Abstract
Summary
This position paper reviews how the National Bone Health Alliance (NBHA) will execute a project to help assure health professionals of the clinical utility of bone turnover markers; the current clinical approaches concerning osteoporosis and the status and use of bone turnover markers in the USA; the rationale for focusing this effort around two specific bone turnover markers; the need to standardize bone marker sample collection procedures, reference ranges, and bone turnover marker assays in clinical laboratories; and the importance of harmonization for future research of bone turnover markers.
Introduction
Osteoporosis is a major global health problem, with the prevalence and incidence of osteoporosis for at-risk populations estimated to be 44 million Americans. The potential of bone markers as an additional tool for health care professionals to improve patient outcomes and impact morbidity and mortality is crucial in providing better health care and addressing rising health care costs. This need to advance the field of bone turnover markers has been recognized by a number of organizations, including the International Osteoporosis Foundation (IOF), National Osteoporosis Foundation, International Federation of Clinical Chemistry, and Laboratory Medicine (IFCC), and the NBHA.
Methods
This position paper elucidates how this project will standardize bone turnover marker sample collection procedures in the USA, establish a USA reference range for one bone formation (serum procollagen type I N propeptide, s-PINP) and one bone resorption (serum C-terminal telopeptide of type I collagen, s-CTX) marker, and standardize bone turnover marker assays used in clinical laboratories. This effort will allow clinicians from the USA to have confidence in their use of bone turnover markers to help monitor osteoporosis treatment and assess future fracture risk. This project builds on the recommendations of the IOF/IFCC Bone Marker Standards Working Group by developing USA reference standards for s-PINP and s-CTX, the markers identified as most promising for use as reference markers.
Results
The goals of this project will be realized through the NBHA and will include its governmental, academic, for-profit, and non-profit sector stakeholders as well as major academic and commercial laboratories. Upon completion, a parallel effort will be pursued to make bone turnover marker measurements reliable and accepted by all health care professionals for facilitating treatment decisions and ultimately be reimbursed by all health insurance payers.
Conclusions
Successful completion of this project will help assure health professionals from the USA of the clinical utility of bone turnover markers and ties in with the parallel effort of the IOF/IFCC to develop worldwide bone turnover reference ranges.
Keywords: Bone turnover markers, Position paper
Introduction
There has been a recognized need to advance the field of bone turnover markers from the International Osteoporosis Foundation (IOF), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), National Osteoporosis Foundation, and the National Bone Health Alliance (NBHA), among others [1, 2]. The scientific triad of standardization, harmonization, and more comprehensive reference population databases are vital steps towards optimization of bone turnover markers for the management of osteoporosis. This position paper is one of two papers: this paper will focus on the current approaches and needs for the advancement of bone turnover markers, while the second paper will explore the scientific data and identify future research needs to help advance this field.
This position paper sets out to review:
How the NBHA, building on the recommendations of the IOF/IFCC Bone Markers Working Group, will execute a project involving experts from academia, government, non-profit organizations, and industry to help assure health professionals of the clinical utility of bone turnover markers;
The current clinical approaches concerning osteoporosis and the status and use of bone turnover markers in the USA;
The rationale for focusing this effort around two specific bone turnover markers: N-terminal propeptides of type I procollagen (s-PINP) and C-terminal cross-linking telopeptides of type 1 collagen (s-CTX);
The need to standardize bone marker sample collection procedures, reference ranges, and bone turnover marker assays in clinical laboratories; and
The importance of harmonization for future research of bone turnover markers.
Previous and ongoing efforts by other groups
Recently, the IOF/IFCC Bone Markers Working Group [3] reviewed the literature to determine the clinical potential of bone turnover markers, which includes the prediction of fracture risk and monitoring the treatment of osteoporosis. The working group also provided recommendations for clinical use and set an appropriate research agenda.
The IOF/IFCC working group also identified one bone resorption marker (serum C-terminal telopeptide of type I collagen) and one bone formation marker (serum procollagen type I N propeptide) to be used as reference markers and measured by standardized assays in observational and intervention studies. This was suggested to compare the performance of alternatives and to enlarge the international experience of the application of markers to clinical medicine. Based on these recommendations, only these two markers, s-PINP and s-CTX, will be addressed in this paper. The origins of these markers are shown in Fig. 1.
Fig. 1.
