Abstract
BACKGROUND
Cancer patients often experience preventable infections, including influenza A and B. These infections can be a cause of significant morbidity and mortality. The increased risk of infection may be because of either cancer itself or treatment-induced immunosuppression.1 Influenza immunization has been shown to decrease the risk of influenza infection in patients with intact immunity.2 In cancer patients, active immunization has been shown to confer protective immunity against several infections at similar rates to healthy individuals, which has translated into decreased duration and severity of infection and potentially improved morbidity and mortality.3
OBJECTIVES
To assess the efficacy of influenza vaccination in stimulating immunological response in patients with cancer during chemotherapy compared to control groups.
To assess the efficacy of influenza vaccination in preventing confirmed influenza and influenza-like illness and/or stimulating immunological response in children with cancer treated with chemotherapy, compared to placebo, no intervention, or different dosage schedules.
To determine the adverse effects associated with influenza vaccination in patients with cancer.
SEARCH METHODS
We searched MEDLINE/PubMed database for articles published from 1964 to 2013 using the search terms “cancer,” “adult,” “influenza vaccination,” and “chemotherapy.”
SELECTION CRITERIA
We included studies based on systematic sampling with defined clinical criteria irrespective of the vaccination status of cancer patients. Studies measure the serological response or clinical response to compare between the study group and the control group. Studies assessed the inactivated influenza vaccines and live attenuated influenza vaccine (LAIV) protective serological reaction and the clinical outcomes after vaccination.
DATA COLLECTION AND ANALYSIS
Two independent authors assessed the methodological quality of included studies and extracted data.
MAIN RESULTS
We included 16 studies (total number of participants = 1,076). None of the included studies reported clinical outcomes. All included studies reported on influenza immunity and adverse reaction on vaccination. We included 6 solid tumor studies and 10 hematological studies. In 12 studies, the serological response to influenza vaccine was compared in patients receiving chemotherapy (n = 425) versus those not receiving chemotherapy (n = 376). In three studies, the serological responses to influenza vaccination in patients receiving chemotherapy are compared to that in healthy adult. Measures used to assess the serological responses included a four-fold rise increase in antibody titer development of hemagglutination inhibition (HI) titer >40, and pre- and post-vaccination geometric mean titers (GMTs). Immune responses in patients receiving chemotherapy were consistently weaker (four-fold rise of 17–52%) than in those who had completed chemotherapy (50–83%) and healthy patients (67–100%). Concerning adverse effects, oncology patients received influenza vaccine, and the side effects described were mild local reactions and low-grade fever. No life-threatening or persistent adverse effects were reported.
AUTHORS’ CONCLUSION
Patients with solid and some of hematological tumors are able to mount a serological response to influenza vaccine, but it remains unclear how much this response protects them from influenza infection or its complications. Meanwhile, influenza vaccine appears to be safe in these patients. While waiting results of randomized controlled trials to give us more details about the clinical benefits of the influenza vaccination, the clinicians should consider the currently proved benefits of influenza vaccination on management of the cancer patients undergoing systematic chemotherapy such as decrease in the duration and severity of the of the disease, and significant decrease in influenza-associated morbidity and mortality in these high-risk patients.3
Keywords: influenza vaccination, cancer patients, chemotherapy
Background
Cancer patients often experience preventable infections, including influenza A and B. These infections can be a cause of significant morbidity and mortality. The increased risk of infection may be because of either cancer itself or treatment-induced immunosuppression.1
Infection often delays the anticancer therapy, worsening the oncologic outcome. Each year, influenza infectious adverse events contribute to up to 36,000 deaths and 226,000 hospitalizations in general population.2 Influenza virus-related mortality can reach up to 9% in cancer patients undergoing active therapy.3
Influenza immunization has been shown to decrease the risk of influenza infection in patients with intact immunity.4 In cancer patients, active immunization has been shown to confer protective immunity against several infections at similar rates to healthy individuals, which has translated into decreased duration and severity of infection and potentially improved morbidity and mortality.5
However, it is currently unclear whether patients undergoing systemic chemotherapy can achieve ideal serologic responses to vaccines. Knowledge regarding vaccine efficacy, safety, and ideal timing in this patient population is still limited.
