Abstract
Background
Triple-negative breast cancer (TNBC) is a special subtype of breast cancer that is characterized by poor prognosis, strong tumor invasion and a high pathologic complete response (pCR) to neoadjuvant chemotherapy (NAC). The pCR rate is a prognostic factor for TNBC. We aimed to evaluate the relationship between pCR and TNBC after NAC and originally tried to identify factors related to achieving pCR for TNBC using a meta-analysis.
Methods
We systematically searched the literature for pCR and breast cancer after NAC and carefully identified eligibility criteria. The association between pCR and breast cancer subtypes was estimated using Review Manager, while pCR rates for TNBC and non-TNBC were determined using Meta-Analyst.
Results
This analysis included a total of 9,460 cases from 27 studies. The summary odds ratio estimating the relationship between pCR and breast cancer subtypes (TNBC vs non-TNBC) was 3.02 (95% confidence interval (CI), 2.66 to 3.42). The TNBC pCR rate was 28.9% (95% CI, 27.0 to 30.8%) and the non-TNBC was 12.5% (95% CI, 11.7 to 13.4%). From subgroup analyses, we identified the factors associated with the highest pCR rates for TNBC.
Conclusions
TNBC has a higher pCR rate than non-TNBC. In the NAC setting, these factors of platinum-containing, more than six cycles, four kinds of drugs, 16 weeks’ treatment duration and sequential chemotherapy may contribute to increasing the pCR rate.
Keywords: breast cancer, neoadjuvant chemotherapy, pathologic complete response, meta-analysis
Background
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that accounts for approximately 15% of all breast cancers [1,2]. TNBC lacks the three important therapeutic markers for clinical regimens of patients with breast cancer: estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). Due to the absence of a therapeutic target (endocrine therapy targets the ER and PR, and trastuzumab targets HER2), the prognosis of patients with TNBC is poorer than that of patients with other types of breast cancer. Patients with TNBC are characterized by early recurrence [3,4] and a significantly shorter survival compared with those with non-TNBCs [5,6].
Neoadjuvant chemotherapy (NAC) is increasingly being used in the treatment of large operable breast cancers or to prevent lymph node metastases, where it is as effective as adjuvant chemotherapy and considered a standard of treatment for patients with locally advanced breast cancer [7]. The advantages of NAC in operable breast cancer include: increasing the rate of success of breast-conserving surgery by downstaging the primary tumor load, early prevention of cancer metastasis in lymphonodi or viscera and providing suggestions for selecting the adjuvant chemotherapy regimen through estimating the clinical response to NAC and avoiding a potentially ineffective treatment in adjuvant chemotherapy.
Interestingly, several clinical studies on NAC for breast cancers have shown that TNBC has lower survival and higher relapse rates among all breast cancer subsets but has a higher rate of pathologic complete response (pCR) to NAC than other phenotypes and patients with pCR have excellent survival [1,8]. In other words, patients with TNBC who do not have pCR are at increased risk of early relapse and death [1,9]. pCR has been proven to be a prognostic factor for breast cancer by von Minckwitz and Xiangnan Kong [10,11]. Consequently, pCR plays a very significant role in predicting prognosis and clinical management for patients with TNBC.
We therefore performed a meta-analysis aiming to report the association between NAC and pCR for TNBC. It was also our purpose to observe which factors are potentially related with pCR in TNBC treated with NAC, such as NAC cycles, drugs and schedules.
Methods
Literature search
The MEDLINE, EMBASE and Cochrane Library databases were systematically searched to September 2013. Publications with the following search words in the title, abstract or key words were included: breast cancer, TNBC, NAC, preoperative chemotherapy, pathologic complete response, pathologic complete remission and pathologic response. The studies identified through the search were independently screened by two authors (KW and AW) for inclusion. Any disagreements were arbitrated by a third author (ZY). We did not limit our search by language, country, race or date.
Inclusion and exclusion criteria
Studies performed using humans regardless of sample size were included if they met the following criteria: papers studying the association between NAC and pCR in TNBCs; all cases definitely diagnosed as breast cancer and where distant metastasis was excluded; ER, PR, HER2 measured by immunohistochemistry (IHC) and/or fluorescence in situ hybridization of primary cancer tissue; pCR explicitly defined; and detailed statistics had to be reported (i.e. patient numbers and percentage of pCR). Any investigations that did not meet all inclusion criteria and cross-sectional studies were excluded. If data were duplicated in more than one paper, the most recent paper was included in the analysis.
Data extraction
Data were independently extracted by two authors (QY and YL) using the same standardized table. The fields extracted included first author, year of publication, NAC schedule (type, number of cycles, interval and treatment duration), and number and percentage of patients achieving pCR in TNBC and non-TNBC. For articles with the same population resources or overlapping datasets, data were extracted and reported as a single trial.