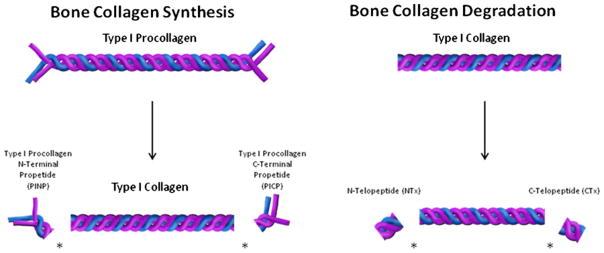
During new bone formation, type I procollagen is a triple helical structure composed of two pro alpha-1 chains shown in purple and one pro alpha-2 chain shown in blue. Type 1 procollagen is made in the osteoblast and secreted into new bone matrix. In the bone matrix, procollagen peptidases cleave off PINP from the amino (N) terminal end and PICP from the carboxy (C)-terminal end of type 1 procollagen resulting in mature type 1 collagen. PINP and PICP make their way into the circulation where their concentration provides information regarding new bone formation. PINP has been selected as the reference marker of bone formation
During bone resorption, type 1 collagen is degraded by cathepsin K to cleave serum s-NTX from the amino (N)-terminal end and s-CTX from the carboxy (C)-terminal end. s-NTX and s-CTX make their way into the circulation where their concentration provides information regarding the level of ongoing bone resorption. s-CTX has been selected as the reference marker of bone resorption. Note: figure is not to scale.
The IOF/IFCC reviewed a few reports that supported the potential of high levels of bone turnover markers to predict fracture risk independently from bone mineral density in postmenopausal women, but highlighted the need for additional research regarding the utility of bone turnover markers before widespread clinical practice use. The review did find that bone turnover markers provide pharmacodynamic information of response of osteoporosis treatment and proposed their usefulness for monitoring treatment in individual patients. However, the clinical utility of bone turnover markers for monitoring treatment in patients is suboptimal for multiple reasons. These include inadequate quality control, limited data comparing the impact of bone turnover changes with treatments over time and biological/analytical variability, and inadequate normative reference population databases.
The IOF/IFCC working group also recommended the development of a reference measurement system. Secondary reference materials will be produced and distributed to the manufacturers of commercial assays in order to calibrate and audit reference measurement procedures which can then be traced to the primary reference material. A reference measurement system consisting of a reference method (i.e., a measurement procedure based on isotope dilution mass spectrometry) and primary reference materials (i.e., highly purified s-CTX) is currently not available to assign target values to secondary reference materials.
As an interim solution, the IOF/IFCC recommended a strategy of harmonization of assays involving comparison studies between different routine clinical assays. This would be done by distributing a panel of human samples which would be compared to the overall mean for all assays. This would identify bias for each commercial assay which can then be corrected to obtain a consensus mean and harmonization of results. This stepwise approach of first harmonizing using an overall mean and then standardizing bone marker measurements by using primary reference materials would advance the application of bone turnover markers in clinical medicine worldwide.
Current approaches and bone turnover markers
Bone mineral density testing is often performed every 1 to 2 years to monitor and/or detect possible bone loss, predict future fracture risk, and measure response to pharmacological therapy. Bone turnover markers may assess a response to treatment earlier than bone mineral density testing to assist clinicians in the management of their patients. Over time, bone mineral density changes slowly in most, but not all, metabolic bone disease states. There has been increased interest in combining these two assessments harmoniously to manage osteoporosis as well as other metabolic bone disease states.
One potential advantage of using bone turnover markers in clinical practice is that they could be used to detect treatment efficacy sooner than dual X-ray absorptiometry (DXA). Early changes in bone turnover markers could be applied to measure the clinical efficacy of an antiresorptive and/or an anabolic treatment, and are valuable to reinforce patient compliance to treatment regimens. Bone turnover markers could also be potentially used as an adjunct to bone density in making clinical decisions to initiate therapy or monitor bone turnover when drug therapy is terminated. All of these monitoring strategies should be aimed at reducing the morbidity and mortality caused by osteoporotic fractures. Bone turnover markers could also be utilized in clinical practice to monitor adherence to therapy and provide feedback to health professionals and patients about whether to continue or change their treatment regimen(s).
Research and development over the past decade have generated sophisticated, widely available bone turnover markers which measure proteins metabolites released from the bone in the breakdown phase of resorption or the renewal phase of formation of bone. For several years, biochemical markers have been used in clinical trials as supportive data and registration for documenting the effects of drugs on the skeleton and in particular on skeletal remodeling. However, shortcomings include considerable short-and long-term biological fluctuations (e.g., diurnal variability), lifestyle and diet, as well as technical variability (inter-and intra-laboratory sample collection and analysis). These shortcomings need to be addressed to optimize the use of bone turnover markers in clinical practice.
Current regulatory status of markers in the USA
The s-PINP assays may be offered as total s-PINP assays which measure both the trimeric and monomer forms of PINP or as intact s-PINP assays that measure the trimeric isomer alone. To date, the FDA has cleared only one serum PINP assay in the USA, the intact s-PINP assay manufactured by Orion Diagnostica utilizing a manual radioimmunoassay.
