 cases. Clin Neuropathol. 1988;7:299–305. [PubMed] [Google Scholar]
- 35.Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 36.Brettschneider J, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braak H, Del Tredici K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 2011;121:589–595. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- 39.Friedhoff P, Schneider A, Mandelkow EM, Mandelkow E. Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry. 1998;37:10223–10230. doi: 10.1021/bi980537d. [DOI] [PubMed] [Google Scholar]
- 40.Wood SJ, et al. α-synuclein fibrillogenesis is nucleation-dependent: implications for the pathogenesis of Parkinson’s disease. J Biol Chem. 1999;274:19509–19512. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
- 41.Meyer-Luehmann M, et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 42.Clavaguera F, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu JW, et al. small misfolded tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem. 2013;288:1856–1870. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freundt EC, et al. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann Neurol. 2012;72:517–524. doi: 10.1002/ana.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tapiola T, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 46.Mollenhauer B, et al. Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett. 2013;532:44–48. doi: 10.1016/j.neulet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 47.van Dijk KD, et al. Reduced α-synuclein levels in cerebrospinal fluid in Parkinson’s disease are unrelated to clinical and imaging measures of disease severity. Eur J Neurol. 2013 doi: 10.1111/ene.12176. [DOI] [PubMed] [Google Scholar]
- 48.Yamada K, et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011;31:13110–13117. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emmanouilidou E, et al. Assessment of α-synuclein secretion in mouse and human brain parenchyma. PLoS ONE. 2011;6:e22225. doi: 10.1371/journal.pone.0022225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emmanouilidou E, et al. Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danzer KM, et al. Exosomal cell-to-cell transmission of α-synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vella LJ, et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol. 2007;211:582–590. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- 54.Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14:389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of tau aggregation by fibrillar species. J Biol Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren PH, et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Münch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci USA. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmes BB, et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen C, et al. α-synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo JL, Lee VM. Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau. FEBS Lett. 2013;587:717–723. doi: 10.1016/j.febslet.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gousset K, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 64.Dietzschold B, et al. Differences in cell-to-cell spread of pathogenic and apathogenic rabies virus in vivo and in vitro. J Virol. 1985;56:12–18. doi: 10.1128/jvi.56.1.12-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cearley CN, et al. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol Ther. 2008;16:1710–1718. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thoulouze MI, et al. The neural cell adhesion molecule is a receptor for rabies virus. J Virol. 1998;72:7181–7190. doi: 10.1128/jvi.72.9.7181-7190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weissmann C, Enari M, Klohn PC, Rossi D, Flechsig E. Transmission of prions. Proc Natl Acad Sci USA. 2002;99 (suppl 4):16378–16383. doi: 10.1073/pnas.172403799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Irwin DJ, et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 2013;70:462–468. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown P, Gajdusek DC, Gibbs CJ, Jr, Asher DM. Potential epidemic of Creutzfeldt-Jakob disease from human growth hormone therapy. N Engl J Med. 1985;313:728–731. doi: 10.1056/NEJM198509193131205. [DOI] [PubMed] [Google Scholar]
- 72.Eisele YS, et al. Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimberlin RH, Walker CA. Pathogenesis of mouse scrapie: effect of route of inoculation on infectivity titres and dose-response curves. J Comp Pathol. 1978;88:39–47. doi: 10.1016/0021-9975(78)90059-2. [DOI] [PubMed] [Google Scholar]
- 74.Aguzzi A, Calella AM. Prions: protein aggregation and infectious diseases. Physiol Rev. 2009;89:1105–1152. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- 75.Will RG. Acquired prion disease: iatrogenic CJD, variant CJD, kuru. Br Med Bull. 2003;66:255–265. doi: 10.1093/bmb/66.1.255. [DOI] [PubMed] [Google Scholar]
- 76.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 77.Iwai A, et al. The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 78.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 79.Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colby DW, et al. Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci USA. 2009;106:20417–20422. doi: 10.1073/pnas.0910350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Makarava N, et al. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol. 2010;119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goedert M, et al. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 83.Westaway D, et al. Degeneration of skeletal muscle, peripheral nerves, and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell. 1994;76:117–129. doi: 10.1016/0092-8674(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 84.Hsiao KK, et al. Serial transmission in rodents of neurodegeneration from transgenic mice expressing mutant prion protein. Proc Natl Acad Sci USA. 1994;91:9126–9130. doi: 10.1073/pnas.91.19.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hill AF, Collinge J. Subclinical prion infection. Trends Microbiol. 2003;11:578–584. doi: 10.1016/j.tim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 86.Brandner S, et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 87.Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR, Collinge J. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature. 2011;470:540–542. doi: 10.1038/nature09768. [DOI] [PubMed] [Google Scholar]
