Abstract
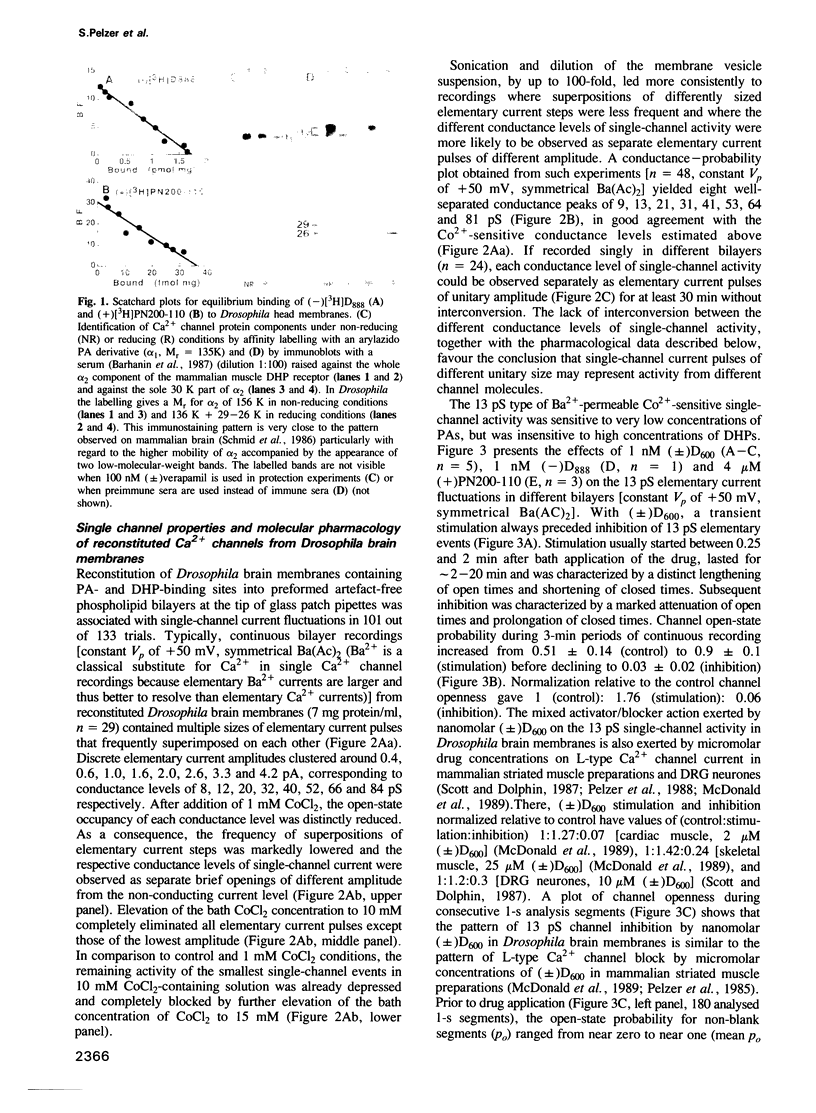
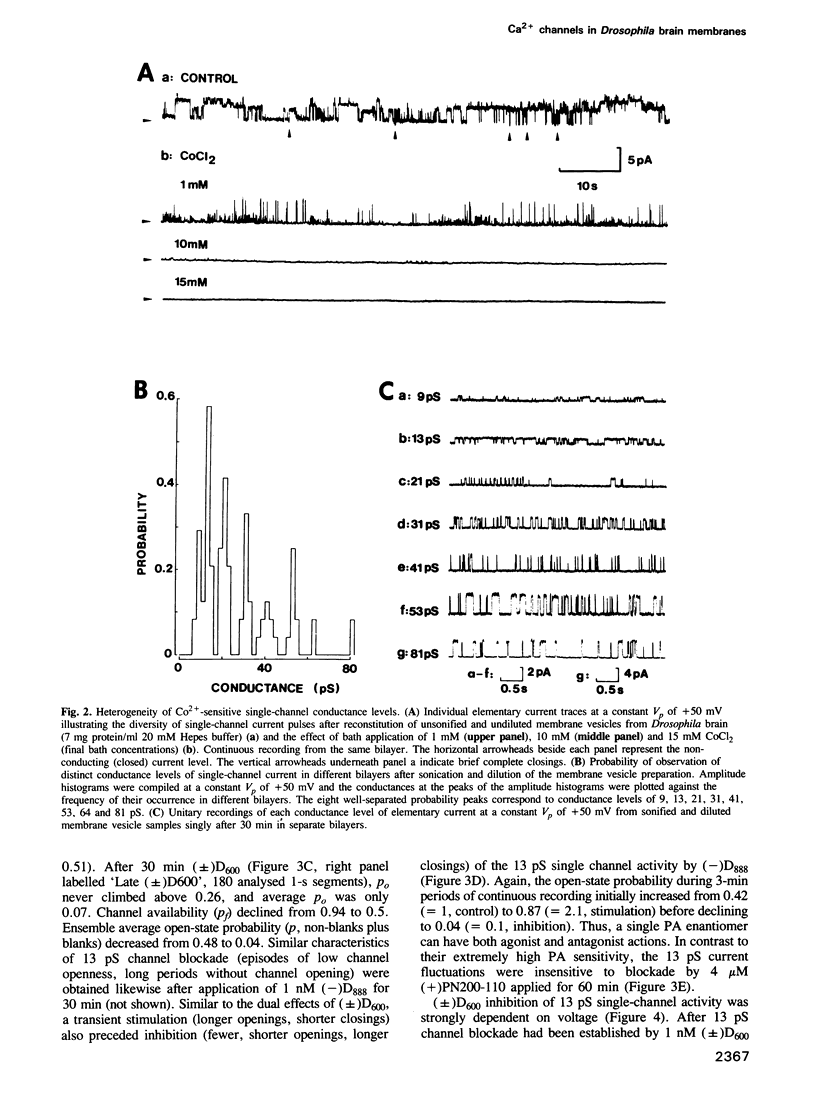
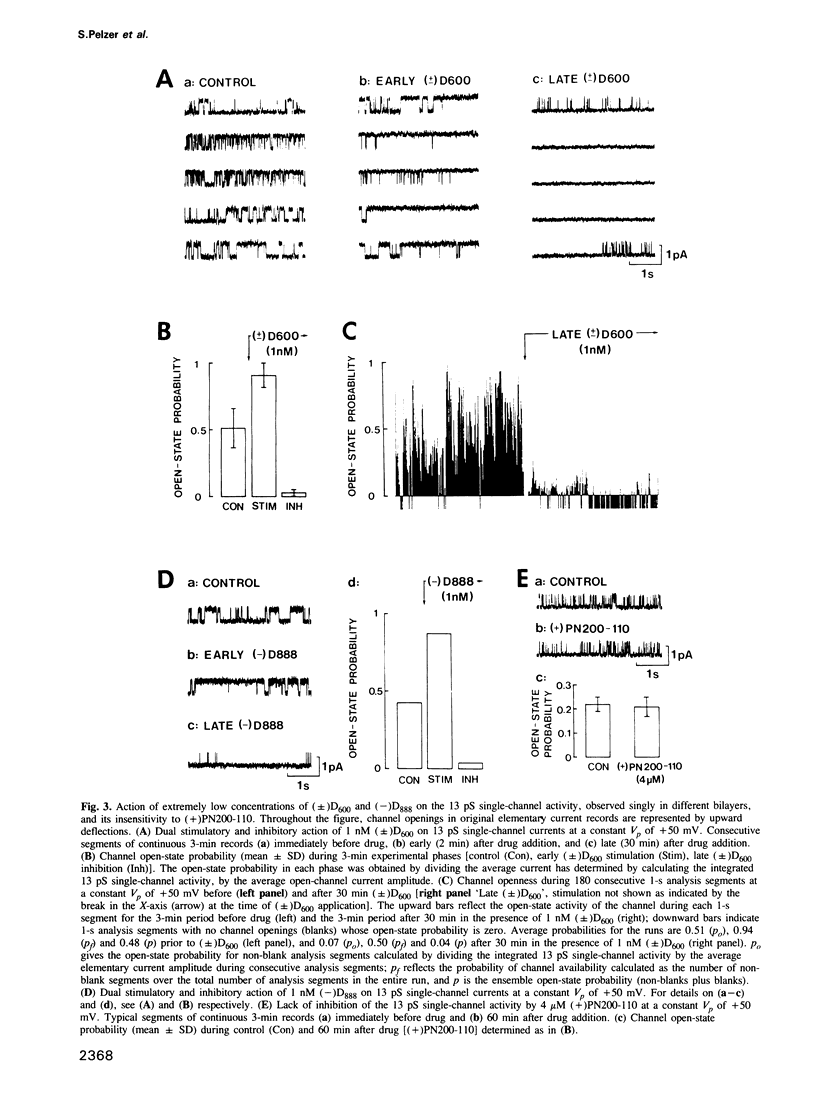
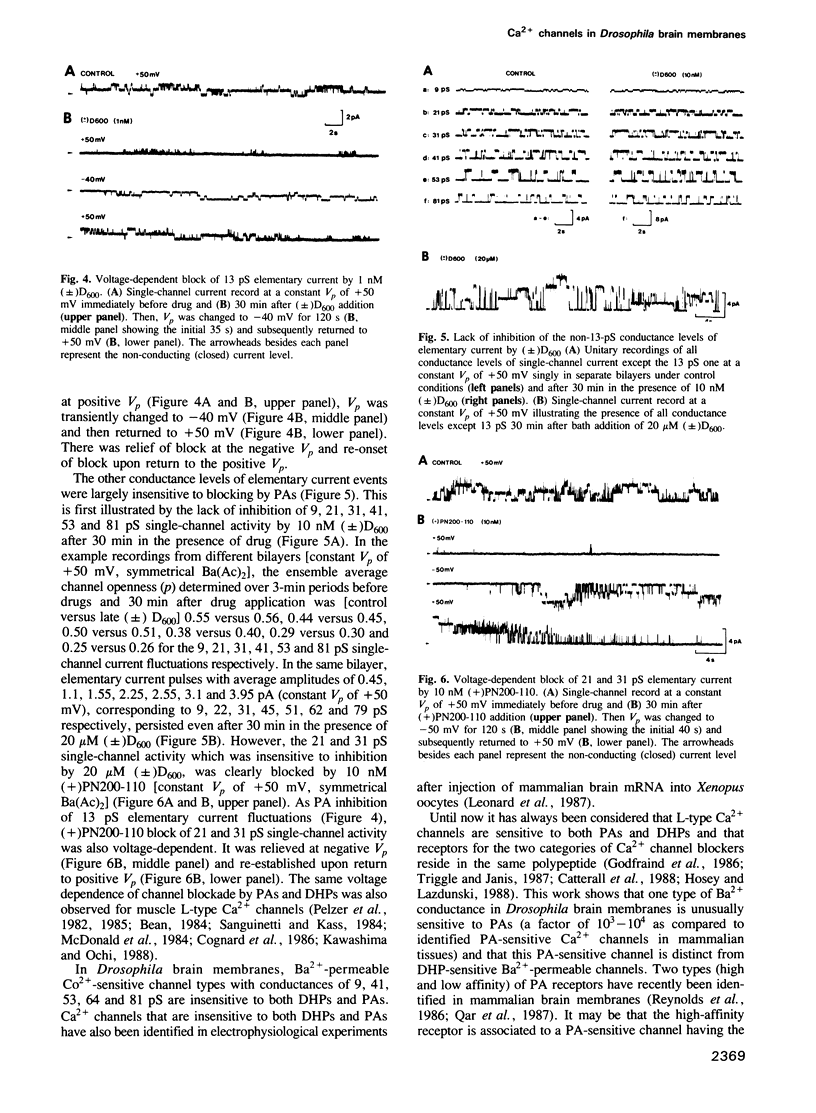
Binding studies as well as affinity labelling and immunoblot techniques were used to identify and characterize the receptors for Ca2+ channel blockers in Drosophila brain membranes. Despite structural analogies with mammalian receptors, Drosophila binding sites for phenylalkylamines and 1,4-dihydropyridines, unlike those described in skeletal and cardiac muscle, were found to be located on separate Ca2+ channels. Single-channel bilayer recordings from reconstituted membranes revealed the presence of eight distinct cobalt-sensitive Ba2+-conducting channels in Drosophila brain membrane preparations. In good agreement with binding studies, the most frequently observed Ca2+ channel type (Ba2+ conductance of 13 pS) was extremely sensitive to phenylalkylamines but not affected by micromolar concentrations of 1,4-dihydropyridines. Distinct 1,4-dihydropyridine-sensitive and phenylalkylamine-insensitive channels were also identified. They had unitary Ba2+ conductances of 21 and 31 pS. A detailed analysis of drug action showed that both 1,4-dihydropyridines and phenylalkylamines first increased channel open state probability before fully blocking channel activity. Other types of channels have been identified with unitary Ba2+ conductances of 9, 41, 53, 64 and 81 pS. They were insensitive to the previously described organic Ca2+ channel blockers. The Drosophila system seems to be a unique model to