Abstract
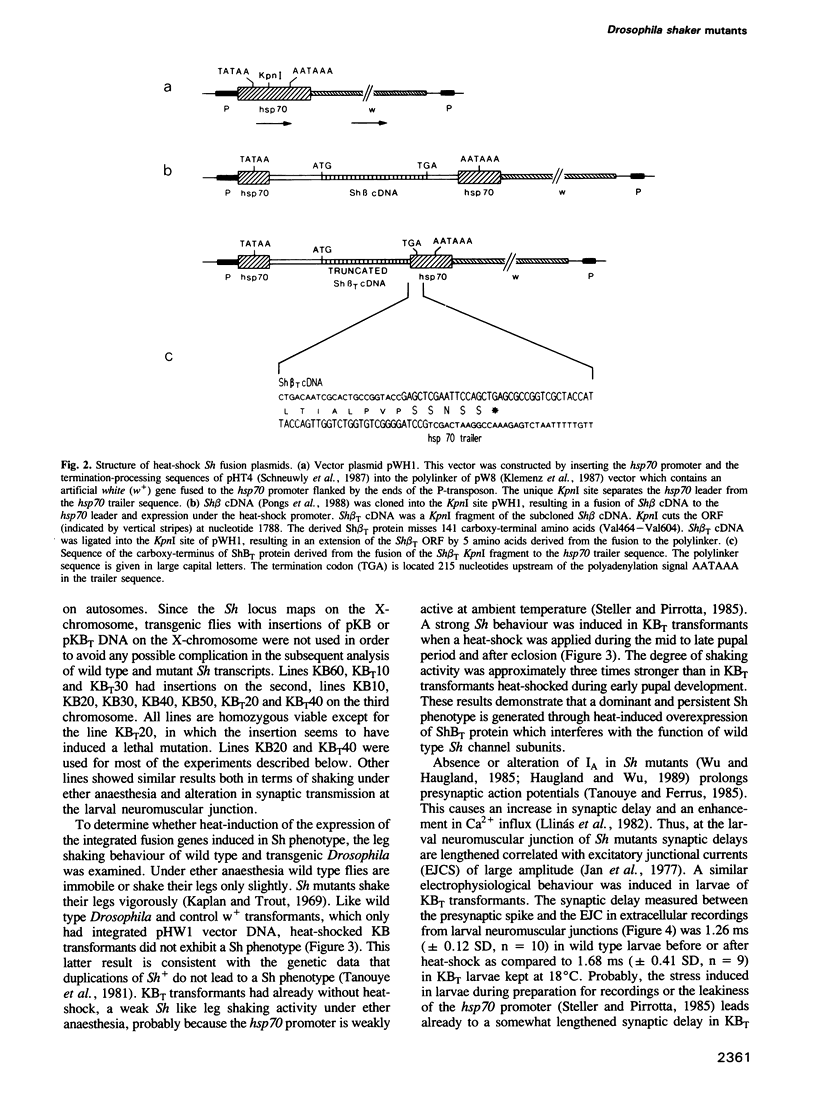
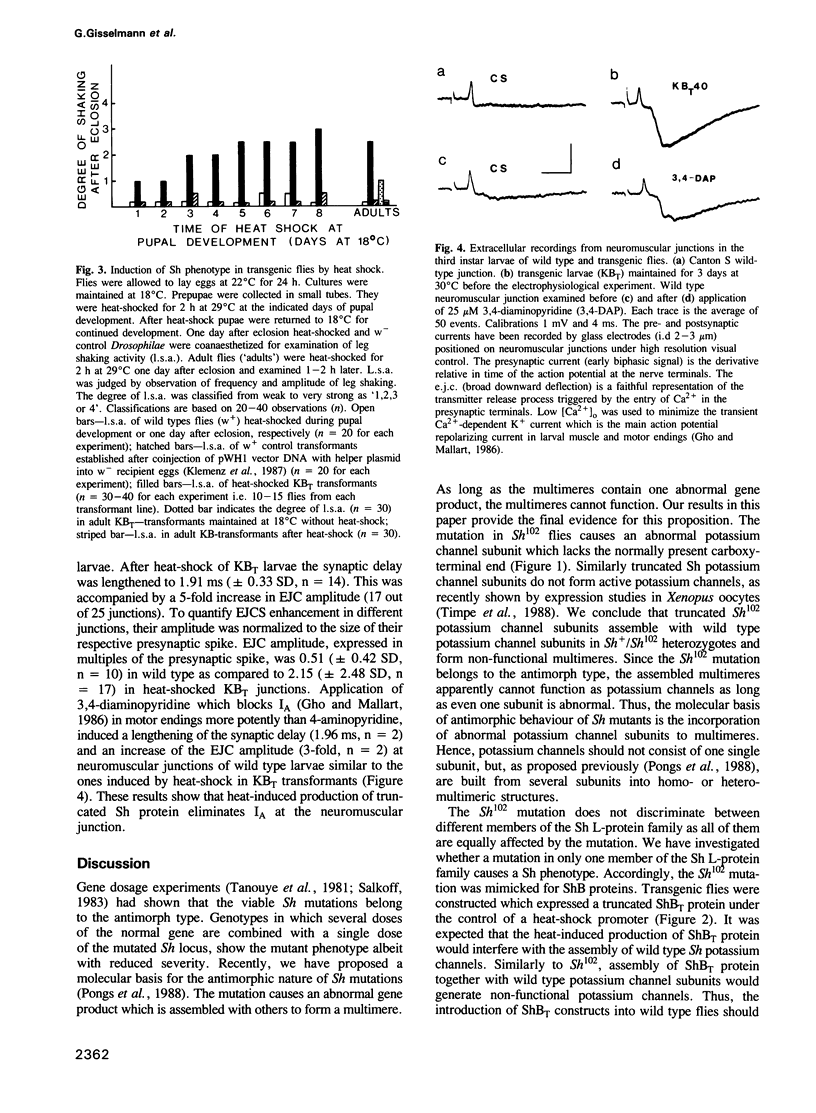
The Shaker locus of Drosophila melanogaster encodes a family of A-type potassium channel subunits. Shaker mutants behave as antimorphs in gene dosage tests. This behaviour is due to the production of truncated A-channel subunits. We propose that they interfere with the function of their normal counterpart by forming multimeric A-channel structures. This hypothesis was tested by constructing transgenic flies carrying a heat-inducible gene encoding a truncated A-type potassium channel subunit together with a normal wild type doses of A-type potassium channel subunits. The altered subunit leads at larval, pupal or adult stages to the transformation of wild type into Shaker flies. The transformed flies exhibited a heat-inducible abnormal leg shaking behaviour and a heat-inducible facilitated neurotransmitter release at larval neuromuscular junctions. By the overexpression of an aberrant A-channel subunit the normal behaviour of transgenic D. melanogaster can be altered in a predictable way.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams T. W., Kandel E. R. Is contiguity detection in classical conditioning a system or a cellular property? Learning in Aplysia suggests a possible molecular site. Trends Neurosci. 1988 Apr;11(4):128–135. doi: 10.1016/0166-2236(88)90137-3. [DOI] [PubMed] [Google Scholar]
- Baumann A., Krah-Jentgens I., Müller R., Müller-Holtkamp F., Seidel R., Kecskemethy N., Casal J., Ferrus A., Pongs O. Molecular organization of the maternal effect region of the Shaker complex of Drosophila: characterization of an I(A) channel transcript with homology to vertebrate Na channel. EMBO J. 1987 Nov;6(11):3419–3429. doi: 10.1002/j.1460-2075.1987.tb02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Crow T. Cellular and molecular analysis of associative learning and memory in Hermissenda. Trends Neurosci. 1988 Apr;11(4):136–147. doi: 10.1016/0166-2236(88)90138-5. [DOI] [PubMed] [Google Scholar]
- Ganetzky B., Wu C. F. Neurogenetics of membrane excitability in Drosophila. Annu Rev Genet. 1986;20:13–44. doi: 10.1146/annurev.ge.20.120186.000305. [DOI] [PubMed] [Google Scholar]
- Gho M., Mallart A. Two distinct calcium-activated potassium currents in larval muscle fibres of Drosophila melanogaster. Pflugers Arch. 1986 Nov;407(5):526–533. doi: 10.1007/BF00657511. [DOI] [PubMed] [Google Scholar]
- Iverson L. E., Tanouye M. A., Lester H. A., Davidson N., Rudy B. A-type potassium channels expressed from Shaker locus cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5723–5727. doi: 10.1073/pnas.85.15.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Dennis M. J. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Kamb A., Tseng-Crank J., Tanouye M. A. Multiple products of the Drosophila Shaker gene may contribute to potassium channel diversity. Neuron. 