- 88.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 89.Roberson ED, et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 90.Ittner LM, et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 91.Dahlgren KN, et al. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 92.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walsh DM, et al. Naturally secreted oligomers of amyloid-β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 94.Wang HW, et al. Soluble oligomers of β-amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- 95.Cohen SI, et al. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci USA. 2013;110:9758–9763. doi: 10.1073/pnas.1218402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding TT, Lee SJ, Rochet JC, Lansbury PT., Jr Annular α-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry. 2002;41:10209–10217. doi: 10.1021/bi020139h. [DOI] [PubMed] [Google Scholar]
- 97.Lashuel HA, et al. α-Synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 98.Winner B, et al. In vivo demonstration that α-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karpinar DP, et al. Pre-fibrillar α-synuclein variants with impaired β-structure increase neurotoxicity in Parkinson’s disease models. EMBO J. 2009;28:3256–3268. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR, Lee VM. Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy. J Biol Chem. 2013;288:15194–15210. doi: 10.1074/jbc.M113.457408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wittmann CW, et al. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 102.Yoshiyama Y, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 103.SantaCruz K, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552–561. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- 105.Aoyagi H, Hasegawa M, Tamaoka A. Fibrillogenic nuclei composed of P301L mutant tau induce elongation of P301L tau but not wild-type tau. J Biol Chem. 2007;282:20309–20318. doi: 10.1074/jbc.M611876200. [DOI] [PubMed] [Google Scholar]
- 106.Yonetani M, et al. Conversion of wild-type α-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. J Biol Chem. 2009;284:7940–7950. doi: 10.1074/jbc.M807482200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Furukawa Y, Kaneko K, Yamanaka K, Nukina N. Mutation-dependent polymorphism of Cu, Zn-superoxide dismutase aggregates in the familial form of amyotrophic lateral sclerosis. J Biol Chem. 2010;285:22221–22231. doi: 10.1074/jbc.M110.113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo JL, et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petkova AT, et al. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 110.Nekooki-Machida Y, et al. Distinct conformations of in vitro and in vivo amyloids of huntingtin-exon1 show different cytotoxicity. Proc Natl Acad Sci USA. 2009;106:9679–9684. doi: 10.1073/pnas.0812083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heilbronner G, et al. Seeded strain-like transmission of β-amyloid morphotypes in APP transgenic mice. EMBO Rep. 2013;14:1017–1022. doi: 10.1038/embor.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morozova OA, March ZM, Robinson AS, Colby DW. Conformational features of tau fibrils from Alzheimer’s disease brain are faithfully propagated by unmodified recombinant protein. Biochemistry. 2013;52:6960–6967. doi: 10.1021/bi400866w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tolnay M, Probst A. The neuropathological spectrum of neurodegenerative tauopathies. IUBMB Life. 2003;55:299–305. doi: 10.1080/1521654032000114348. [DOI] [PubMed] [Google Scholar]
- 114.Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(suppl 2):II6–II15. doi: 10.1007/BF03161076. [DOI] [PubMed] [Google Scholar]
- 115.Mayeux R, et al. A population-based investigation of Parkinson’s disease with and without dementia—relationship to age and gender. Arch Neurol. 1992;49:492–497. doi: 10.1001/archneur.1992.00530290076015. [DOI] [PubMed] [Google Scholar]
- 116.Morris JC, Drazner M, Fulling K, Grant EA, Goldring J. Clinical and pathological aspects of parkinsonism in Alzheimer’s disease. A role for extranigral factors? Arch Neurol. 1989;46:651–657. doi: 10.1001/archneur.1989.00520420071025. [DOI] [PubMed] [Google Scholar]
- 117.Galpern WR, Lang AE. Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol. 2006;59:449–458. doi: 10.1002/ana.20819. [DOI] [PubMed] [Google Scholar]
- 118.Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- 119.Galloway PG, Bergeron C, Perry G. The presence of tau distinguishes Lewy bodies of diffuse Lewy body disease from those of idiopathic Parkinson disease. Neurosci Lett. 1989;100:6–10. doi: 10.1016/0304-3940(89)90651-4. [DOI] [PubMed] [Google Scholar]
- 120.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and α-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- 121.Lu JX, et al. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Korecka JA, Verhaagen J, Hol EM. Cell-replacement and gene-therapy strategies for Parkinson’s and Alzheimer’s disease. Regen Med. 2007;2:425–446. doi: 10.2217/17460751.2.4.425. [DOI] [PubMed] [Google Scholar]
- 123.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 124.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 125.Schenk DB, Seubert P, Grundman M, Black R. Aβ immunotherapy: lessons learned for potential treatment of Alzheimer’s disease. Neurodegener Dis. 2005;2:255–260. doi: 10.1159/000090365. [DOI] [PubMed] [Google Scholar]
- 126.Banks WA, et al. Passage of amyloid β protein antibody across the blood-brain barrier in a mouse model of Alzheimer’s disease. Peptides. 2002;23:2223–2226. doi: 10.1016/s0196-9781(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 127.Couch JA, et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci Transl Med. 2013;5:183ra157. doi: 10.1126/scitranslmed.3005338. [DOI] [PubMed] [Google Scholar]
- 128.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 129.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 130.Wolynes PG, Onuchic JN, Thirumalai D. Navigating the folding routes. Science. 1995;267:1619–1620. doi: 10.1126/science.7886447. [DOI] [PubMed] [Google Scholar]
- 131.Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C. Darwinian evolution of prions in cell culture. Science. 2010;327:869–872. doi: 10.1126/science.1183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghaemmaghami S, et al. Conformational transformation and selection of synthetic prion strains. J Mol Biol. 2011;413:527–542. doi: 10.1016/j.jmb.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of α-synuclein and tau: cellular models of neurodegenerative diseases. J Biol Chem. 2010;285:34885–34898. doi: 10.1074/jbc.M110.148460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luk KC, et al. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen AK, et al. Induction of amyloid fibrils by the C-terminal fragments of TDP-43 in amyotrophic lateral sclerosis. J Am Chem Soc. 2010;132:1186–1187. doi: 10.1021/ja9066207. [DOI] [PubMed] [Google Scholar]
- 136.Nonaka T, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]