Abstract
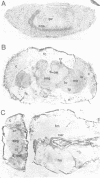
The proteasome is a multicatalytic proteinase complex composed of nonidentical protein subunits. We have isolated a cDNA clone encoding the 35 kd proteasome subunit of Drosophila melanogaster and propose the designation PROS-35 for the corresponding gene. The deduced amino acid sequence reveals a region of striking homology to a tyrosine phosphorylation site of viral and cellular proteins suggesting a potential regulatory function for the 35 kd subunit within the proteinase complex. Immunocytochemical experiments reveal a tissue-dependent differential distribution of the proteasome between the nucleus and cytoplasm. In addition developmental analysis shows that the proteasome is highly expressed in the CNS of stage-16 embryos and in cardia, ventriculus and ovaries of adult flies. These data suggest a tissue- and development-dependent distribution of the proteasome in D. melanogaster.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Darlix J. L., Khandjian E. W., Simon M., Spahr P. F. Characterization of the prosome from Drosophila and its similarity to the cytoplasmic structures formed by the low molecular weight heat-shock proteins. EMBO J. 1985 Feb;4(2):399–406. doi: 10.1002/j.1460-2075.1985.tb03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Tanaka K., Goldberg A. L., Welch W. J. Identity of the 19S 'prosome' particle with the large multifunctional protease complex of mammalian cells (the proteasome). Nature. 1988 Jan 14;331(6152):192–194. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- Chasan R., Anderson K. V. The role of easter, an apparent serine protease, in organizing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1989 Feb 10;56(3):391–400. doi: 10.1016/0092-8674(89)90242-0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Dahlmann B., Rutschmann M., Kuehn L., Reinauer H. Activation of the multicatalytic proteinase from rat skeletal muscle by fatty acids or sodium dodecyl sulphate. Biochem J. 1985 May 15;228(1):171–177. doi: 10.1042/bj2280171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLotto R., Spierer P. A gene required for the specification of dorsal-ventral pattern in Drosophila appears to encode a serine protease. Nature. 1986 Oct 23;323(6090):688–692. doi: 10.1038/323688a0. [DOI] [PubMed] [Google Scholar]
- Driscoll J., Goldberg A. L. Skeletal muscle proteasome can degrade proteins in an ATP-dependent process that does not require ubiquitin. Proc Natl Acad Sci U S A. 1989 Feb;86(3):787–791. doi: 10.1073/pnas.86.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburg P. E., Haass C., Kloetzel P. M., Niedel B., Kopp F., Kuehn L., Dahlmann B. Drosophila small cytoplasmic 19S ribonucleoprotein is homologous to the rat multicatalytic proteinase. Nature. 1988 Jan 14;331(6152):190–192. doi: 10.1038/331190a0. [DOI] [PubMed] [Google Scholar]
- Falkenburg P. E., Kloetzel P. M. Identification and characterization of three different subpopulations of the Drosophila multicatalytic proteinase (proteasome). J Biol Chem. 1989 Apr 25;264(12):6660–6666. [PubMed] [Google Scholar]
- Gregory R. J., Kammermeyer K. L., Vincent W. S., 3rd, Wadsworth S. G. Primary sequence and developmental expression of a novel Drosophila melanogaster src gene. Mol Cell Biol. 1987 Jun;7(6):2119–2127. doi: 10.1128/mcb.7.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Kloetzel P. M. The Drosophila proteasome undergoes changes in its subunit pattern during development. Exp Cell Res. 1989 Jan;180(1):243–252. doi: 10.1016/0014-4827(89)90228-0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann E., Geisler N., Weber K. SDS-PAGE strongly overestimates the molecular masses of the neurofilament proteins. FEBS Lett. 1984 May 7;170(1):81–84. doi: 10.1016/0014-5793(84)81373-3. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Escher C., Wolf D. H. Proteinase yscE of yeast shows homology with the 20 S cylinder particles of Xenopus laevis. FEBS Lett. 1988 Oct 24;239(1):35–40. doi: 10.1016/0014-5793(88)80540-4. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Hügle B., Grund C., Franke W. W. The 22 S cylinder particles of Xenopus laevis. I. Biochemical and electron microscopic characterization. Eur J Cell Biol. 1983 Nov;32(1):143–156. [PubMed] [Google Scholar]
