Abstract
Background
Osteosarcoma is the most common primary bone cancer in growing adolescents and young adults. The prognostic role of C-reactive protein (CRP) in patients with osteosarcoma is not fully investigated. The purpose of this study is to perform a meta-analysis and literature review on the role of CRP in osteosarcoma and to assess the potential role of serum CRP as a prognostic factor for patients with osteosarcoma.
Methods
A detailed literature search was made in Medline for related research publications written in English. Methodological quality of the studies was also evaluated. The data were extracted and assessed by two reviewers independently. Analysis of pooled data were performed, risk ratio (RR) and corresponding confidence intervals (CIs) were calculated and summarized respectively.
Results
Final analysis of 397 patients from 2 eligible studies was performed. Combined RR of CRP expression suggested that the raised serum CRP level had an adverse prognostic effect on overall survival of patients with osteosarcoma (n = 397 in 2 studies; RR = 0.35; 95% CI: 0.18–0.68; p = 0.002). In the uni- and multivariate survival analysis, response rate and CRP levels were the only independent prognostic variables.
Conclusions
The results of this meta-analysis suggest that CRP expression confers a worse prognosis in patients with osteosarcoma. Large prospective studies are necessary to provide solid data to confirm the prognostic significance of CRP.
Introduction
The two most common primary bone malignancies, osteosarcoma (OS) and Ewing sarcoma (ES), are both aggressive, highly metastatic cancers that occur in children and young adults [1], [2]. OS originates from primitive bone-forming mesenchymal cells and ranks the eighth most common cancer of childhood in the United States. About 15–20% patients present with overt lung metastases at initial diagnosis and 40% patients develop metastases at a later stage [3], [4]. Despite multidisciplinary treatments including surgery, neoadjuvant and adjuvant chemotherapy, the overall five-year survival rate for OS remains 60–70% [5]. The mechanism underlying OS metastasis is still limited, neovascularization, invasion, anoikis resistance, chemoresistance, and evasion of the immune response are considered to be required for progression and metastasis in OS [6]. OS metastasis causes a pejorative prognosis [7], [8], [9]. In order to improve the clinical outcomes for patients with poor prognosis, it is urgent to find new diagnostic and therapeutic approaches to identify high-risk patients and block metastasis in this disease.
C-reactive protein (CRP) is an acute phase protein, which is produced principally by hepatocytes with serum level correlated with systemic inflammation [10]. It belongs to a highly conserved phylogenetically ancient family called pentraxins, which have five identical non-covalently linked subunits [11]. CRPs are characterized by their homopentameric structure and calcium-dependent ligand-binding affinity for the phosphocholine (PhC) moiety [12], [13]. The wide distribution of PhC in polysaccharides of pathogens and in cellular membranes enables CRP to recognize a range of pathogenic targets as well as membranes of damaged and necrotic host cells [13]. Expression of CRP is principally induced at the transcriptional level by interleukin-6 (IL-6), which can be synergistically enhanced by IL-1 and tumor necrosis factor (TNF). In humans, the CRP level is low (0.1–0.5 µg/ml) under normal conditions, but can increase its serum level up to 100-fold during systemic inflammation [12], which probably allows CRP to be the single most useful molecule for monitoring acute-phase reactions. CRP is a nonspecific but sensitive marker of inflammation. Increase CRP level is considered to be an important risk factor for inflammatory disease [14], atherosclerosis, myocardial infarction, peripheral vascular disease, and ischemic stroke [15], [16], [17], [18], [19], [20]. It is positively associated with weight loss, anorexia-cachexia syndrome, extent of disease, and recurrence in advanced/high-grade cancer [21], [22]. Its role as a predictor of survival has been shown in multiple myeloma, melanoma, lymphoma, ovarian, renal, pancreatic, and gastrointestinal tumors [17], [21], [23], [24], [25].
In this study, we sought to perform a meta-analysis and systematic review to evaluate whether CRP was correlated with outcome of OS patients. A pool of research data published between 2007 and 2013 on the study of the correlation between CRP and patients' survival, histological response to adjuvant chemotherapy and recurrence in OS were analyzed. Our analysis indicated that CRP had an unfavorable impact on OS patients' overall survival.
Methods
Search strategy and selection criteria
Medline, Pubmed and Web of Science were searched using the search terms: ‘c-reactive protein’, ‘prognosis’ and ‘osteosarcoma’. All searched data were retrieved. Authors' bibliographies and references of selected studies were also searched for other relevant studies. The most complete study was chosen to avoid duplication if same patient populations were reported in several publications.
Two reviewers estimated the eligibility of studies independently by reviewing titles and abstracts found by the search. The inclusion criteria included: 1) CRP evaluated in the primary OS tissues; 2) relationship displayed between CRP serum levels and OS clinicopathological variables or prognosis; 3) CRP serum levels detested by latex-enhanced immuno-turbidimetric test; 4) publications written in English; 5) data provided sufficient information to analyze risk ratio (RR) and 95% confidence interval (CI). The exclusion criteria included: 1) duplicate data set on the same patient populations; 2) articles published in non-English; 3) case reports, editorials, letters, reviews and conference abstracts. The detailed information of 13 relevant citations was listed in Table 1.