A fully automated total s-PINP assay manufactured by Roche Diagnostics is available in Europe. More recently, Immunodiagnostic Systems (IDS) introduced a fully automated s-PINP assay that measures the intact trimeric form alone. Since these s-PINP assays employ different antibodies, differences in specificity may be observed in samples with varying trimeric and monomer ratios in certain disease states (i.e., renal failure) and storage conditions. In addition, the PINP trimer is the only form that is not affected as renal function declines.
Regarding s-CTX, both Roche Diagnostics and IDS offer fully automated assays on their respective platforms in the USA as well as Europe. These s-CTX assays employ the same primary (capture) antibody and detect the C-terminal octapeptide.
Pre-analytical sources of variation and control
The successful use of bone turnover markers in clinical trials and practice requires maximizing the accuracy and precision of the assays. To that effect, sources of pre-analytical and analytical variation need to be controlled to facilitate the detection of any true change in bone turnover markers. To ensure accuracy, samples for bone turnover markers must be collected, processed, and stored following established standardized procedures.
Pre-analytical sources of variation in bone turnover marker measurements may be divided into controllable variations such as circadian rhythm, meal status and exercise, as well as uncontrollable variations such as age, menopausal status, gender, and disease state [3]. Among the controllable variations, circadian rhythm is a key contributor, especially in resorption markers such as s-CTX. Peak s-CTX levels that normally occur in the early morning hours can be twice as high as the trough levels that occur in the mid-afternoon [4].
Therefore, it is critical that resorption markers be collected consistently at the same time of the day, preferably early morning, so current reference ranges based on similar collection conditions may be used. This also ensures that serial measurements will have clinical meaning. Another important pre-analytical consideration is the marked influence of s-CTX by meal status. Therefore samples must be collected in the fasted state. Most bone formation markers have relatively small circadian variation and little impact by food intake [5]. Nevertheless, when s-CTX, a resorption marker, and s-PINP, a formation marker, are measured in the same sample, early morning samples after an overnight fast are recommended.
Bone turnover marker samples should be stored appropriately and analyzed within a defined stability time period to avoid analyte deterioration. Because of the robustness of s-PINP, it may be analyzed when required collection conditions for s-CTX are not met. Careful pre-analytical control will ensure consistent bone turnover marker results.
EDTA plasma is the preferred sample type for s-CTX because of its superior analyte stability. The use of EDTA plasma is especially helpful when stringent sample handling conditions cannot be guaranteed. Although Roche Diagnostics and IDS both list serum and EDTA plasma as acceptable sample types for s-PINP, Orion Diagnostica recommends only serum in their FDA-cleared radio immunoassay package insert.
Analytical control
The accuracy of measurements depends on the antibody specificity, calibrator standardization, the analytical platform, and other assay specifics. Analytical sources of variation are related to the assays used and the execution of the measurements.
Intra-assay (within run) and inter-assay (among run) precision is normally monitored by including three levels of quality control samples with each test run of patient samples. The matrix of the control samples should be similar to that of patient samples in order to detect assay performance issues that include reagents, calibrations and instrument and operator techniques. In clinical trial support, serial bone turnover marker samples from each subject are usually analyzed together in a batch to ensure consistency. In clinical practice, where batch analysis is often not an option, it is crucial that acceptable inter-assay precision be maintained.
Assay accuracy must be ensured through external surveillance (external proficiency testing, whereby blinded samples are analyzed on a regular schedule). This ensures the short-term and long-term consistency of assay performance. Among lab standardization to have the same accuracy target is essential for a universal reference range and proper medical decision points to be adopted.
Requirements to ensure clinical utility
In addition to the requirements for establishing an assay’s analytic validity, it is also essential to address the assay’s clinical validity and utility, which ultimately guide the clinical use of a test. The definition of clinical validity is how well a test detects the presence, absence, or risk of a disease or condition. Clinical validity is largely dependent on the intrinsic properties of the test. Clinical utility, on the other hand, indicates whether a test is relevant to clinical practice. Thus, for a test to have clinical utility, it must provide information about the diagnosis, prognosis, prevention, treatment, management, or disease outcomes. Table 1 contains a list of examples of conditions for ensuring clinical utility of a bone turnover marker assay.
Table 1.
Conditions for ensuring the clinical utility of a bone turnover marker assay
| Condition for clinical utility | Potential application to osteoporosis |
|---|---|
|
|
Harmonization
Because of the lack of a reference measurement system, bone marker measurements cannot be calibrated and linked to a higher order standard such as primary reference material or reference measurement procedure. Therefore, bone marker measurements cannot be standardized at this time. However, through a harmonization approach, bone marker measurements can be linked to panels of human samples that have values assigned through a generally agreed upon process, such as the overall mean obtained from different measurement procedures. Standardization and harmonization will lead to measurement results comparable across laboratories, time, and location.