Although the Centers for Disease Control and Prevention (CDC) recommends annual influenza vaccination for high-risk population, including health care workers, the elderly, and patients who are immunosuppressed or have chronic medical conditions and malignancies, only less than 50% of patients receiving chemotherapy routinely receive the influenza vaccination.3,5,6
Influenza virulence
In the United States, annual epidemics of influenza occur typically during the late fall through early spring. Influenza viruses can cause disease among persons in any age group, but rates of infection are the highest among children.7–9 Rates of serious illness and death are highest among persons aged ≥65 years, children aged <2 years, and persons of any age who have medical conditions that place them at increased risk for complications from influenza.7,10 Influenza may also result in secondary bacterial pneumonias, sinusitis, ear infection, or a worsening of chronic respiratory condition.11,12 There are main two types of influenza virus, type A and B, that are responsible for the majority of cases of severe disease in humans. Influenza A is further classified based on the presence of two surface antigens, hemagglutinin (HA) and neuraminidase (NA), and influenza B is separated into two genetic lineages, Yamagata and Victoria.2,12 Any changes in the amino acid sequences of HA and/or NA result in seasonal epidemics. In April 2009, a novel influenza A (H1N1) virus, often referred to as pdm 2009 A/H1N1, that is similar to but genetically and antigenically distinct from influenza A (H1N1) viruses previously identified in swine was determined to be the cause of respiratory illnesses that spread across North America and were identified in many areas of the world by May 2009.13,14 Influenza morbidity caused by the pdm 2009 A/H1N1 remained above seasonal baselines throughout spring and summer 2009 and was the cause of the first pandemic since 1968. The pdm 2009 A/H1N1 has now mostly replaced the H1N1 virus that was previously circulating in humans.2
Cancer and immunization
Cancer increases the risk of complications from influenza, including recurrent hospitalization and death.15 In cancer patients, altered humoral and cellular immunity has been noted.16,17 Moreover, many cancer patients are treated with cytostatic and immunosuppressive drugs, and chemotherapy has also been associated with various disorders.17 For these reasons, patients with cancer may be considered a high-risk group who are at risk of particularly serious post-influenza complications and who should be immunized against influenza before every epidemic season. In a study of patients with solid tumors, such as lung and breast cancer, who were not undergoing systemic chemotherapy, the patients were able to mount protective antibody titers to influenza vaccination that approached the level of healthy controls.17 Protective antibodies after vaccination in this population were significant when compared with patients receiving chemotherapy, and there were no major complications attributable to vaccination.18 Patient with lung cancer, in particular, developed protective antibody responses to influenza vaccine, which did not appear to be affected by systemic steroid treatment, recent chemotherapy, or lung cancer histology.5 Influenza vaccination coverage is currently low among cancer patients undergoing systemic treatment: only 18% in the 18–49-year group and 32% in the 50–64-year group.19 CDC recommends that people who live with or care for a person at high risk for flu-related problems get the flu shot too. This means that if you are being treated for cancer, your family members, caregivers, and children at home should get the flu shot.2
Methods
The MEDLINE/PubMed database was reviewed for articles published from 1964 to 2013 using the search terms “cancer,” “adult,” “influenza vaccination,” and “chemotherapy.” Studies included in this review meet one of these criteria:
Study based on systematic sampling with defined clinical criteria irrespective of the vaccination status of cancer patients.
Study assessed inactivated influenza vaccines and live attenuated influenza vaccine (LAIV) protective serological reaction among cancer patients.
Study assessed the clinical outcomes and immune response after vaccination among cancer patients.
Study showing the efficacy of influenza vaccination among different types of cancer.
Study assessed the potential benefits and complications of vaccination among cancer patients.
Study assessed the timing of vaccination.
Study reported overall vaccine efficacy against all circulating influenza strains.
Search methods
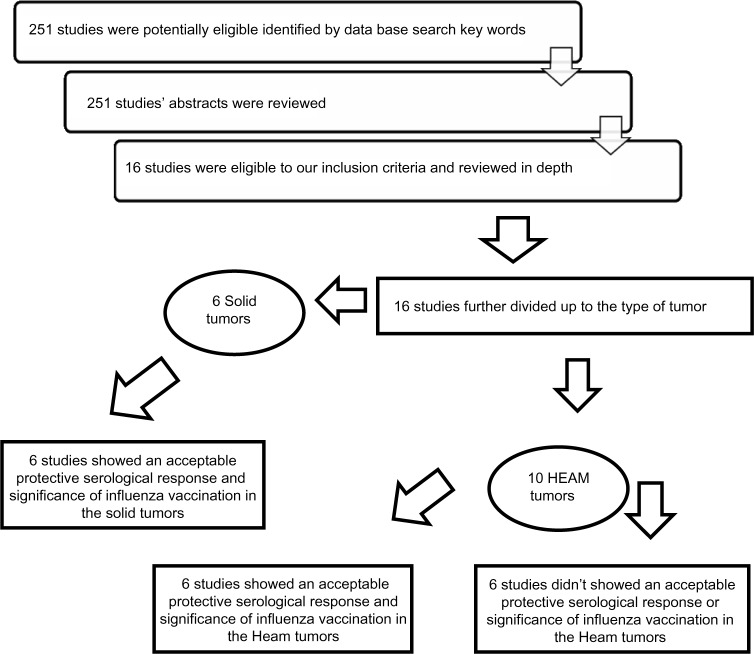
A total of 251 studies were identified in initial PubMed database search of key words. In all, 52 studies were selected based on reviewing the abstracts of the 251 studies. These 52 studies were handling the same topic of interest of our review. After reviewing the 52 studies, only 16 studies met our inclusion criteria and reviewed the full articles in depth. We further divided the articles’ outcomes among the selected population having different types of underlying cancer disease, solid versus hematological tumors (Fig. 1). We focused in our analysis of the selected papers on the time vaccination in patients on chemotherapy versus patients out of therapy. We kept our attention also on the type of outcome measurement of the vaccination efficacy and protective titer used in the studies versus clinically based influenza flue-like illness disease.