Statistical analysis
The Cochrane Collaboration Review Manager 5.1 and Meta-Analyst Beta 3.13 statistical software were used for this meta-analysis. The χ2 and I2 test methods were used to evaluate the heterogeneity of the odds ratios (ORs) in the studies. When I2 < 50% and P > 0.05 for χ2, indicating heterogeneity in the results, the heterogeneity in the studies was considered acceptable and the fixed-effect model with the Mantel–Haenszel method was used for the two-arm meta-analysis or the inverse variance method was used for the single-arm meta-analysis. Otherwise, a random-effect model with the DerSimonian and Laird method was adapted for both the one- and two-arm meta-analyses. Each study was weighted according to the sample size.
Subgroup analyses were executed for NAC cycles, drugs and schedules. The sensitivity was analyzed by excluding small cases studies (defined as <100 cases) and changing the effect model to estimate confidence. Potential publication bias was evaluated using funnel plots. An asymmetric plot indicates there was potential publication bias; otherwise, the plot should be shaped like a funnel.
Ethical standards
This study complies with the current laws of China.
Results
Eligible studies
We identified 516 studies in the three databases and their bibliographies of relevant clinical trials. After excluding duplicates (n = 126), the titles and abstracts of all remaining studies (n = 390) were reviewed. Of these 390 studies, we excluded 353 that did not meet the selection criteria. After reviewing the full text of the remaining 37 studies, we ultimately included 27 studies [1,8,9,12-35] in the final analysis. Ten studies were excluded from the final review for these reasons: insufficient data (n = 1) [36], cross-sectional study (n = 1) [37], distant metastasis (n = 5) [38-42], undefined pCR (n = 1) [43] or undefined hormone receptor (n = 1) [44]. The same populations were reviewed in two papers [23,45], the data were extracted and reported as a single study. Figure 1 shows a flow diagram with the numbers of relevant studies.
Figure 1.
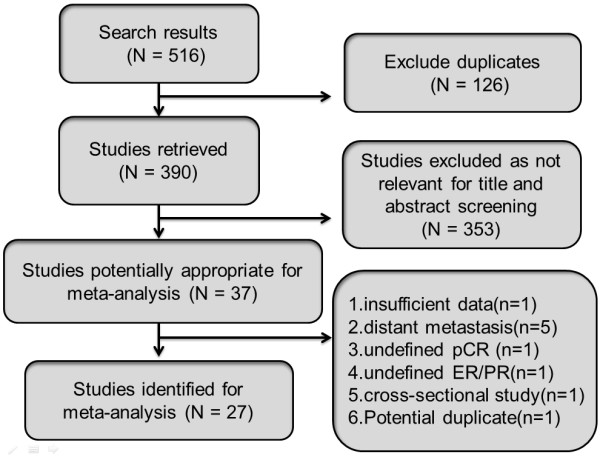
Flow chart used to identify relevant literature. ER, estrogen receptor; pCR, pathologic complete response; PR, progesterone receptor.
Study characteristics
In all, 27 studies published between 2005 and 2012 were included in this meta-analysis. Table 1 shows the main characteristics of all of the studies. A total of 9,460 cases from the 27 studies that had pathological results and clinical data were included. Enrollment of participants across the studies was from 1985 to 2009. Most studies enrolled patients who had been diagnosed with breast cancer stages II and III (n = 17), but some studies also recruited stage I patients (n = 8) and a few studies recruited non-metastatic patients (n = 3). According to these studies, the percentage of patients achieving pCR after NAC was 3.1 to 66.7% (TNBC, 14.6 to 66.7%; non-TNBC, 3.1 to 39.0%). NAC consisted of an anthracycline and/or taxane with other chemotherapeutic regimens.
Table 1.