1988 Jul;1(5):421–430. doi: 10.1016/0896-6273(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Kaplan W. D., Trout W. E., 3rd The behavior of four neurological mutants of Drosophila. Genetics. 1969 Feb;61(2):399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Weber U., Gehring W. J. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 1987 May 26;15(10):3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T., Simon J. A., Sutton C. A. New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell. 1983 Dec;35(2 Pt 1):403–410. doi: 10.1016/0092-8674(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pongs O., Kecskemethy N., Müller R., Krah-Jentgens I., Baumann A., Kiltz H. H., Canal I., Llamazares S., Ferrus A. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J. 1988 Apr;7(4):1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Salkoff L. Genetic and voltage-clamp analysis of a Drosophila potassium channel. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):221–231. doi: 10.1101/sqb.1983.048.01.025. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Klemenz R., Gehring W. J. Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. 1987 Feb 26-Mar 4Nature. 325(6107):816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. Expression of the Drosophila white gene under the control of the hsp70 heat shock promoter. EMBO J. 1985 Dec 30;4(13B):3765–3772. doi: 10.1002/j.1460-2075.1985.tb04146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye M. A., Ferrus A. Action potentials in normal and Shaker mutant Drosophila. J Neurogenet. 1985 Sep;2(4):253–271. doi: 10.3109/01677068509102322. [DOI] [PubMed] [Google Scholar]
- Tanouye M. A., Ferrus A., Fujita S. C. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6548–6552. doi: 10.1073/pnas.78.10.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye M. A., Kamb C. A., Iverson L. E., Salkoff L. Genetics and molecular biology of ionic channels in Drosophila. Annu Rev Neurosci. 1986;9:255–276. doi: 10.1146/annurev.ne.09.030186.001351. [DOI] [PubMed] [Google Scholar]
- Timpe L. C., Jan L. Y. Gene dosage and complementation analysis of the Shaker locus in Drosophila. J Neurosci. 1987 May;7(5):1307–1317. doi: 10.1523/JNEUROSCI.07-05-01307.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpe L. C., Jan Y. N., Jan L. Y. Four cDNA clones from the Shaker locus of Drosophila induce kinetically distinct A-type potassium currents in Xenopus oocytes. Neuron. 1988 Oct;1(8):659–667. doi: 10.1016/0896-6273(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Timpe L. C., Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature. 1988 Jan 14;331(6152):143–145. doi: 10.1038/331143a0. [DOI] [PubMed] [Google Scholar]
- Wu C. F., Haugland F. N. Voltage clamp analysis of membrane currents in larval muscle fibers of Drosophila: alteration of potassium currents in Shaker mutants. J Neurosci. 1985 Oct;5(10):2626–2640. doi: 10.1523/JNEUROSCI.05-10-02626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]