Comparable results are needed to be able to:
Detect the effect of treatments in patients over time independent of the laboratory that perform the measurements;
Compare a result from a patient to a reference range that was generated by the laboratory at an earlier time point or by another laboratory; and
Guide treatment decisions by comparing a patient result to a clinical decision point that was defined using bone marker data obtained in a research laboratory.
Studies assessing the comparability of bone turnover marker measurements performed in clinical and research laboratories found high variability among laboratories and assays [6, 7]. This variability profoundly limits the use of research findings in patient care and prevents the formulation and implementation of clinical practice guidelines.
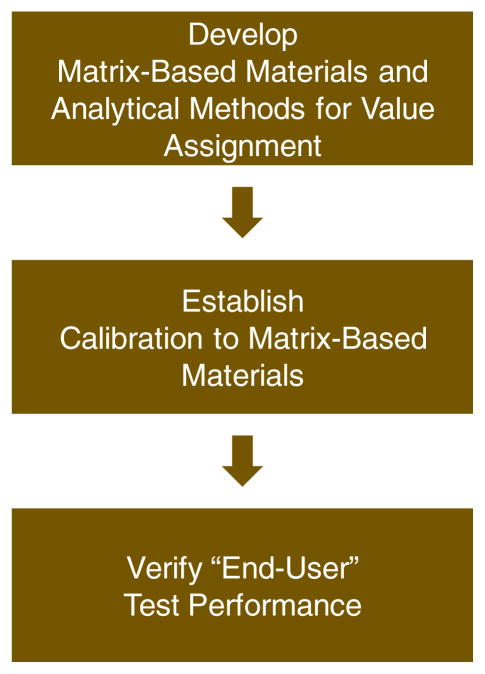
These problems in measurement variability can be minimized through a formal process that first harmonizes bone turnover marker measurements and later allows to standardize them once a reference measurement system is in place. The proposed process is in line with the IOF/IFCC-recommended strategy. It was successfully applied to a number of clinical analytes and can be adopted for harmonizing bone turnover marker measurements. It can be divided into three basic steps (Fig. 2).
Fig. 2.
Basic steps to harmonization for biochemical bone markers
In the first step, a measurement harmonization system is created consisting of special analytical methods and panels of human samples. These special analytical methods are used to assign target concentrations to the human samples. Because these analytical methods and human samples form a point of reference for bone turnover marker measurements, their performance such as precision, recovery, and specificity need to be well defined and at a higher level than the performance of analytical methods used in patient care. The human samples are intended for use as calibrators and controls of routine assays in step 2.
In the second step, routine assays are calibrated using the human samples developed in step 1. After the assay is calibrated, its calibration is verified through blinded challenges performed regularly. For automated immunoassays systems, this calibration step is commonly performed by the assay manufacturer who calibrates the in-house master assay and uses the master assay to calibrate the routine assay.
Calibration of assays and the verification of calibration is a collaborative activity conducted between the assay manufacturer and an independent organization performing the challenges and certifying appropriateness of calibration. Examples of successful collaborations include the Centers for Disease Control and Prevention (CDC) Cholesterol Reference Method Laboratory Network [6], and by the National Glycohemoglobin Standardization Program [7].
In the third step, the measurement performance of the end user assay is assessed to verify that calibrations of end user assays performed by the assay manufacturers are done correctly and lead to consistent and comparable measurement results in patient care and research. This can be accomplished by performing informal inter-laboratory comparison studies, using data from accuracy-based proficiency testing programs, and monitoring data from appropriate quality assurance materials used in laboratories.
An example of the latter is the CDC Lipid Standardization Program [6], which provides serum materials to clinical and research laboratories, monitors measurement performance over time, and certifies laboratories when they meet certain performance criteria. It is important to point out that such performance assessments can only be useful if the materials used in the assessment process are commutable [8]. In other words, the materials must demonstrate the same performance with the respective assays as do actual patient samples. The issue of commutability of serum materials can be avoided by well-characterized panels of patient samples.
It needs to be emphasized that reagents, calibrator lots, and instrumentation change over time, and each change requires new calibration and verification of measurement performance. Otherwise, comparability of measurement results cannot be assured over time and measurement harmonization cannot be maintained.
In conclusion, the harmonization of bone turnover marker measurements is technically feasible and can be accomplished by adopting procedures successfully used for other analytes such as blood lipids and HbA1c. Additional guidance for implementing harmonization was developed by the American Association of Clinical Chemistry [9] and is currently being refined. Guidance documents to establish specific components needed in this process such as commutable reference materials are available from organizations such as the Clinical and Laboratory Standards Institute [10].