Characteristics of the eligible studies
| Author | Year | Tumor stage | Neoadjuvant chemotherapy |
TNBC |
pCR rate of TNBC |
non-TNBC |
pCR rate of non-TNBC | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| pCR | no-pCR | pCR | no-pCR | |||||||
| Rouzier R [8] |
2005 |
I, II, III |
12 weeks of P followed by FAC 4 courses (weekly P (80 mg/m2) × 12 + FAC × 4 or 3 weekly P (225 mg/m2) × 4 + FAC × 4) |
10 |
12 |
45.50% |
11 |
49 |
18.30% |
3.71 (1.28, 10.76) |
| Carey LA [1] |
2007 |
II, III |
A 60 mg/m2 + C 600 mg/m2 every 2 weeks or 3 weeks for 4 cycles, either alone or as the first component of a sequential AC-taxane neoadjuvant regimen |
9 |
25 |
26.50% |
8 |
69 |
10.40% |
3.10 (1.08, 8.93) |
| Goldstein NS [12] |
2007 |
IIA to IIIC |
FAC every 3 weeks × 6 FEC every 3 weeks × 6 AC every 2 weeks (dose dense) × 4 then paclitaxel every 2 weeks (dose dense) × 4 AC every 3 weeks × 4 then P every 1 week × 4 |
12 |
9 |
57.10% |
16 |
31 |
34.00% |
2.58 (0.90, 7.41) |
| Keam B [13] |
2007 |
II, III |
D (75 mg/m2 or 60 mg/m2) and A (60 mg/m2 or 50 mg/m2) by intravenous infusion every 3 weeks for 3 cycles |
8 |
39 |
17.00% |
3 |
95 |
3.10% |
6.50 (1.64, 25.78) |
| Liedtke C [9] |
2008 |
I, II, III |
FAC; FEC; weekly or once every 3 weeks P/D followed by FAC; weekly or once every 3 weeks P/D followed by FEC |
57 |
198 |
22.40% |
98 |
765 |
11.40% |
2.25 (1.56, 3.23) |
| Bidard FC [14] |
2008 |
I, II, III |
FEC (F 500 mg/m2, E 100 mg/m2, C 500 mg/m2) or FAC (F 500 mg/m2, A 60 mg/m2, C 500 mg/m2), every 3 weeks for 4 to 6 cycles |
21 |
99 |
17.50% |
7 |
166 |
4.00% |
5.03 (2.06, 12.26) |
| Julka PK [15] |
2008 |
IIA to IIIB |
4 cycles (21 days) of Gem 1,200 mg/m2 + A 60 mg/m2, 4 cycles of Gem 1,000 mg/m2 plus Cis 70 mg/m2 |
7 |
7 |
50.00% |
6 |
16 |
27.30% |
2.67 (0.65, 10.88) |
| Sánchez-Muñoz A [16] |
2008 |
II, III |
Schedule A: E 90 mg/m2 + C 600 mg/m2 d1 for 3 cycles followed by a second sequence with P 150 mg/m2 + Gem 2,500 mg/m2 d1 ± trastuzumab 2 mg/kg/week according to HER2 status Schedule B: A 40 mg/m2 d1 + P 150 mg/m2 + Gem (2,000 mg/m2) d2, 2 weekly for 6 cycles |
14 |
10 |
58.30% |
17 |
58 |
22.70% |
4.78 (1.80, 12.66) |
| Sirohi B [17] |
2008 |
M0 |
F 200 mg/m2 daily with E 60 mg/m2 and Cis 60 mg/m2 both repeating 3 weekly for 6 courses |
1 |
5 |
16.70% |
5 |
49 |
9.30% |
1.96 (0.19, 20.26) |
| Darb-Esfahani S [18] |
2009 |
T2 to 3, N0 to 2, M0 |
A 50 mg/m2 + D 75 mg/m2 every 14 days for 4 cycles or 4 cycles A 60 mg/m2 plus C 600 mg/m2 every 21 days followed by D 100 mg/m2 every 21 days for 4 cycles |
8 |
25 |
24.20% |
5 |
78 |
6.00% |
4.99 (1.50, 16.65) |
| Sikov WM [19] |
2009 |
IIA to IIIB |
Cb (AUG = 6) every 4 weeks and P 80 mg/m2 weekly for 16 weeks, and weekly trastuzumab was added for HER2(+) status |
8 |
4 |
66.70% |
16 |
25 |
39.00% |
3.13 (0.81, 12.11) |
| Bhargava R [20] |
2010 |
I, II, III |
Anthracycline-based therapy: AC, FEC; taxane-based therapy: T/P + Cb. In many cases a sequential combination of anthracycline and taxane was given: AC-T. The total number of cycles ranged from 4 to 10 with an average of 6 |
24 |
55 |
30.40% |
24 |
256 |
8.60% |
4.65 (2.46, 8.80) |
| Chang HR [21] |
2010 |
II, III |
D (75 mg/m2) and Cb (AUC = 6) were administered every 3 weeks for 4 cycles. Patients with HER2(+) tumors were randomized to receive either additional weekly trastuzumab preoperatively or TC alone |
6 |
5 |
54.50% |
13 |