Figure 1.
Review strategy.
Note: N.B, Two studies included solid and heamatological tumors together at the same study and showed an acceptable protective serological response and significance of influenza vaccination in solid tumors and non-significance of influenza vaccinations in patients with hematological tumors at the same study.
Data extraction
The two review authors independently performed data extraction using standardized forms. We extracted data on the characteristics of the participants (tumor type, anticancer treatment received, timing of vaccination, measurement of the vaccination efficacy, and series of vaccine administrations) and outcomes measures (immunological response to vaccination, laboratory response, hospitalization, length of stay in the hospital, and pneumonia). We examined and discussed the articles until consensus was made.
Assessment of risk of bias in included studies
The two review author’s independently assessed trial quality. We assessed mythologies of the included study and contacted study authors for additional information where necessary. Disagreements were resolved by discussion among the review authors.
Main results
We included 16 studies (total number of participants = 1,076). None of the included studies reported clinical outcomes. All included studies reported on influenza immunity and adverse reaction on vaccination. We included 6 solid tumor studies and 10 hematological studies. In 12 studies, the serological response to influenza vaccine was compared in patients receiving chemotherapy (n = 425) versus those not receiving chemotherapy (n = 376). In three studies, the serological responses to influenza vaccination in patients receiving chemotherapy are compared to those in healthy adult. Measures used to assess the serological responses included a four-fold rise increase in antibody titer development of hemagglutination inhibition (HI) titer >40, and pre- and post-vaccination geometric mean titers (GMTs). Immune responses in patients receiving chemotherapy were consistently weaker (four-fold rise of 17–52%) than in those who had completed chemotherapy (50–83%) and healthy patients (67–100%). Concerning adverse effects, oncology patients received influenza vaccine, and the side effects described were mild local reactions and low-grade fever. No life-threatening or persistent adverse effects were reported.
Outcomes
Influenza immunity (difference in pre- and post-influenza vaccination HI antibody titer)
All the 16 sampled studies determined the efficacy of vaccination by measuring HI titer, a serum antibody titer of 40 or four-fold rise increase in HI titer which is normally considered protective in healthy individuals. Seven studies defined as protective the development of HI titer of >40. Two studies defined as protective the development of HI titer of >20.
Laboratory-confirmed influenza infection within the epidemic period
They were not reported outcome measures. There are no stated methods used to identify influenza infection.
Influenza-like illness, pneumonia, hospitalization, length of stay, delay in chemotherapy, and mortality
They were not reported as an outcome measure in any of the included studies.
Comparisons related to the efficacy of the influenza vaccination in adult cancer patients during chemotherapy compared with that in other control groups
Comparison 1: Influenza immunity in vaccinated patients receiving chemotherapy with those in vaccinated patients off chemotherapy.
Three studies20–22 (Table 3) reported on this comparison. Result on protective HI titer, four-fold rise in antibody titer in pre and post-vaccination immune assay. The analysis shows that the serological responses to influenza vaccination in adult receiving chemotherapy were weaker than in those completed chemotherapy.
Table 3.
Outcomes of immunogenicity studies of influenza vaccination in adult patients with hematological tumors which didn’t recommend vaccination.