Table 1. All 13 citations identified through database searching.
| Author | Included/excluded | Comments |
| Mulrooney DA et al. 2013 [98] | Excluded | Pilot study of vascular health in survivors of osteosarcoma |
| Nakamura T et al. 2013 [5] | Included | Determine whether circulating CRP predicts survival in bone sarcoma |
| Peng TI et al. 2012 [99] | Excluded | Osteosarcoma 143B cells were utilized for study of mitochondrial dynamics |
| Fujiwara T et al. 2011 [100] | Excluded | A study of macrophage infiltration in prediction of human ewing sarcoma |
| Funovics PT et al. 2011 [22] | Included | A study of CRP as independent prognostic factor for survival in osteosarcoma patients |
| Jansson AF et al.2009 [101] | Excluded | A study of clinical score for nonbacterial osteitis in patients |
| Schwinger W et al. 2005 [102] | Excluded | A study of effect of interleukin-2 treatment in pretreated solid tumor patients including osteosarcoma |
| Patino WD et al. 2005 [103] | Excluded | A study of biomarkers such as osteosarcoma (FOS) gene in atherosclerosis |
| Das T et al. 2003 [97] | Excluded | A study of glycosylation in human CRP under different pathological conditions |
| Trapani S et al. 2000 [104] | Excluded | A study of incidence of occult cancer in children presenting with musculoskeletal symptoms |
| Kleinerman ES et al. 1995 [105] | Excluded | A study of combined therapy on patients with relapsed osteosarcoma |
| Asano T et al. 1993 [106] | Excluded | A study of a novel biologic agent for osteosarcoma therapy |
| Kleinerman ES et al. 1992 [107] | Excluded | Phase II study of liposomal muramyl tripeptide in osteosarcoma |
Data extraction and study assessment
Two reviewers (JY, DW) extracted data from selected studies independently. Any discontent was discussed and reached a consensus for all issues. The following items were collected from each study: first author's name, year of publication, country, number of patients, age, sex and CRP expression. Duplicated studies were avoided by checking the authors' name of the institutions.
Statistic analysis
Hazard ratio (HR) and its variance for each individual study were extracted or analyzed based on the published data according to the methods described previously [26], [27]. A HR>1 was regarded as a risk factor for worse survival in patients with elevated CRP serum levels. This impact of CRP on survival was regarded as statistically significant if the corresponding 95% CI for the summary HR did not overlap 1 unit. Odds ratios (OR) were utilized to measure the relationship of CRP and histological response to neoadjuvant chemotherapy, serum CRP level and tumor size. All P values were two sided. Statistical analyses were conducted using GraphPad Prism (GraphPad Software Inc., LaJolla, California; version 4.00, 2003).
Results
Study selection and characteristics
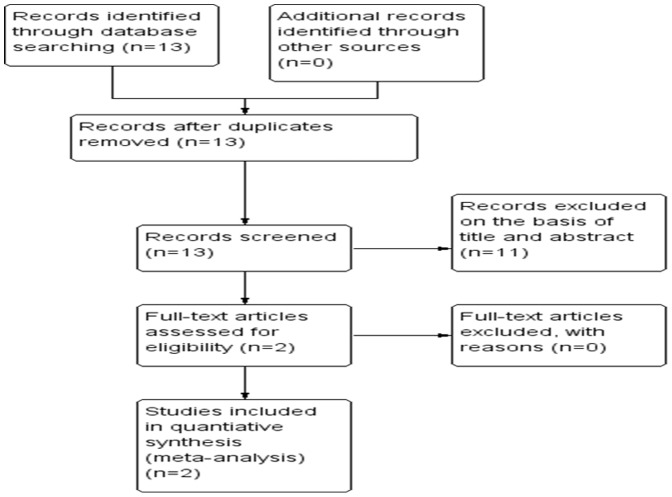
13 relevant citations were selected for initial review utilizing search strategies as described above. Of these, 11 were initially excluded after reading the abstracts (Figure 1). As a result, the systematic literature search generated a total of 2 datasets [5], [22] and 397 patients for ultimate analysis. These data were published from 2011 to 2013 to fit the inclusion criteria for our meta-analysis.
Figure 1. Flow chart of study selection.
A HR on 5 year disease-specific survival could be extracted from these studies, respectively. The survival data were summarized from 2 eligible studies [5], [22] and analyzed the correlation between CRP and histological response to neoadjuvant chemotherapy.
Methodological quality of the studies
2 selected studies [5], [22] were evaluated by two reviewers with high levels of methodological quality (>6 stars) according to Newcastle-Ottawa quality assessment scale [28].
Impact of CRP on EFS of OS patients
Two studies [5], [22] with a total of 397 OS patients regarding CRP and disease-specific survival were meta-analyzed. Because of heterogeneity (I 2 = 19%), a random effect model was used in this analysis. The pooled HR was 2.16, illustrating that CRP was significantly correlated with the poor survival of OS patients (Figure 2). The representative figure of the correlation between survival and CRP levels were shown as Figure 3.
Figure 2. Forest plot showing the association between CRP level and disease-specific survival of osteosarcoma.
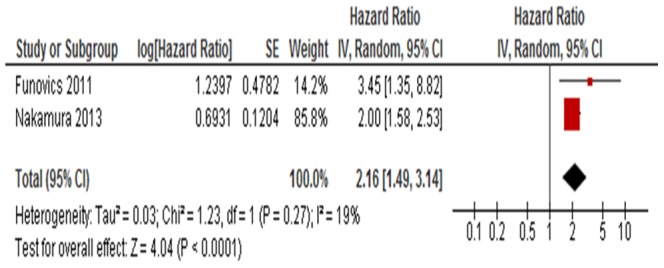
The summary HR and 95% CIs were shown (according to the random-effects estimations).
Figure 3. Representative figure of the correlation between survival and CRP levels.