analyse the properties of several different types of Ca2+ channels and particularly those of channel types that are uniquely blocked by phenylalkylamines or uniquely blocked by 1,4-dihydropyridines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsotto M., Barhanin J., Fosset M., Lazdunski M. The 1,4-dihydropyridine receptor associated with the skeletal muscle voltage-dependent Ca2+ channel. Purification and subunit composition. J Biol Chem. 1985 Nov 15;260(26):14255–14263. [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Seagar M. J., Takahashi M. Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle. J Biol Chem. 1988 Mar 15;263(8):3535–3538. [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- Cognard C., Romey G., Galizzi J. P., Fosset M., Lazdunski M. Dihydropyridine-sensitive Ca2+ channels in mammalian skeletal muscle cells in culture: electrophysiological properties and interactions with Ca2+ channel activator (Bay K8644) and inhibitor (PN 200-110). Proc Natl Acad Sci U S A. 1986 Mar;83(5):1518–1522. doi: 10.1073/pnas.83.5.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Hosey M. M., Barhanin J., Schmid A., Vandaele S., Ptasienski J., O'Callahan C., Cooper C., Lazdunski M. Photoaffinity labelling and phosphorylation of a 165 kilodalton peptide associated with dihydropyridine and phenylalkylamine-sensitive calcium channels. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1137–1145. doi: 10.1016/s0006-291x(87)80188-2. [DOI] [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Kawashima Y., Ochi R. Voltage-dependent decrease in the availability of single calcium channels by nitrendipine in guinea-pig ventricular cells. J Physiol. 