- Kloetzel P. M., Falkenburg P. E., Hössl P., Glätzer K. H. The 19S ring-type particles of Drosophila. Cytological and biochemical analysis of their intracellular association and distribution. Exp Cell Res. 1987 May;170(1):204–213. doi: 10.1016/0014-4827(87)90130-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1986 May;83(9):2850–2854. doi: 10.1073/pnas.83.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGuire M. J., DeMartino G. N. Purification and characterization of a high molecular weight proteinase (macropain) from human erythrocytes. Biochim Biophys Acta. 1986 Sep 26;873(2):279–289. doi: 10.1016/0167-4838(86)90055-5. [DOI] [PubMed] [Google Scholar]
- Newcomb R., Scheller R. H. Proteolytic processing of the Aplysia egg-laying hormone and R3-14 neuropeptide precursors. J Neurosci. 1987 Mar;7(3):854–863. doi: 10.1523/JNEUROSCI.07-03-00854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rivett A. J. Purification of a liver alkaline protease which degrades oxidatively modified glutamine synthetase. Characterization as a high molecular weight cysteine proteinase. J Biol Chem. 1985 Oct 15;260(23):12600–12606. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Schuldt C., Kloetzel P. M. Analysis of cytoplasmic 19 S ring-type particles in Drosophila which contain hsp 23 at normal growth temperature. Dev Biol. 1985 Jul;110(1):65–74. doi: 10.1016/0012-1606(85)90064-8. [DOI] [PubMed] [Google Scholar]
- Shilo B. Z., Weinberg R. A. DNA sequences homologous to vertebrate oncogenes are conserved in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6789–6792. doi: 10.1073/pnas.78.11.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. A., Kornberg T. B., Bishop J. M. Three loci related to the src oncogene and tyrosine-specific protein kinase activity in Drosophila. Nature. 1983 Apr 28;302(5911):837–839. doi: 10.1038/302837a0. [DOI] [PubMed] [Google Scholar]
- Siwicki K. K., Eastman C., Petersen G., Rosbash M., Hall J. C. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1988 Apr;1(2):141–150. doi: 10.1016/0896-6273(88)90198-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ichihara A. Half-life of proteasomes (multiprotease complexes) in rat liver. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1309–1315. doi: 10.1016/0006-291x(89)92253-5. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ii K., Ichihara A., Waxman L., Goldberg A. L. A high molecular weight protease in the cytosol of rat liver. I. Purification, enzymological properties, and tissue distribution. J Biol Chem. 1986 Nov 15;261(32):15197–15203. [PubMed] [Google Scholar]
- Tanaka K., Yoshimura T., Ichihara A., Kameyama K., Takagi T. A high molecular weight protease in the cytosol of rat liver. II. Properties of the purified enzyme. J Biol Chem. 1986 Nov 15;261(32):15204–15207. [PubMed] [Google Scholar]
- Tanaka K., Yoshimura T., Kumatori A., Ichihara A., Ikai A., Nishigai M., Kameyama K., Takagi T. Proteasomes (multi-protease complexes) as 20 S ring-shaped particles in a variety of eukaryotic cells. J Biol Chem. 1988 Nov 5;263(31):16209–16217. [PubMed] [Google Scholar]
- Tsukahara T., Ishiura S., Sugita H. An ATP-dependent protease and ingensin, the multicatalytic proteinase, in K562 cells. Eur J Biochem. 1988 Nov 1;177(2):261–266. doi: 10.1111/j.1432-1033.1988.tb14371.x. [DOI] [PubMed] [Google Scholar]
- Vincent W. S., 3rd, Gregory R. J., Wadsworth S. C. Embryonic expression of a Drosophila src gene: alternate forms of the protein are expressed in segmental stripes and in the nervous system. Genes Dev. 1989 Mar;3(3):334–347. doi: 10.1101/gad.3.3.334. [DOI] [PubMed] [Google Scholar]
- Waxman L., Fagan J. M., Goldberg A. L. Demonstration of two distinct high molecular weight proteases in rabbit reticulocytes, one of which degrades ubiquitin conjugates. J Biol Chem. 1987 Feb 25;262(6):2451–2457. [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Cation-sensitive neutral endopeptidase: isolation and specificity of the bovine pituitary enzyme. J Neurochem. 1980 Nov;35(5):1172–1182. doi: 10.1111/j.1471-4159.1980.tb07873.x. [DOI] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex. J Neurochem. 1983 Mar;40(3):842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]