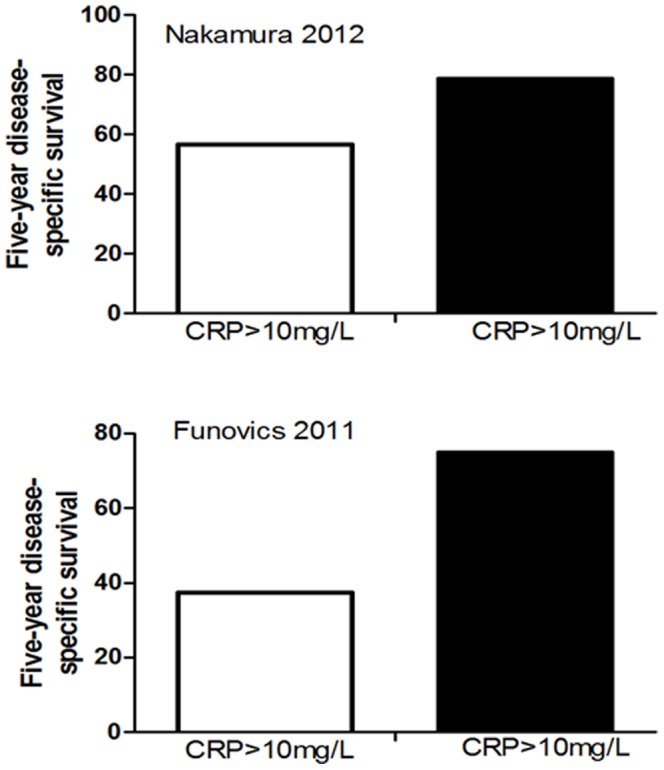
CRP and histological response to neoadjuvant chemotherapy
Two of the selected studies [5], [22] consisting of 397 patients dealt with the correlation between CRP and histological response to neoadjuvant chemotherapy. Due to heterogeneity (I 2 = 19%), a random effect model was selected for analysis. In the uni- and multivariate survival analysis, response rate and CRP levels were the only independent prognostic variables (Table 2).
Table 2. Univariate and multivariate analysis for predictive factors for survival (CI, confidence interval).
| Variable | Univariate p | Multivariate p | Hazard risk(95% CI) | |||
| Funovics | Nakamura | Funovics | Nakamura | Funovics | Nakamura | |
| Sex | 0.281 | 0.92 | 0.410 | 1.5 (0.6–3.7) | ||
| Age | 0.003 | 0.6 | 0.003 | 1.1 (1.0–1.1) | ||
| Tumor size | 0.153 | 0.002 | 0.267 | 0.1 | 1.0 (1.0–1.1) | 0.598 (0.32 to 1.115) |
| Histological subtype | 0.052 | 0.519 | 0.6 (0.1–3.3) | |||
| Tumor grade | 0.002 | |||||
| Response to chemotherapy | 0.002 | 0.04 | 0.024 | 10.8 (1.4–85.6) | ||
| C-reactive protein | <0.001 | <0.0001 | 0.031 | 0.02 | 1.4 (1.0–1.8) | 0.598 (0.333 to 0.892) |
Assessment of publication bias
Due to the less number of studies selected in our meta-analysis, we did not draft funnel plot to display publication bias.
Discussion
CRP is a representative acute phase reactant due to its rapid production and short half-life in circulation. CRP is mainly produced by hepatocytes in the liver in response to inflammatory cytokines, the level of CRP increases when there is inflammation throughout the body. Extrahepatic production of CRP has also been found in tumor cells, monocytes, lymphocytes and neuron as a part of local inflammation, but the amount is too little to affect serum CRP [21]. CRP is a sensitive but non-specific serum biomarker induced by infectious and non-infectious processes [29] including cancer and tissue damage [21]. In addition to its role as a very sensitive indicator of current disease activity for inflammation, the role of serum CRP has recently been re-evaluated by extending its clinical use to the diagnosis of cardiovascular diseases as well as risk prediction of cancer [21].
The linkage of inflammation and cancer has been extensively investigated since the first study reported by Virchow et al [30]. Actually inflammation is the seventh hallmark for cancer initiation and progression, it represents the linkage between intrinsic factors (oncogenes, genome instability) and extrinsic factors (immune and stromal components) [31]. Cancer cells in tumor tissues are always embedded by a persistent inflammatory state, called tumor microenvironment. The leucocytes, lymphocytes and macrophages along with cytokines/chemokines in tumor microenvironment serve as mediators to induce genomic instability, epigenetic changes and subsequent malignancy phenotype of cancer cells [32], [33]. CRP and other acute phase proteins are induced to be synthesized in hepatocytes by cytokines/chemokines and released into circulation. The circulating CRP acts on tumor cells, which lead to tumor cell lysis and facilitate tumor progression [21].