| ARTIClE’S TITLE | SELECTED POPULATION | TREATMENT AT TIME OF VACCINATION | MEASURE OF EFFICACY | RESPONSE | OUTCOMES |
|---|---|---|---|---|---|
| Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma22 | Multiple myeloma (n = 48) | Treated within 1 week of vaccination (n = 16); autologous SCT with melphalan/TBI conditioning 6 months prior (n = 7); interferon-alpha (n = 21); unknown (n = 4) | HI titer >40 | 19% to all three strains | Poor response lead to question for routine single dose influenza vaccination in multiple myeloma patients |
| Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine24 | Heamatologic malignancies (n = 70) | Treatment, described as high or low intensity, with or without monoclonal antibodies (n = 59) | HI titer >40 | 21%, 26% and 16% post vaccination response to each influenzastrain; 4/70 responded to all 3 subtypes | Unclear benefit from 2 doses regimen |
| Impaired serum antibody response to inactivated influenza A and B vaccine in cancer patients36 | Lymphoma, CLL (n = 23); controls (n = 27) | Chlorambucil, cyclophos (n = 21); untreated (n = 2) | 4 fold rise increase in HI titer | 17% v 93% for controls | Does not recommend vaccination for patients with hematologic malignancies |
| The influence of chemotherapy on response of patients with hematologic malignancies to influenza vaccine. Cancer41 | NHL or LPP disorders (n = 25); controls (n = 28) | Untreated, on maintenance with daily oral alkylator and/or steroid, or received weekly or biweekly combination chemotherapy | 4 fold rise increase in HI titer | 36% v 82% for controls | Not recommended, with good response showed in untreated patient with response close to the control |
| Efficacy of the influenza vaccine in patients with malignant lymphoma20 | Lymphoma (n = 29); controls (n = 29) | Treated (n = 21); completed treatment 3 months prior (n = 8) | 4 fold rise increase in HI titer | 3% v 24% for controls | Unclear benefit from influenza vaccination |
| Antibody response to influenza immunization in adult patients with malignant disease21 | Lymphoma (n = 29); controls (n = 15) | Untreated (n = 10); treated (n = 19) | 4 fold rise increase in HI titer | 30%–40% response for lymphoma v 96% for controls | Significant decreased response compared with both solid tumor patients and controls |
Abbreviations: CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin’s lymphoma; LPP, Lymphoproliferative; HI, heamagglutinin inhibition.
Comparison 2: Influenza immunity in vaccinated patients receiving chemotherapy with those in vaccinated healthy adult
A total of 12 studies (Tables 1–3) reported on this comparison. Result on protective HI titer, four-fold rise in antibody titer in pre- and post-vaccination immune assay. The analysis shows that the serological response in patients receiving chemotherapy was weaker than those in healthy controls. Meanwhile, these patients under chemotherapy still mount a protective (HI) immune antibody titer.
Table 1.
Outcomes of immunogenicity studies of influenza vaccination in adult patients with solid tumors which recommend vaccination.
| ARTICLE’S TITLE | SELECTED POPULATION | TREATMENT AT TIME OF VACCINATION | MEASURE OF EFFICACY | RESPONSE | OUTCOMES |
|---|---|---|---|---|---|
| Responses of Patients with Neoplastic Diseases to Influenza Virus Vaccine18 | Patients with cancer (n = 17); patients with solid tumors (n = 15); controls (n = 15) | Multi-agent chemotherapy (n = 8); single-agent chemotherapy (n = 7); immunotherapy (n = 2) | HI titer >40 | 41%–47%, v 67% for controls | Vaccination recommended |
| Sero-conversion after influenza vaccination in patients with lung cancer3 | Lung cancer (n = 59) | Received chemotherapy in the preceding month (n = 14); receiving oral steroids (n = 22) | HI titer >40 | 83% | Vaccination recommended |
| Humoral immune response after vaccination against influenza in patients with breast cancer17 | Breast cancer (n = 9); controls (n = 19) | Mitomycin or CMF (n = 6) | HI titer >40 | 89% v 100% controls | Vaccination recommended |
| Randomized trial of influenza vaccine with granulocyte macrophage colony-stimulating factor or placebo in cancer patients35 | Unspecified tumor types (n = 133), observed over 3 year period | N/A | HI titer >40 | 21% to 60% responded, depending on year and influenza subtype | Vaccination recommended |
| Impaired serum antibody response to inactivated influenza A and B vaccine in cancer patients36 | Breast cancer (n = 13); not reported (n = 3); controls (n = 27) | Breast cancer patients received Cyclophosphamide, MTX, FU (n13) | 4 Fold increase in HI titer | 50% v 93% controls | Recommended vaccination for patient with solid tumors but doesn’t recommended for hematological malignancies |
| Antibody response to influenza immunization in adult patients with malignant disease21 | Various tumor types (n = 53); controls (n = 15) | Various regimens, including XRT (n = 39); untreated (n = 14) | 4 Fold increase in HI titer | Significantly lower rates of Sero-conversion v controls | Recommended vaccination for solid tumor and poor for hematological tumors |
Abbreviations: MTX, methotrexate; FU, fluorouracil; cyclophosphamide; XRT, radiation therapy; CMF, cyclophosphamide, methotrexate, fluorouracil; HI, heamagglutinin inhibition.