Thus, basic steps for harmonization of bone turnovers markers include the development of matrix-based material and analytical methods for value assignment, establishment of calibration to matrix-based materials, and verification of end user test performance.
Reference population databases
Young healthy
There is a need to establish robust bone turnover marker reference ranges for both healthy young and older women as well as healthy young and older men. Currently, reference ranges have been established utilizing either the Roche Diagnostics (Elecsys) or Immunodiagnostic (IDS) immunoassay systems separately and never in the same population using both devices [11–14].
There are many justifications for first establishing more robust normal reference ranges in a young healthy population. In order to define an “abnormal” range, one first has to know what defines a “normal” range. The fewer the biological variables introduced into that definition, the more scientifically valid the normal reference range becomes.
Though reference databases for bone turnover markers have been published, additional data are needed, especially regarding males and older age groups. There is no data establishing a consistent reference population database at any age measured in the same population by these two currently commercially available devices [15–22]. In addition, in order for clinicians to know whether two serial bone turnover marker measurements are different, there must be data establishing the least significant change, which has currently never been performed simultaneously on both immunoassay machines in the same population [19–22]. Serial bone turnover marker measurements require knowledge of the least significant change in order to interpret whether or not a change is significant or nonsignificant.
Defining normal reference ranges provides the basis to start to address several practical and scientific needs [23]:
Building level of trust in bone turnover marker results provided by laboratories to clinicians;
Relating a level of bone turnover markers (elevated) to short-term as well as long-term fracture risk in untreated patients;
Predicting rates of change (loss) in bone mineral density in untreated patients; and
Determining what change in bone turnover markers is necessary to conclude that an appropriate pharmacologic response to an osteoporosis therapy has been met, either defined by increase in bone mineral density or better yet reductions in fracture risk [24–50].
Young normal persons with no disease that may affect bone will have fewer abnormal biological variables that may affect bone turnover markers. Bone loss is minimal in the younger age range from age 25 to 35 for young women and men. The lower end of the age range avoids the period of “consolidation,” and the upper end avoids the early changes of menopause in women. Larger sample size for reference intervals for young individuals may allow us to better interpret the relevance of clinical trial bone turnover marker data.
Older healthy
While premenopausal or young male reference range determinations have value, this range may not necessarily be the correct range for clinical management decisions in older adults. Predicting rates of bone loss, risk for fracture, or effect of pharmacological treatment using a normal, healthy young person’s reference range may not be the appropriate age range.
For example, a potential major use of s-CTX and s-PINP is the identification of untreated patients that are at an increased rate of bone loss or fracture risk. This application would require data acquired mostly in older persons [24–30]. There are other scientific reasons why an older population normal reference range for bone turnover markers has merit, and requires defining as well.
These include:
An older healthy population could exclude the lower age of 60 years to avoid the changes of early menopause and the upper age limit of 75 years to exclude very elderly people who commonly have comorbidities that accelerate bone loss.
Defining the age-matched levels of the population for whom therapy is targeted in much the same way as age-matched data may be valuable for the use of bone densitometry.
Having knowledge of the reference ranges of younger healthy and older healthy age groups provides a foundation for studying the relationships between drug discontinuation, bone turnover, and bone strength.
Reference intervals for postmenopausal women and older men are needed to detect changes in bone turnover markers with age that relate to renal clearance of the marker, since renal function declines with age. The data relating bone turnover markers to strength as a function of renal function are just beginning to be defined [51, 52].
Thus, once an adequately powered reference population database is established for all ages, future studies can advance the understanding of the relationship between bone turnover marker levels or changes to outcomes. We will also be able to know how the results compare between immunoassay devices (Roche and IDS), with the hope of avoiding the limitations that currently exist in clinical practice when trying to compare bone mineral density results from different DXA manufacturers.
Summary, conclusions, and next steps
Using bone turnover markers to personalize patient care for those with osteoporosis provides complimentary information in conjunction with the use of bone mineral density measurements. The meritage of standardization and harmonization of sample collection, measurement procedures, and reference ranges in the appropriate populations are critical to optimizing the potential benefits of bone marker use. Following this work, many practical questions will need to be addressed concerning how to use markers in the day-to-day management of patients.
These efforts that need to come to fruition concerning the field of bone turnover markers can positively impact clinical practice, health care costs, and reimbursement decisions, as well as solidifying research efforts to help the bone turnover marker field. With these efforts, we hope that bone markers can be more fully utilized by clinicians and work in tandem with the other currently available approaches to manage bone diseases.