In most prevalent studies, serum CRP levels have been demonstrated to be highly elevated in cancer patients compared to healthy individuals. The prognostic significance of serum CRP was found in various cancer patients and in the advanced stage of cancers, including renal cell carcinoma [34], [35], [36], [37], [38], [39], [40], bladder [41], [42], [43], [44], breast [45], [46], [47], [48], [49], [50], stomach [51], , colon and rectum [56], , head and neck [62], [63], [64], esophagus [25], [65], [66], [67], [68], [69], prostate [70], [71], [72], [73], [74], lung [75], [76], [77], [78], [79] and pancreatic cancer [80], [81]. The high CRP levels have also been associated with aggressive diseases and poor survival outcomes for these malignancies. Its role as a predictor of survival has been shown in multiple myeloma, melanoma, lymphoma, ovarian, renal, pancreatic, and gastrointestinal tumors [23]. Its predictive ability for the survival and individualized therapy of patients with advanced cancers can be improved by combining CRP with other parameters such as tumor necrosis factor (TNF), serum albumin, and serum B12 [21], [82]
With similarity of the role of CRP in other malignancies, elevated level of CRP is also associated with poor prognosis of soft-tissue sarcoma patients [5], [83], [84]. This current review and meta-analysis demonstrated that CRP had an unfavourable impact on disease-specific survival of OS patients. Funovics et al [22] have revealed that pre-operative circulating CRP levels before tumor resection are associated with disease-specific outcomes, that is, patients with CRP levels higher than the cut-off value (1 mg/dl) had a significant worse survival. Therefore, the level of circulating pre-operative CRP was considered to be an independent prognostic factor for survival in patients with high-grade OS [22]. Similar observations were obtained for pre-treatment CRP by Nakamu et al, the elevated levels of pre-treatment CRP are demonstrated to be correlated with a decreased disease-specific survival and an increased rate of local recurrence in patients with a sarcoma of bone [5]. Although pre-treatment serum CRP is a significant prognostic factor and a monitor of tumor aggressiveness in OS patients, it does not apply to Ewing's sarcoma and chondrosarcom.
The reasons why CRP levels correlated with prognosis in tumor patients including OS remain to be determined. An experimental data obtained using transgenic mouse models provides a possible explanation, which showed that serum CRP levels are involved in host defenses against infections [85], [86], [87]. The strong correlation between CRP levels and cancer risk and/or poor prognosis may be due to (1) causality: elevated CRP levels cause cancer, (2) reverse causality: occult cancer increases CRP levels, (3) or confounding: a third factor, e.g. inflammation, increases both CRP levels and the risk of cancer [17], [88]. In addition, the chronic elevation of CRP observed in obese subjects may potentiate leptin resistance, contributing to the carcinogenesis, tumor progression and poor outcomes of tumor patients [89], [90], [91].
The CRP gene is located on the chromosome 1(1q21–q23). The generation of CRP in hepatocytes is induced at the transcriptional level by IL-6, and can be synergistically increased by IL-1β. The synergistic induction of CRP gene expression by IL-6 plus IL-1 requires transcriptional factors CCAAT/enhancer binging protein (C/EBP family α/β/δ) [92], [93], as well as signal transducers and activators of transcription (STAT3). The nuclear factor κB (NF-κB) subunits p50 and p65 through protein kinase C signaling pathway also participates in cytokine-induced CRP synthesis [94], [95]. In addition, transcriptional complex formation of c-Fos, STAT3, and hepatocyte NF-1 alpha is essential for cytokine-driven CRP gene expression [96]. As a result, the interaction of these transcriptional factors induce maximal production of CRP in hepatocytes. Alternatively the unique glycosylated molecular variants of CRP can be induced and subsequently influence it biological function in different clinical pathological conditions [97].
It's still unclear that the relationship between CRP and cancer is a causal one, since so many conditions can elevate CRP levels without increasing cancer risk. Some studies have been conducted to determine if elevated CRP levels and cancer are directly related. Many clinical findings reveal that CRP tends to increase in cancer patients, but the direct relation has not yet been established. The increase in CRP levels could be due to cancerous illness causing inflammation in the body or vise versa. Irrespective of relatively small number of patients, as well as the fact that the analysis was retrospective, the current meta-analysis indicated that the CRP levels can be used as a key indicator of prognosis for osteosarcoma. Nevertheless, additional research in the future especially larger prospective studies will be needed to provide more definitive answers to confirm these results. Overall, measurement of plasma CRP is simple, rapid, cost effective, suitable and provides valuable information for patients with cancer. We believe that development of agents decreasing CRP levels will provide a promising benefit in the prevention and treatment of many different types of human malignancies.