Comparison 3: Influenza immunity in vaccinated patients receiving chemotherapy in solid tumors with those receiving chemotherapy in hematological tumors
In all, 16 studies (Tables 1–3) reported on this comparison. The analysis shows 6 studies (Table 1) with solid tumors mounted a protective HI titer and 12 studies with hematological tumors, and concluded with vaccination recommendation. Six studies (Table 2) with hematological tumors mounted a protective HI titer and concluded with vaccination recommendation, whereas the other six studies (Table 3) with hematological tumors did not find a significance of vaccination and they did not recommend vaccination.
Table 2.
Outcomes of immunogenicity studies of influenza vaccination in adult patients with hematological tumors which recommend vaccination.
| ARTICLE’S TITLE | SELECTED POPULATION | TREATMENT AT TIME OF VACCINATION | MEASURE OF EFFICACY | RESPONSE | OUTCOMES |
|---|---|---|---|---|---|
| Response to influenza A vaccine among high-risk patients37 | Hematologic malignancies (n = 31); controls (n = 41) | Maintenance treatment (n = 14); cyclic chemotherapy (n = 7); untreated (n = 10) | 4 fold rise increase in HI titer | 52% v 78% for controls in first strain; 32% v 56% for controls in second strain | Vaccination recommended |
| Antibody response to a two-dose influenza vaccine regimen in adult lymphoma patients on chemotherapy23 | Lymphoma (n = 41) | Doxorubicin (66%); cyclophos (56%); etoposide (46%); ara-c(39%); cisplatin (39%);bleomycin (37%); VCR (29%); mesna (29%); steroids (100%); some alpha-interferon | 4 fold rise increase in HI titer | 42% after single shot; 50% after second vaccination | Recommended vaccination of 2 doses regimen |
| Humoral response to hemagglutinin components of influenza vaccine in patients with non-Hodgkin malignant lymphoma38 | NHL (n = 32); controls (n = 32) | Immunosuppressive drugs (n = 16); “not subjected to this therapy” (n = 11); unaccounted for (n = 5) | HI MFI, seroprotection, RR | MFI from 9.3 to 12.2 v27.6 to 44.3 for controls; lymphoma Sero-protection rate increased from 59% to 69% v 91% to 97% for controls and RR went from 47% to 69% v 84% to 88% for controls | Vaccination recommended |
| Influenza immunization of adult patients with malignant diseases29 | Hematologic malignancies (n = 21); solid tumors (n = 21); controls (n = 96) | N/A | HI titer >20 | GMT 55.6, v 110 for controls; 67% response rate v 94% for controls | Vaccination recommended |
| Influenza virus vaccine in B-cell chronic lymphocytic leukemia patients39 | CLL (n = 43); controls (n = 10) | Untreated (n = 26); Chlorambucil <20 days before vaccination (n = 17) | HI titer >20 | 56% v 100% for controls at 60 days Post-vaccination | Vaccination recommended |
| Influenza vaccine in chronic lymphoproliferative disorders and multiple myeloma40 | LPP disorders (n = 34); controls (n = 34) | Treatment with combination of multiple regimens, including cyclophosphamide, prednisone, CHOP, MOPP, ABVD, melphalan, VAD, XRT (n = 24) | HI titer >40 | 76%, 62%, and 65% seroprotection to 3 strains v 97%, 82% and 97% for controls | Vaccination recommended |
Abbreviations: XRT, radiation therapy; CLL, chronic lymphocytic leukemia; HD, Hodgkin’s lymphoma; GMT, geometric mean titre; NHL, non-Hodgkin’s lymphoma; LPP, Lymphoproliferative; MFI, mean fold increase; HI, heamagglutinin inhibition.
Comparison 4: Influenza immunity in two vaccination schedules in patients receiving systemic chemotherapy
Two studies23,24 reported on this comparison. One study (Table 1) reported significance of two vaccination schedules. The other study (Table 2) showed no significance of two doses of vaccination.