Osteoporosis is a major global health problem with the prevalence and incidence of osteoporosis for at-risk populations estimated to be 44 million Americans. In the USA, osteoporotic fractures currently cost US$19 billion annually; these costs are projected to rise to over US$25 billion by the year 2025 [53]. The annual incidence of osteoporotic fractures in women is greater than the combined rates of heart attack, stroke, and breast cancer. The potential of bone markers as an additional tool for health professionals to improve patient outcomes by impacting morbidity and mortality is crucial in providing better healthcare and addressing rising healthcare costs.
The goals elucidated in this position paper will be addressed by the National Bone Health Alliance, a public–private partnership on bone health that includes the involvement of the governmental, academic, for-profit and nonprofit sectors, including the major academic and commercial laboratories, to collectively agree on a common, harmonized approach to address the pre-analytical and analytical variables identified as well as the standardization of bone marker sample collection procedures, reference ranges, and bone turnover marker assays in clinical laboratories. As stated, a second paper will be produced to help elucidate the research efforts to date and will hope to identify gaps and potential research projects to answer additional needs in the bone marker field. In addition, NBHA will pursue a parallel effort to make bone turnover marker measurements reliable and accepted by all health professionals for facilitating management decisions upon project completion and ultimately be reimbursed by all health insurance payers.
Footnotes
Conflicts of interest None.
Contributor Information
D. Bauer, Email: dbauer@psg.ucsf.edu, University of California, San Francisco, USA
J. Krege, Eli Lilly and Co., Inc., Indianapolis, USA
N. Lane, University of California Davis Health System, Sacramento, USA
E. Leary, Pacific Biomarkers, Seattle, USA
C. Libanati, Amgen, Thousand Oaks, USA
P. Miller, Colorado Center for Bone Research, Lakewood, USA
G. Myers, American Association for Clinical Chemistry, Washington, USA
S. Silverman, Cedars-Sinai Medical Center, Los Angeles, USA
H. W. Vesper, Centers for Disease Control and Prevention, Atlanta, USA
D. Lee, National Bone Health Alliance, Washington, USA
M. Payette, Roche Diagnostics, Indianapolis, USA
S. Randall, National Osteoporosis Foundation, Washington, USA
References
- 1.NOF. NOF clinician’s guide to prevention and treatment of osteoporosis. NOF; Washington: 2010. [Google Scholar]
- 2.Schafer ALL, Vittinghoff E, Ramachandran R, Mahmoud N, Bauer DC. Laboratory reproducibility of biochemical markers of bone turnover in clinical practice. Osteoporos Int. 2010;21:439–455. doi: 10.1007/s00198-009-0974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporosis Int. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 4.Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type 1 collagen (serum s-CTX): effects of gender, age, menopausal status, posture, daylight, serum cortisol and fasting. Bone. 2002;31:57–61. doi: 10.1016/s8756-3282(02)00791-3. [DOI] [PubMed] [Google Scholar]
- 5.Clowes JA, Hannon RA, Yap TA, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002;30:886–890. doi: 10.1016/s8756-3282(02)00728-7. [DOI] [PubMed] [Google Scholar]
- 6.Rifai N, Cooper GR, Brown WV, Friedewald W, Havel RJ, Myers GL, Warnick GR. Clinical chemistry journal has contributed to progress in lipid and lipoprotein testing for fifty years. Clin Chem. 2004;50:1861–1870. doi: 10.1373/clinchem.2004.038976. [DOI] [PubMed] [Google Scholar]
- 7.Little RR, Rohlfing CL, Sacks DB National Glycohemoglobin Standardization Program (NGSP) Steering Committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57:205–214. doi: 10.1373/clinchem.2010.148841. [DOI] [PubMed] [Google Scholar]
- 8.Miller WG, Myers GL, Rej R. Why commutability matters. Clin Chem. 2006;52:553–554. doi: 10.1373/clinchem.2005.063511. [DOI] [PubMed] [Google Scholar]
- 9.Miller WG, Myers GL, Gantzer LM, Kahn SE, Schönbrunner ER, Thienpont LM, Bunk DM, Christenson RH, Eckfeldt JH, Lo SF, Nübling CM, Sturgeon CM. Roadmap for harmonization of clinical laboratory measurement procedures. Clin Chem. 2011;57:1108–1117. doi: 10.1373/clinchem.2011.164012. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute (CLSI) [Accessed 16 Jan 2012]; www.CLSI.org.
- 11. [Accessed 16 Jan 2012];Serum CrossLaps®ELISA, Immunodiagnostic systems, Inc. technical manual. http://www.idsplc.com.
- 12.CrossLaps/Serum, Roche Diagnostics. [Accessed 16 Jan 2012];Technical manual. http://www.roche.com.
- 13. [Accessed 16 Jan 2012];intact PINP, Immunodiagnostic Systems, Inc. technical manual. http://www.idsplc.com.
- 14. [Accessed 16 Jan 2012];total PINP, Roche Diagnostics technical manual. http://www.roche.com.