Supporting Information
PRISMA checklist.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Gill J, Ahluwalia MK, Geller D, Gorlick R (2013) New targets and approaches in osteosarcoma. Pharmacol Ther 137: 89–99. [DOI] [PubMed] [Google Scholar]
- 2. Sadikovic B, Park PC, Selvarajah S, Zielenska M (2013) Array comparative genomic hybridization in osteosarcoma. Methods Mol Biol 973: 227–247. [DOI] [PubMed] [Google Scholar]
- 3. Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, et al. (1997) Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. J Clin Oncol 15: 76–84. [DOI] [PubMed] [Google Scholar]
- 4. Ferguson WS, Goorin AM (2001) Current treatment of osteosarcoma. Cancer Invest 19: 292–315. [DOI] [PubMed] [Google Scholar]
- 5. Nakamura T, Grimer RJ, Gaston CL, Watanuki M, Sudo A, et al. (2013) The prognostic value of the serum level of C-reactive protein for the survival of patients with a primary sarcoma of bone. Bone Joint J 95-B: 411–418. [DOI] [PubMed] [Google Scholar]
- 6. Zhu L, McManus MM, Hughes DP (2013) Understanding the Biology of Bone Sarcoma from Early Initiating Events through Late Events in Metastasis and Disease Progression. Front Oncol 3: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mialou V, Philip T, Kalifa C, Perol D, Gentet JC, et al. (2005) Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer 104: 1100–1109. [DOI] [PubMed] [Google Scholar]
- 8. Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, et al. (2000) Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res 6: 572–577. [PubMed] [Google Scholar]
- 9. Dunn D, Dehner LP (1977) Metastatic osteosarcoma to lung: a clinicopathologic study of surgical biopsies and resections. Cancer 40: 3054–3064. [DOI] [PubMed] [Google Scholar]
- 10. Marnell L, Mold C, Du Clos TW (2005) C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol 117: 104–111. [DOI] [PubMed] [Google Scholar]
- 11. Shrive AK, Cheetham GM, Holden D, Myles DA, Turnell WG, et al. (1996) Three dimensional structure of human C-reactive protein. Nat Struct Biol 3: 346–354. [DOI] [PubMed] [Google Scholar]
- 12. Pepys MB (1981) C-reactive protein fifty years on. Lancet 1: 653–657. [DOI] [PubMed] [Google Scholar]
- 13. Volanakis JE (2001) Human C-reactive protein: expression, structure, and function. Mol Immunol 38: 189–197. [DOI] [PubMed] [Google Scholar]
- 14. Deodhar SD (1989) C-reactive protein: the best laboratory indicator available for monitoring disease activity. Cleve Clin J Med 56: 126–130. [DOI] [PubMed] [Google Scholar]
- 15. Devaraj S, Singh U, Jialal I (2009) The evolving role of C-reactive protein in atherothrombosis. Clin Chem 55: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340: 448–454. [DOI] [PubMed] [Google Scholar]
- 17. Heikkila K, Ebrahim S, Lawlor DA (2007) A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health 61: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jialal I, Devaraj S, Venugopal SK (2004) C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension 44: 6–11. [DOI] [PubMed] [Google Scholar]
- 19.Kushner I, Rzewnicki D, Samols D (2006) What does minor elevation of C-reactive protein signify? Am J Med 119: 166 e117–128. [DOI] [PubMed]
- 20. Venugopal SK, Devaraj S, Jialal I (2005) Effect of C-reactive protein on vascular cells: evidence for a proinflammatory, proatherogenic role. Curr Opin Nephrol Hypertens 14: 33–37. [DOI] [PubMed] [Google Scholar]
- 21. Wang CS, Sun CF (2009) C-reactive protein and malignancy: clinico-pathological association and therapeutic implication. Chang Gung Med J 32: 471–482. [PubMed] [Google Scholar]
- 22. Funovics PT, Edelhauser G, Funovics MA, Laux C, Berzaczy D, et al. (2011) Pre-operative serum C-reactive protein as independent prognostic factor for survival but not infection in patients with high-grade osteosarcoma. Int Orthop 35: 1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahmoud FA, Rivera NI (2002) The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep 4: 250–255. [DOI] [PubMed] [Google Scholar]
- 24. Hefler LA, Concin N, Hofstetter G, Marth C, Mustea A, et al. (2008) Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res 14: 710–714. [DOI] [PubMed] [Google Scholar]
- 25. Nozoe T, Saeki H, Sugimachi K (2001) Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg 182: 197–201. [DOI] [PubMed] [Google Scholar]
- 26. Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, et al. (2010) Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 375: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 28. Cota GF, de Sousa MR, Fereguetti TO, Rabello A (2013) Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 7: e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lyu YX, Yu XC, Zhu MY (2013) Comparison of the diagnostic value of procalcitonin and C-reactive protein after hematopoietic stem cell transplantation: a systematic review and meta-analysis. Transpl Infect Dis 15: 290–299. [DOI] [PubMed] [Google Scholar]
- 30. Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357: 539–545. [DOI] [PubMed] [Google Scholar]
- 31.Terlizzi M, Casolaro V, Pinto A, Sorrentino R (2014) Inflammasome: Cancer's friend or foe? Pharmacol Ther. [DOI] [PubMed]
- 32. Smith GR, Missailidis S (2004) Cancer, inflammation and the AT1 and AT2 receptors. J Inflamm (Lond) 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwartsburd PM (2003) Chronic inflammation as inductor of pro-cancer microenvironment: pathogenesis of dysregulated feedback control. Cancer Metastasis Rev 22: 95–102. [DOI] [PubMed] [Google Scholar]