Discussion
Cancer and influenza vaccination issue is still unconcluded especially in patient with cancer undergoing systemic chemotherapy. This specific group of patients seems to have an increased risk of infection for which influenza vaccine may offer additional protection and significant benefit.5,17,25 There are positive data in the majority of the studies reviewed showing vaccine efficacy and serological protective levels in patients with solid tumors such as breast cancer and lung cancer.5,17 On the other hand, some other studies found that immunization has no benefit in providing adequate sero-conversion, especially in patients with lymphomas; hence, they did not recommend the vaccination in these patients.24,26,27
Serological response
Matsuzaki et al compared the serological response of children with cancer to influenza vaccine with those of healthy children, although the response in children actively receiving chemotherapy was significantly weaker than that in children having completed chemotherapy. However, they recommended vaccination and suggested other large and outside Japan studies needed in future.28 Robertson et al confirmed that patients with multiple myeloma are susceptible to infection with Streptococcus pneumoniae and influenza and demonstrate impaired ability to mount a good humoral response to vaccination. In particular, the extremely poor response to influenza vaccination calls into question the policy of vaccinating patients with myeloma using a conventional single-shot influenza vaccine. This small study was unable to address many other clinical variables that will be of relevance such as vaccination responses in MGUS (monoclonal gammopathy of uncertain significance) or untreated myeloma and in early versus late diseases, and emphasized that further studies are needed.22
Safety and timing
Safety is a very important issue in vaccination in these particular patients. Sommer et al added that the use of inactivated vaccines in cancer patients is safe, but the ability of a cancer patient to mount an immune response is significantly dependent on the time of vaccine administration in relation to time of chemotherapy administration and added that patients with solid tumors receiving myelosuppressive chemotherapy should be vaccinated only with inactivated or attenuated vaccines, as clinically indicated. They emphasized that vaccines should be administered at a minimum of two weeks before or post-chemotherapy administration for optimal benefit to the patient.6 Gross et al reported that continual chemotherapy is required during the induction and maintenance treatment of individuals with malignancies, and the decision to interrupt chemotherapy for one month is to permit an adequate antibody response to influenza immunization. Timing was very controversial. Ortbals et al confirmed that influenza infection is more severe in patients with neoplastic disease, may require interruption of antineoplastic therapy, and depresses immune responses that theoretically might adversely affect the course of an infection or increase infection rates in patients with cancer. Therefore, vaccination to prevent influenza is indicated in these patients.29
Brown et al concluded that two months are needed for influenza vaccination after completion of chemotherapy.30 Chrisholm et al added children with acute lymphocytic leukemia (ALL), and supported the recommendation that all children receiving chemotherapy for cancer and those within six months of completing therapy should undergo annual influenza immunization.
Cost and dosage series
Molinari et al conducted a study to measure the annual impact of influenza vaccination and the cost and concluded immunization against influenza can effectively reduce the annual economic burden of influenza in the United States.31
CDC or Advisory committee on immunization practices (ACIP) still doesn’t recommend increasing in dose series in healthy adults although a study of non-Hodgkin lymphoma (NHL) receiving chemotherapy proved that 50% had a high HI titer who received two doses of influenza vaccination versus only 42% for the patients who received one dose of influenza vaccination.25,32,23 It has been proven in healthy adult population that higher dosages of influenza vaccination provide a good result and improvement in the level of HI titer.33 However, other studies showed that high dosage influenza vaccination in NHL produces better responses and is well tolerated by the patients.34
Debate is still ongoing on the optimal vaccination of patient with cancer undergoing chemotherapy. Many studies handled this issue in depth, but each study has its supportive and unsupportive evidences regarding the timing of vaccination. Some studies supported vaccination during chemotherapy cycle,29 whereas other studies reveal the opposite meaning that the patients who completed the course chemotherapy have better responses than patients vaccinated at active treatment course.
Authors’ Conclusions
Implication for practices
In national guidelines, it is recommended that patients who are being treated for cancer should be vaccinated against influenza. The full picture and the clinical evidence from randomized controlled studies to support this recommendation are still lacking. We can conclude from the articles included in this review that patients with solid and hematological tumors are able to mount an immune response to influenza vaccine, but it remains unclear whether this immune response fully protects them from influenza infection or its complications. We can emphasize that influenza vaccine appears to be safe in these patients. While awaiting results of randomized controlled trials addressing the complete picture of the clinical benefits from influenza vaccination, the clinicians must consider the benefits of influenza vaccination such as decrease in the duration and severity of the disease and significant decrease in influenza-associated morbidity and mortality in these high-risk patients.5
Implication for research
A well-designed prospective, multi-center, randomized controlled trial of influenza vaccination in patients being treated for cancer is necessary. This trial should have a low risk of bias and should measure carefully the clinical relevant outcomes, including laboratory-confirmed influenza infection, hospitalizations, hospital length of stay, and pneumonia. This study should be conducted in large-scale population. The purpose of this study is to give the clinical evidence of benefits of influenza vaccination in patients with cancer undergoing systemic chemotherapy.
Limitations
The included studies used different immunization schedules according to guidelines from different eras. These studies were published in the last 50 years. Studies’ population is from different age groups. Age is a possible confounder for immune response. The included studies had relatively small sample sizes. The results described are all based on separate small studies. Larger trials are needed to verify the result of these studies.