1988 Aug;402:219–235. doi: 10.1113/jphysiol.1988.sp017201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. P., Nargeot J., Snutch T. P., Davidson N., Lester H. A. Ca channels induced in Xenopus oocytes by rat brain mRNA. J Neurosci. 1987 Mar;7(3):875–881. doi: 10.1523/JNEUROSCI.07-03-00875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan E. S., Schofield P. R., Burt D. R., Rhee L. M., Wisden W., Köhler M., Fujita N., Rodriguez H. F., Stephenson A., Darlison M. G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988 Sep 1;335(6185):76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. Cat ventricular muscle treated with D600: characteristics of calcium channel block and unblock. J Physiol. 1984 Jul;352:217–241. doi: 10.1113/jphysiol.1984.sp015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Papazian D. M., Schwarz T. L., Tempel B. L., Timpe L. C., Jan L. Y. Ion channels in Drosophila. Annu Rev Physiol. 1988;50:379–394. doi: 10.1146/annurev.ph.50.030188.002115. [DOI] [PubMed] [Google Scholar]
- Pauron D., Qar J., Barhanin J., Fournier D., Cuany A., Pralavorio M., Berge J. B., Lazdunski M. Identification and affinity labeling of very high affinity binding sites for the phenylalkylamine series of Ca+ channel blockers in the Drosophila nervous system. Biochemistry. 1987 Oct 6;26(20):6311–6315. doi: 10.1021/bi00394a003. [DOI] [PubMed] [Google Scholar]