- 34. Naito S, Kinoshita H, Kondo T, Shinohara N, Kasahara T, et al. (2013) Prognostic factors of patients with metastatic renal cell carcinoma with removed metastases: a multicenter study of 556 patients. Urology 82: 846–851. [DOI] [PubMed] [Google Scholar]
- 35. de Martino M, Klatte T, Seemann C, Waldert M, Haitel A, et al. (2013) Validation of serum C-reactive protein (CRP) as an independent prognostic factor for disease-free survival in patients with localised renal cell carcinoma (RCC). BJU Int 111: E348–353. [DOI] [PubMed] [Google Scholar]
- 36. Steffens S, Kohler A, Rudolph R, Eggers H, Seidel C, et al. (2012) Validation of CRP as prognostic marker for renal cell carcinoma in a large series of patients. BMC Cancer 12: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ito K, Yoshii H, Sato A, Kuroda K, Asakuma J, et al. (2011) Impact of postoperative C-reactive protein level on recurrence and prognosis in patients with N0M0 clear cell renal cell carcinoma. J Urol 186: 430–435. [DOI] [PubMed] [Google Scholar]
- 38. Saito K, Tatokoro M, Fujii Y, Iimura Y, Koga F, et al. (2009) Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol 55: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 39. Karakiewicz PI, Hutterer GC, Trinh QD, Jeldres C, Perrotte P, et al. (2007) C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer 110: 1241–1247. [DOI] [PubMed] [Google Scholar]
- 40. Komai Y, Saito K, Sakai K, Morimoto S (2007) Increased preoperative serum C-reactive protein level predicts a poor prognosis in patients with localized renal cell carcinoma. BJU Int 99: 77–80. [DOI] [PubMed] [Google Scholar]
- 41. Eggers H, Seidel C, Schrader AJ, Lehmann R, Wegener G, et al. (2013) Serum C-reactive protein: a prognostic factor in metastatic urothelial cancer of the bladder. Med Oncol 30: 705. [DOI] [PubMed] [Google Scholar]
- 42. Saito K, Urakami S, Komai Y, Yasuda Y, Kubo Y, et al. (2012) Impact of C-reactive protein kinetics on survival of patients with advanced urothelial carcinoma treated by second-line chemotherapy with gemcitabine, etoposide and cisplatin. BJU Int 110: 1478–1484. [DOI] [PubMed] [Google Scholar]
- 43. Gakis G, Todenhofer T, Renninger M, Schilling D, Sievert KD, et al. (2011) Development of a new outcome prediction model in carcinoma invading the bladder based on preoperative serum C-reactive protein and standard pathological risk factors: the TNR-C score. BJU Int 108: 1800–1805. [DOI] [PubMed] [Google Scholar]
- 44. Yoshida S, Saito K, Koga F, Yokoyama M, Kageyama Y, et al. (2008) C-reactive protein level predicts prognosis in patients with muscle-invasive bladder cancer treated with chemoradiotherapy. BJU Int 101: 978–981. [DOI] [PubMed] [Google Scholar]
- 45. Han Y, Mao F, Wu Y, Fu X, Zhu X, et al. (2011) Prognostic role of C-reactive protein in breast cancer: a systematic review and meta-analysis. Int J Biol Markers 26: 209–215. [DOI] [PubMed] [Google Scholar]
- 46. Allin KH, Nordestgaard BG, Flyger H, Bojesen SE (2011) Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res 13: R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Albuquerque KV, Price MR, Badley RA, Jonrup I, Pearson D, et al. (1995) Pre-treatment serum levels of tumour markers in metastatic breast cancer: a prospective assessment of their role in predicting response to therapy and survival. Eur J Surg Oncol 21: 504–509. [DOI] [PubMed] [Google Scholar]
- 48. Al Murri AM, Bartlett JM, Canney PA, Doughty JC, Wilson C, et al. (2006) Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer 94: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, et al. (2009) Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 27: 3437–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alkhateeb AA, Leitzel K, Ali SM, Campbell-Baird C, Evans M, et al. (2012) Elevation in inflammatory serum biomarkers predicts response to trastuzumab-containing therapy. Plos one 7: e51379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mohri Y, Tanaka K, Ohi M, Toiyama Y, Yasuda H, et al. (2012) Inflammation-based prognostic score as a predictor of postoperative gastric cancer recurrence. Anticancer Res 32: 4581–4584. [PubMed] [Google Scholar]
- 52. Shimura T, Kitagawa M, Yamada T, Ebi M, Mizoshita T, et al. (2012) C-reactive protein is a potential prognostic factor for metastatic gastric cancer. Anticancer Res 32: 491–496. [PubMed] [Google Scholar]
- 53. Woo Y, Hyung WJ, Obama K, Kim HI, Pak KH, et al. (2012) Elevated high-sensitivity C-reactive protein, a marker of advanced stage gastric cancer and postgastrectomy disease recurrence. J Surg Oncol 105: 405–409. [DOI] [PubMed] [Google Scholar]
- 54. Nozoe T, Iguchi T, Adachi E, Matsukuma A, Ezaki T (2011) Preoperative elevation of serum C-reactive protein as an independent prognostic indicator for gastric cancer. Surg Today 41: 510–513. [DOI] [PubMed] [Google Scholar]
- 55. Elahi MM, McMillan DC, McArdle CS, Angerson WJ, Sattar N (2004) Score based on hypoalbuminemia and elevated C-reactive protein predicts survival in patients with advanced gastrointestinal cancer. Nutr Cancer 48: 171–173. [DOI] [PubMed] [Google Scholar]
- 56. Toiyama Y, Fujikawa H, Koike Y, Saigusa S, Inoue Y, et al. (2013) Evaluation of preoperative C-reactive protein aids in predicting poor survival in patients with curative colorectal cancer with poor lymph node assessment. Oncol Lett 5: 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takasu C, Shimada M, Kurita N, Iwata T, Nishioka M, et al. (2013) Impact of C-reactive protein on prognosis of patients with colorectal carcinoma. Hepatogastroenterology 60: 507–511. [DOI] [PubMed] [Google Scholar]