Footnotes
ACADEMIC EDITOR: William C.S. Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
Author Contributions
MS, NK conceived and designed the study. MS analyzed the data. MS wrote the first draft of the manuscript. MS contributed to the writing of the manuscript. MS, NK agreed with manuscript results and conclusions. MS, NK jointly developed the structure and arguments of the paper. MS, NK made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Borella L, Webster RG. The immunosuppressive effects of long-term combination chemotherapy in children with acute leukemia in remission. Cancer Res. 1971;31:420–6. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Y Update: Recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep. 2010;59:989–92. [PubMed] [Google Scholar]
- 3.Loulergue P, Mir O, Alexandre J, Ropert S, Goldwasser F, Launay O. Low influenza vaccination rate among patients receiving chemotherapy for cancer. Ann Oncol. 2008;19:1658. doi: 10.1093/annonc/mdn531. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention X Updated recommendations for use of meningococcal conjugate vaccines – Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72–6. [PubMed] [Google Scholar]
- 5.Anderson H, Petrie K, Berrisford C, Charlett A, Thatcher N, Zambon M. Seroconversion after influenza vaccination in patients with lung cancer. Br J Cancer. 1999;80:219–20. doi: 10.1038/sj.bjc.6690342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer AL, Wachel BK, Smith JA. Evaluation of vaccine dosing in patients with solid tumors receiving myelosuppressive chemotherapy. J Oncol Pharm Pract. 2006;12:143–54. doi: 10.1177/1078155206070868. [DOI] [PubMed] [Google Scholar]
- 7.Monto AS, Kioumehr F. The Tecumseh Study of Respiratory Illness. IX. Occurrence of influenza in the community, 1966–1971. Am J Epidemiol. 1975;102:553–63. doi: 10.1093/oxfordjournals.aje.a112193. [DOI] [PubMed] [Google Scholar]
- 8.Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974–76. N Engl J Med. 1978;298:587–92. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 9.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 10.Barker WH. Excess pneumonia and influenza associated hospitalization during influenza epidemics in the United States, 1970–78. Am J Public Health. 1986;76:761–5. doi: 10.2105/ajph.76.7.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 12.Pollyea DA, Brown JM, Horning SJ. Utility of influenza vaccination for oncology patients. J Clin Oncol. 2010;28:2481–90. doi: 10.1200/JCO.2009.26.6908. [DOI] [PubMed] [Google Scholar]
- 13.Novel Swine-Origin Influenza A (H1N1)Virus Investigation Team X. Dawood FS, Jain S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 14.New influenza A (H1N1) virus: global epidemiological situation, June 2009. Releve epidemiologique hebdomadaire/Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations. 2009;84:249–57. [PubMed] [Google Scholar]
- 15.Cooksley CD, Avritscher EB, Bekele BN, Rolston KV, Geraci JM, Elting LS. Epidemiology and outcomes of serious influenza-related infections in the cancer population. Cancer. 2005;104:618–28. doi: 10.1002/cncr.21203. [DOI] [PubMed] [Google Scholar]
- 16.Steinherz PG, Brown AE, Gross PA, et al. Influenza immunization of children with neoplastic diseases. Cancer. 1980;45:750–6. doi: 10.1002/1097-0142(19800215)45:4<750::aid-cncr2820450423>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Brydak LB, Guzy J, Starzyk J, Machala M, Gozdz SS. Humoral immune response after vaccination against influenza in patients with breast cancer. Support Care Cancer. 2001;9:65–8. doi: 10.1007/s005200000186. [DOI] [PubMed] [Google Scholar]
- 18.Ganz PA, Shanley JD, Cherry JD. Responses of patients with neoplastic diseases to influenza virus vaccine. Cancer. 1978;42:2244–7. doi: 10.1002/1097-0142(197811)42:5<2244::aid-cncr2820420523>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Fiore AE, Shay DK, Haber P, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56:1–54. [PubMed] [Google Scholar]
- 20.Mazza JJ, Yale SH, Arrowood JR, et al. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005;3:214–20. doi: 10.3121/cmr.3.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shildt RA, Luedke DW, Kasai G, El-Beheri S, Laham MN. Antibody response to influenza immunization in adult patients with malignant disease. Cancer. 1979;44:1629–35. doi: 10.1002/1097-0142(197911)44:5<1629::aid-cncr2820440514>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Robertson JD, Nagesh K, Jowitt SN, et al. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer. 2000;82:1261–5. doi: 10.1054/bjoc.1999.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo W, Whimbey E, Elting L, Couch R, Cabanillas F, Bodey G. Antibody response to a two-dose influenza vaccine regimen in adult lymphoma patients on chemotherapy. Eur J Clin Microbiol Infect Dis. 1993;12:778–82. doi: 10.1007/BF02098469. [DOI] [PubMed] [Google Scholar]