- Pelzer D., Trautwein W., McDonald T. F. Calcium channel block and recovery from block in mammalian ventricular muscle treated with organic channel inhibitors. Pflugers Arch. 1982 Aug;394(2):97–105. doi: 10.1007/BF00582909. [DOI] [PubMed] [Google Scholar]
- Qar J., Galizzi J. P., Fosset M., Lazdunski M. Receptors for diphenylbutylpiperidine neuroleptics in brain, cardiac, and smooth muscle membranes. Relationship with receptors for 1,4-dihydropyridines and phenylalkylamines and with Ca2+ channel blockade. Eur J Pharmacol. 1987 Sep 11;141(2):261–268. doi: 10.1016/0014-2999(87)90271-8. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Snowman A. M., Snyder S. H. (-)-[3H] desmethoxyverapamil labels multiple calcium channel modulator receptors in brain and skeletal muscle membranes: differentiation by temperature and dihydropyridines. J Pharmacol Exp Ther. 1986 Jun;237(3):731–738. [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Schmid A., Barhanin J., Mourre C., Coppola T., Borsotto M., Lazdunski M. Antibodies reveal the cytolocalization and subunit structure of the 1,4-dihydropyridine component of the neuronal Ca2+ channel. Biochem Biophys Res Commun. 1986 Sep 30;139(3):996–1002. doi: 10.1016/s0006-291x(86)80276-5. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Dolphin A. C. Activation of a G protein promotes agonist responses to calcium channel ligands. Nature. 1987 Dec 24;330(6150):760–762. doi: 10.1038/330760a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Triggle D. J., Janis R. A. Calcium channel ligands. Annu Rev Pharmacol Toxicol. 1987;27:347–369. doi: 10.1146/annurev.pa.27.040187.002023. [DOI] [PubMed] [Google Scholar]