- 15.Glover SJ, Garnero P, Naylor K, Rogers A, Eastell R. Establishing a reference range for bone turnover markers in young, healthy women. Bone. 2008;42:623–630. doi: 10.1016/j.bone.2007.12.218. [DOI] [PubMed] [Google Scholar]
- 16.Glover S, Gall M, Schoenborn-Kellenberger O, Wagener M, Garnero P, Boonen S, Cauley J, Black D, Delmas P, Eastell R. Establishing a reference interval for bone turnover markers in 637 healthy, young, pre-menopausal women from UK, France, Belgium and the USA. J Bone Miner Res. 2009;24:389–397. doi: 10.1359/jbmr.080703. [DOI] [PubMed] [Google Scholar]
- 17.de Papp AE, Bone HG, Caulfield MP, Kagan R, Buinewicz A, Chen E, Rosenberg E, Reitz RE. A cross-sectional study of bone turnover markers in healthy premenopausal women. Bone. 2007;40:1222–1230. doi: 10.1016/j.bone.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Adami S, Bianchi G, Brandi ML, Giannini S, Ortolani S, Dimunno O, Frediani B, Rossini M. Determinants of bone turnover markers in healthy premenopausal women. Calcif Tissue Int. 2008;82:341–347. doi: 10.1007/s00223-008-9126-5. [DOI] [PubMed] [Google Scholar]
- 19.Cavalier E, Delanaye P. Letter to the editor: Defining a “reference population”: no easy task. JBMR. 2009;24:1638. doi: 10.1359/jbmr.090322. [DOI] [PubMed] [Google Scholar]
- 20.Eastell R, Glover SJ, Gall M, Schoenborn-Kellenberger O, Garnero P, Black D. Defining a “reference population”: no easy task. JBMR. 2009;24:1639. doi: 10.1359/jbmr.090322. [DOI] [PubMed] [Google Scholar]
- 21.Linnet K. Two-stage transformation systems for normalization of reference distributions evaluated. Vlin Vhrm. 1987;33:381–386. [PubMed] [Google Scholar]
- 22.Baim S, Miller PD. Perspective: assessing the clinical utility of serum CTX in postmenopausal osteoporosis and its use in predicting risk of osteonecrosis of the jaw. J Bone Miner Res. 2009;24:561–573. doi: 10.1359/jbmr.090203. [DOI] [PubMed] [Google Scholar]
- 23.Keaveny TM, Kopperdahl DL, Melton LJ, III, Hoffman PF, Amin S, Riggs BL, Khosla S. Age-dependence of femoral neck strength in white women and men. J Bone Miner Res. 2010;25(5):994–1001. doi: 10.1359/jbmr.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, McClung MR, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA IOF-IFCC Bone Markers Standard Working Group. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 25.Melton LJ, III, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res. 1997;12:1083–1091. doi: 10.1359/jbmr.1997.12.7.1083. [DOI] [PubMed] [Google Scholar]
- 26.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–1536. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 27.Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20:1813–1819. doi: 10.1359/JBMR.050609. [DOI] [PubMed] [Google Scholar]
- 28.Garnero P. Markers of bone turnover for the prediction of fracture risk. Osteoporosis Int. 2000;11(Suppl 6):S55–S65. doi: 10.1007/s001980070006. [DOI] [PubMed] [Google Scholar]
- 29.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 30.Ivaska KK, Gerdhem P, Vaananen HK, Akesson K, Obrant KJ. Bone turnover markers and prediction of fracture: a prospective follow-up study of 1,040 elderly women for a mean of 9 years. J Bone Miner Res. 2010;25:393–403. doi: 10.1359/jbmr.091006. [DOI] [PubMed] [Google Scholar]
- 31.Bauer DC, Garnero P, Harrison SL, Cauley JA, Eastell R, Ensrud KE, Orwoll E. Osteoporotic fractures in men (MrOs) Research Group. Biochemical markers of bone turnover, hip bone loss, and fracture in older men: the MrOs Study. J Bone Miner Res. 2009;24(12):2032–2038. doi: 10.1359/JBMR.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, Greenspan SL. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int. 2000;66:100–103. doi: 10.1007/pl00005830. [DOI] [PubMed] [Google Scholar]
- 33.Recker R, Stakkestad JA, Chesnut CH, III, Christiansen C, Skag A, Hoiseth A, Ettinger M, Mahoney P, Schimmer RC, Delmas PD. Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone. 2004;34:89–93. doi: 10.1016/j.bone.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Papapolpous S, Schimmer RC. Changes in bone remodeling and anti-fracture efficacy of intermittent bisphosphonate therapy: implications from clinical studies with ibandronate. Ann Rheum Dis. 2007;66(7):853–858. doi: 10.1136/ard.2006.064931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 36.Christiansen C, Riis BJ, Rodbro P. Prediction of rapid bone loss in postmenopausal women. Lancet. 1987;1:1105–1108. doi: 10.1016/s0140-6736(87)91671-0. [DOI] [PubMed] [Google Scholar]