- 24.Ljungman P, Nahi H, Linde A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: a randomised study. Br J Haematol. 2005;130:96–8. doi: 10.1111/j.1365-2141.2005.05582.x. [DOI] [PubMed] [Google Scholar]
- 25.Bridges CB, Thompson WW, Meltzer MI, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA. 2000;284:1655–63. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 26.Mackay HJ, McGee J, Villa D, et al. Evaluation of pandemic H1N1 (2009) influenza vaccine in adults with solid tumor and hematological malignancies on active systemic treatment. J Clin Virol. 2011;50:212–6. doi: 10.1016/j.jcv.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Feery BJ, Sullivan JR, Hurley TH, Evered MG. Immunization with influenza vaccine in patients with haematological malignant disease. Med J Aust. 1977;1:292–4. doi: 10.5694/j.1326-5377.1977.tb130704.x. [DOI] [PubMed] [Google Scholar]
- 28.Matsuzaki A, Suminoe A, Koga Y, Kinukawa N, Kusuhara K, Hara T. Immune response after influenza vaccination in children with cancer. Pediatr Blood Cancer. 2005;45:831–7. doi: 10.1002/pbc.20470. [DOI] [PubMed] [Google Scholar]
- 29.Ortbals DW, Liebhaber H, Presant CA, Van Amburg AL, III, Lee JY. Influenza immunization of adult patients with malignant diseases. Ann Intern Med. 1977;87:552–7. doi: 10.7326/0003-4819-87-5-552. [DOI] [PubMed] [Google Scholar]
- 30.Brown AE, Steinherz PG, Miller DR, et al. Immunization against influenza in children with cancer: results of a three-dose trial. J Infect Dis. 1982;145:126. doi: 10.1093/infdis/145.1.126. [DOI] [PubMed] [Google Scholar]
- 31.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 32.Gross PA, Weksler ME, Quinnan GV, Jr, Douglas RG, Jr, Gaerlan PF, Denning CR. Immunization of elderly people with two doses of influenza vaccine. J Clin Microbiol. 1987;25:1763–5. doi: 10.1128/jcm.25.9.1763-1765.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J. 1973;49:152–8. doi: 10.1136/pgmj.49.569.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safdar A, Rodriguez MA, Fayad LE, et al. Dose-related safety and immunogenicity of baculovirus-expressed trivalent influenza vaccine: a double-blind, controlled trial in adult patients with non-Hodgkin B cell lymphoma. J Infect Dis. 2006;194:1394–7. doi: 10.1086/508493. [DOI] [PubMed] [Google Scholar]
- 35.Schafer AI, Churchill WH, Ames P, Weinstein L. The influence of chemotherapy on response of patients with hematologic malignancies to influenza vaccine. Cancer. 1979;43:25–30. doi: 10.1002/1097-0142(197901)43:1<25::aid-cncr2820430103>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 36.Ramanathan RK, Potter DM, Belani CP, et al. Randomized trial of influenza vaccine with granulocyte-macrophage colony-stimulating factor or placebo in cancer patients. J Clin Oncol. 2002;20:4313–8. doi: 10.1200/JCO.2002.02.041. [DOI] [PubMed] [Google Scholar]
- 37.Stiver HG, Weinerman BH. Impaired serum antibody response to inactivated influenza A and B vaccine in cancer patients. Can Med Assoc J. 1978;119:733, 5, 8. [PMC free article] [PubMed] [Google Scholar]
- 38.Hodges GR, Davis JW, Lewis HD, Jr, et al. Response to influenza A vaccine among high-risk patients. South Med J. 1979;72:29–32. doi: 10.1097/00007611-197901000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Brydak LB, Machala M, Centkowski P, Warzocha K, Bilinski P. Humoral response to hemagglutinin components of influenza vaccine in patients with non-Hodgkin malignant lymphoma. Vaccine. 2006;24:6620–3. doi: 10.1016/j.vaccine.2006.05.100. [DOI] [PubMed] [Google Scholar]
- 40.Gribabis DA, Panayiotidis P, Boussiotis VA, Hannoun C, Pangalis GA. Influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol. 1994;91:115–8. doi: 10.1159/000204315. [DOI] [PubMed] [Google Scholar]
- 41.Rapezzi D, Sticchi L, Racchi O, Mangerini R, Ferraris AM, Gaetani GF. Influenza vaccine in chronic lymphoproliferative disorders and multiple myeloma. Eur J Haematol. 2003;70:225–30. doi: 10.1034/j.1600-0609.2003.00028.x. [DOI] [PubMed] [Google Scholar]