- 58. Koike Y, Miki C, Okugawa Y, Yokoe T, Toiyama Y, et al. (2008) Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. J Surg Oncol 98: 540–544. [DOI] [PubMed] [Google Scholar]
- 59. Nozoe T, Mori E, Takahashi I, Ezaki T (2008) Preoperative elevation of serum C-reactive protein as an independent prognostic indicator of colorectal carcinoma. Surg Today 38: 597–602. [DOI] [PubMed] [Google Scholar]
- 60. Wong VK, Malik HZ, Hamady ZZ, Al-Mukhtar A, Gomez D, et al. (2007) C-reactive protein as a predictor of prognosis following curative resection for colorectal liver metastases. Br J Cancer 96: 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chung YC, Chang YF (2003) Serum C-reactive protein correlates with survival in colorectal cancer patients but is not an independent prognostic indicator. Eur J Gastroenterol Hepatol 15: 369–373. [DOI] [PubMed] [Google Scholar]
- 62. Khandavilli SD, Ceallaigh PO, Lloyd CJ, Whitaker R (2009) Serum C-reactive protein as a prognostic indicator in patients with oral squamous cell carcinoma. Oral Oncol 45: 912–914. [DOI] [PubMed] [Google Scholar]
- 63. Zeng YC, Xue M, Chi F, Xu ZG, Fan GL, et al. (2012) C-reactive protein level predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Tumour Biol 33: 891–895. [DOI] [PubMed] [Google Scholar]
- 64. Chen HH, Chen IH, Liao CT, Wei FC, Lee LY, et al. (2011) Preoperative circulating C-reactive protein levels predict pathological aggressiveness in oral squamous cell carcinoma: a retrospective clinical study. Clin Otolaryngol 36: 147–153. [DOI] [PubMed] [Google Scholar]
- 65. Wang CY, Hsieh MJ, Chiu YC, Li SH, Huang HW, et al. (2009) Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol 92: 270–275. [DOI] [PubMed] [Google Scholar]
- 66. Gockel I, Dirksen K, Messow CM, Junginger T (2006) Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol 12: 3746–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guillem P, Triboulet JP (2005) Elevated serum levels of C-reactive protein are indicative of a poor prognosis in patients with esophageal cancer. Dis Esophagus 18: 146–150. [DOI] [PubMed] [Google Scholar]
- 68. Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, et al. (2003) Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol 83: 248–252. [DOI] [PubMed] [Google Scholar]
- 69. Feng JF, Zhao HG, Liu JS, Chen QX (2013) Significance of preoperative C-reactive protein as a parameter in patients with small cell carcinoma of the esophagus. Onco Targets Ther 6: 1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pond GR, Armstrong AJ, Wood BA, Leopold L, Galsky MD, et al. (2012) Ability of C-reactive protein to complement multiple prognostic classifiers in men with metastatic castration resistant prostate cancer receiving docetaxel-based chemotherapy. BJU Int 110: E461–468. [DOI] [PubMed] [Google Scholar]
- 71. Ito M, Saito K, Yasuda Y, Sukegawa G, Kubo Y, et al. (2011) Prognostic impact of C-reactive protein for determining overall survival of patients with castration-resistant prostate cancer treated with docetaxel. Urology 78: 1131–1135. [DOI] [PubMed] [Google Scholar]
- 72. McCall P, Catlow J, McArdle PA, McMillan DC, Edwards J (2011) Tumoral C-reactive protein and nuclear factor kappa-B expression are associated with clinical outcome in patients with prostate cancer. Cancer Biomark 10: 91–99. [DOI] [PubMed] [Google Scholar]
- 73. Prins RC, Rademacher BL, Mongoue-Tchokote S, Alumkal JJ, Graff JN, et al. (2012) C-reactive protein as an adverse prognostic marker for men with castration-resistant prostate cancer (CRPC): confirmatory results. Urol Oncol 30: 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beer TM, Lalani AS, Lee S, Mori M, Eilers KM, et al. (2008) C-reactive protein as a prognostic marker for men with androgen-independent prostate cancer: results from the ASCENT trial. Cancer 112: 2377–2383. [DOI] [PubMed] [Google Scholar]
- 75. Tomita M, Shimizu T, Ayabe T, Nakamura K, Onitsuka T (2012) Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res 32: 3535–3538. [PubMed] [Google Scholar]
- 76. Hong S, Kang YA, Cho BC, Kim DJ (2012) Elevated serum C-reactive protein as a prognostic marker in small cell lung cancer. Yonsei Med J 53: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. O'Dowd C, McRae LA, McMillan DC, Kirk A, Milroy R (2010) Elevated preoperative C-reactive protein predicts poor cancer specific survival in patients undergoing resection for non-small cell lung cancer. J Thorac Oncol 5: 988–992. [DOI] [PubMed] [Google Scholar]
- 78. Gagnon B, Abrahamowicz M, Xiao Y, Beauchamp ME, MacDonald N, et al. (2010) Flexible modeling improves assessment of prognostic value of C-reactive protein in advanced non-small cell lung cancer. Br J Cancer 102: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Koch A, Fohlin H, Sorenson S (2009) Prognostic significance of C-reactive protein and smoking in patients with advanced non-small cell lung cancer treated with first-line palliative chemotherapy. J Thorac Oncol 4: 326–332. [DOI] [PubMed] [Google Scholar]
- 80. Sanjay P, de Figueiredo RS, Leaver H, Ogston S, Kulli C, et al. (2012) Preoperative serum C-reactive protein levels and post-operative lymph node ratio are important predictors of survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. JOP 13: 199–204. [PubMed] [Google Scholar]
- 81. Pine JK, Fusai KG, Young R, Sharma D, Davidson BR, et al. (2009) Serum C-reactive protein concentration and the prognosis of ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol 35: 605–610. [DOI] [PubMed] [Google Scholar]