- 37.Lenora J, Ivaska KK, Obrant KJ, Gerdhem P. Prediction of bone loss using biochemical markers of bone turnover. Osteoporos Int. 2007;18:1297–1305. doi: 10.1007/s00198-007-0379-z. [DOI] [PubMed] [Google Scholar]
- 38.Rogers A, Hannon RA, Eastell R. Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res. 2000;15:1398–1404. doi: 10.1359/jbmr.2000.15.7.1398. [DOI] [PubMed] [Google Scholar]
- 39.Greenspan SL, Resnick NM, Parker RA. Early changes in biochemical markers of bone turnover are associated with long-term changes in bone mineral density in elderly women on alendronate, hormone replacement therapy, or combination therapy: a three-year, double-blind, placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab. 2005;90:2762–2767. doi: 10.1210/jc.2004-1091. [DOI] [PubMed] [Google Scholar]
- 40.Hannon RA, Bluhmson A, Naylor KE, Eastell R. Response of biochemical markers of bone turnover to hormone replacement therapy. J Bone Miner Res. 1998;13:1124–1133. doi: 10.1359/jbmr.1998.13.7.1124. [DOI] [PubMed] [Google Scholar]
- 41.Kim SW, Park DJ, Park KS, Kim SY, Cho BY, Lee HK, Shin CS. Early changes in biochemical markers of bone turnover predict bone mineral density response to antiresorptive therapy in Korean postmenopausal women with osteoporosis. Endocr J. 2005;52:667–674. doi: 10.1507/endocrj.52.667. [DOI] [PubMed] [Google Scholar]
- 42.Ravn P, Clemmesen B, Christiansen C. Biochemical markers can predict the response in bone mass during alendronate treatment in early postmenopausal women. Alendronate Osteoporosis Prevention Study Group. Bone. 1999;24:237–244. doi: 10.1016/s8756-3282(98)00183-5. [DOI] [PubMed] [Google Scholar]
- 43.Ravn P, Hosking D, Thompson D, Cizza G, Wasnich RD, McClung M, Yates AJ, Bjarnason NH, Christiansen C. Monitoring of alendronate treatment and prediction of effect on bone mass by biochemical markers in the early postmenopausal intervention cohort study. J Clin Endocrinol Metab. 1999;84:2363–2368. doi: 10.1210/jcem.84.7.5847. [DOI] [PubMed] [Google Scholar]
- 44.Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res. 1998;13:1431–1438. doi: 10.1359/jbmr.1998.13.9.1431. [DOI] [PubMed] [Google Scholar]
- 45.Greenspan SL, Rosen HN, Parker RA. Early changes in serum N-telopeptide and C-telopeptide cross-linked collagen type 1 predict long-term response to alendronate therapy in elderly women. J Clin Endocrinol Metab. 2000;85:3537–3540. doi: 10.1210/jcem.85.10.6911. [DOI] [PubMed] [Google Scholar]
- 46.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–1056. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 47.Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, Thompson DE, Ewing SK, Delmas PD. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004;19:1250–1258. doi: 10.1359/JBMR.040512. [DOI] [PubMed] [Google Scholar]
- 48.Reginster JY, Sarkar S, Zegels B, Henrotin Y, Bruyere O, Agnusdei D, et al. Reduction in PINP, a marker of bone metabolism, with raloxifene treatment and its relationship with vertebral fracture risk. Bone. 2004;34:344–351. doi: 10.1016/j.bone.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Reginster JY, Collette J, Neuprez A, Zegels B, Deroisy R, Bruyere O. Role of biochemical markers of bone turnover as prognostic indicator of successful osteoporosis therapy. Bone. 2008;42:832–836. doi: 10.1016/j.bone.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH. PINP as an aid for monitoring patients treated with teriparatide. Bone. 2011;48:798–803. doi: 10.1016/j.bone.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Porter D, Pienowski D, Monier-Faugere MC, Mallluche H. Difference in bone quality between high versus low turnover renal osteodystrophy. JASN. 2012 doi: 10.1681/ASN.2010121253. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nickolas T, Cremers S, Zhang A, Stein E, Cohen A, Chauncey R, Nikkel L, Liu X, Boutroy S, Yin M, Staron R, Leonard M, McMahon D, Dworakowski E, Shane E. Biochemical markers of bone turnover discriminate fracture status in patients with pre-dialysis chronic kidney disease. JASN. 2012 (in press) [Google Scholar]
- 53.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]