- 82. Andersson BA, Lewin F, Lundgren J, Nilsson M, Rutqvist LE, et al. (2014) Plasma tumor necrosis factor-alpha and C-reactive protein as biomarker for survival in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol 140: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, et al. (2013) Validation of the prognostic relevance of plasma C-reactive protein levels in soft-tissue sarcoma patients. Br J Cancer. [DOI] [PMC free article] [PubMed]
- 84. Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, et al. (2012) Clinical significance of pretreatment serum C-reactive protein level in soft tissue sarcoma. Cancer 118: 1055–1061. [DOI] [PubMed] [Google Scholar]
- 85. Zimmerman MA, Selzman CH, Cothren C, Sorensen AC, Raeburn CD, et al. (2003) Diagnostic implications of C-reactive protein. Arch Surg 138: 220–224. [DOI] [PubMed] [Google Scholar]
- 86. Szalai AJ, VanCott JL, McGhee JR, Volanakis JE, Benjamin WH Jr (2000) Human C-reactive protein is protective against fatal Salmonella enterica serovar typhimurium infection in transgenic mice. Infect Immun 68: 5652–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Szalai AJ, Briles DE, Volanakis JE (1995) Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J Immunol 155: 2557–2563. [PubMed] [Google Scholar]
- 88. Allin KH, Nordestgaard BG (2011) Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci 48: 155–170. [DOI] [PubMed] [Google Scholar]
- 89.Hribal ML, Fiorentino TV, Sesti G (2013) Role of C Reactive Protein (CRP) in Leptin Resistance. Curr Pharm Des. [DOI] [PMC free article] [PubMed]
- 90. Choi J, Joseph L, Pilote L (2013) Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 14: 232–244. [DOI] [PubMed] [Google Scholar]
- 91. Guo S, Liu M, Wang G, Torroella-Kouri M, Gonzalez-Perez RR (2012) Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim Biophys Acta 1825: 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cha-Molstad H, Young DP, Kushner I, Samols D (2007) The interaction of C-Rel with C/EBPbeta enhances C/EBPbeta binding to the C-reactive protein gene promoter. Mol Immunol 44: 2933–2942. [DOI] [PubMed] [Google Scholar]
- 93. Zhang D, Sun M, Samols D, Kushner I (1996) STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J Biol Chem 271: 9503–9509. [DOI] [PubMed] [Google Scholar]
- 94. Cha-Molstad H, Agrawal A, Zhang D, Samols D, Kushner I (2000) The Rel family member P50 mediates cytokine-induced C-reactive protein expression by a novel mechanism. J Immunol 165: 4592–4597. [DOI] [PubMed] [Google Scholar]
- 95. Voleti B, Agrawal A (2005) Regulation of basal and induced expression of C-reactive protein through an overlapping element for OCT-1 and NF-kappaB on the proximal promoter. J Immunol 175: 3386–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nishikawa T, Hagihara K, Serada S, Isobe T, Matsumura A, et al. (2008) Transcriptional complex formation of c-Fos, STAT3, and hepatocyte NF-1 alpha is essential for cytokine-driven C-reactive protein gene expression. J Immunol 180: 3492–3501. [DOI] [PubMed] [Google Scholar]
- 97. Das T, Sen AK, Kempf T, Pramanik SR, Mandal C (2003) Induction of glycosylation in human C-reactive protein under different pathological conditions. Biochem J 373: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mulrooney DA, Ness KK, Huang S, Solovey A, Hebbel RP, et al. (2013) Pilot study of vascular health in survivors of osteosarcoma. Pediatr Blood Cancer 60: 1703–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Peng TI, Hsiao CW, Reiter RJ, Tanaka M, Lai YK, et al. (2012) mtDNA T8993G mutation-induced mitochondrial complex V inhibition augments cardiolipin-dependent alterations in mitochondrial dynamics during oxidative, Ca(2+), and lipid insults in NARP cybrids: a potential therapeutic target for melatonin. J Pineal Res 52: 93–106. [DOI] [PubMed] [Google Scholar]
- 100. Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, et al. (2011) Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol 179: 1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jansson AF, Muller TH, Gliera L, Ankerst DP, Wintergerst U, et al. (2009) Clinical score for nonbacterial osteitis in children and adults. Arthritis Rheum 60: 1152–1159. [DOI] [PubMed] [Google Scholar]
- 102. Schwinger W, Klass V, Benesch M, Lackner H, Dornbusch HJ, et al. (2005) Feasibility of high-dose interleukin-2 in heavily pretreated pediatric cancer patients. Ann Oncol 16: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 103. Patino WD, Mian OY, Kang JG, Matoba S, Bartlett LD, et al. (2005) Circulating transcriptome reveals markers of atherosclerosis. Proc Natl Acad Sci U S A 102: 3423–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Trapani S, Grisolia F, Simonini G, Calabri GB, Falcini F (2000) Incidence of occult cancer in children presenting with musculoskeletal symptoms: a 10-year survey in a pediatric rheumatology unit. Semin Arthritis Rheum 29: 348–359. [DOI] [PubMed] [Google Scholar]
- 105. Kleinerman ES, Meyers PA, Raymond AK, Gano JB, Jia SF, et al. (1995) Combination therapy with ifosfamide and liposome-encapsulated muramyl tripeptide: tolerability, toxicity, and immune stimulation. J Immunother Emphasis Tumor Immunol 17: 181–193. [DOI] [PubMed] [Google Scholar]
- 106. Asano T, Kleinerman ES (1993) Liposome-encapsulated MTP-PE: a novel biologic agent for cancer therapy. J Immunother Emphasis Tumor Immunol 14: 286–292. [DOI] [PubMed] [Google Scholar]
- 107. Kleinerman ES, Jia SF, Griffin J, Seibel NL, Benjamin RS, et al. (1992) Phase II study of liposomal muramyl tripeptide in osteosarcoma: the cytokine cascade and monocyte activation following administration. J Clin Oncol 10: 1310–1316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)