47 |
21.70% |
4.34 (1.14, 16.51) |
| Chavez-Macgregor M [22] |
2010 |
M0 |
Taxane administered: P 175 to 250 mg/m2 on d1, 3 weekly for 4 cycles; P 80 mg/m2 weekly for 12 doses; or D 100 mg/m2 on d1, 3 weekly for 4 cycles Anthracycline regimens (3 to 6 cycles): F 500 mg/m2, E 100 mg/m2 and C 500 mg/m2 on d1, d3 weekly; F 500 mg/m2 on d1, d4, E 75 mg/m2 and C 500 mg/m2 on d1, 3 weekly |
95 |
395 |
19.40% |
165 |
1419 |
10.40% |
2.07 (1.57, 2.73) |
| Chen XS [23] |
2010 |
T3 to 4, any N, M0; any T, N2 to 3, M0 |
VE: V 25 mg/m2 d1, d8 + E 60 mg/m2 d1, d3 weekly; PCb: P 80 mg/m2 + Cb AUC = 2 d1, d8, d15, 4 weekly; CEF: C 500 mg/m2, E 75 mg/m2 and F 500 mg/m2 d1, 3 weekly; CTF: C 500 mg/m2, THP 50 mg/m2, and F 500 mg/m2 d1, 3 weekly; CEF➝T: D 75 mg/m2 d1, 3 weekly; ED: E 60 mg/m2 + D 75 mg/m2, 3 weekly |
9 |
44 |
17.00% |
19 |
153 |
11.00% |
2.07 (0.86, 4.96) |
| Huober J [24] |
2010 |
I, II, III |
6 to 8 cycles of TAC (D 75 mg/m2, A 50 mg/m2, C 500 mg/m2 on d1, every 3 weeks) or 2 cycles of TAC followed by four cycles of V 25 mg/m2 on d1, d8 + capecitabine 1,000 mg/m2 orally twice a day on d1 to d14 every 3 weeks |
198 |
311 |
38.90% |
147 |
820 |
15.20% |
3.55 (2.77, 4.56) |
| Kim SI [25] |
2010 |
M0 |
A (50 mg/m2, d1) + D (75 mg/m2, d1) chemotherapy (AT) every 3 weeks for 3 cycles |
16 |
60 |
21.10% |
10 |
181 |
5.20% |
4.83 (2.08, 11.21) |
| Pierga JY [26] |
2010 |
II, III |
E (75 mg/m2) + C (750 mg/m2) intravenously every 3 weeks for 4 cycles followed by D (100 mg/m2) every 3 weeks for 4 cycles with or without trastuzumab (8 mg/kg at first infusion then 6 mg/kg) every 3 weeks |
23 |
55 |
29.50% |
14 |
57 |
19.70% |
1.70 (0.80, 3.64) |
| Straver ME [27] |
2011 |
T1 to 3, N0 to 2, M0 |
AC (6 cycles of A 60 mg/m2 and C 600 mg/m2 every 3 weeks) or AD (6 cycles of A 50 mg/m2 and D 75 mg/m2, every 3 weeks) |
16 |
41 |
28.10% |
13 |
181 |
6.70% |
5.43 (2.43, 12.17) |
| Bernsdorf M [28] |
2011 |
T2 to 3, N0 to 3b, M0 |
4 cycles of EC (E 90 mg/m2 and C 600 mg/m2) plus 12 weeks of daily treatment with gefitinib 250 mg or EC plus 12 weeks’ treatment with placebo. Chemotherapy was administered every 3 weeks |
12 |
70 |
14.60% |
1 |
47 |
2.10% |
8.06 (1.01, 64.06) |
| Iwata H [29] |
2011 |
T1c to 3, N0, M0; T1 to 3, N1, M0 |
4 cycles of D (75 mg/m2) administered intravenously every 21 days followed by 4 cycles of FEC (F 500 mg/m2, E 100 mg/m2 and C 500 mg/m2) administered intravenously on d1 every 21 days before surgery |
14 |
15 |
48.30% |
16 |
84 |
16.00% |
4.90 (1.99, 12.09) |
| Loo CE [30] |
2011 |
T2 to 4, N1 to 3, M0 |
Either ER(+) or (–), received 6 courses of AC, administered in a dose-dense schedule (every 2 weeks). A minority received 6 courses of capecitabine + D or doxorubicin + D |
16 |
41 |
28.10% |
22 |
119 |
15.60% |
2.11 (1.01, 4.40) |
| Medioni J [31] |
2011 |
II, III |
Six 2-weekly courses of Gem 1,000 mg/m2 + D 75 mg/m2 on d1, d15 and V 25 mg/m2 + E 100 mg/m2 on d29, d43. Patients with an objective response on d56 then received another cycle of Gem + D on d57 and V + E on d71 |
9 |
13 |
40.90% |
7 |
43 |
14.00% |
4.25 (1.32, 13.65) |
| Nakahara H [32] |
2011 |
T1 to 4 |
HER2(–) tumors started with CE (E 75 mg/m2 × d1 + C 100 mg × daily for 14 days with 7 days’ rest) for 4 or 6 cycles. HER2(+) tumors initiated with CE (E 90 mg/m2 × d1 or E 50 mg/m2 × d1, d8 and C 100 mg × daily for 14 days with 7 days’ rest) |
5 |
13 |
27.80% |
3 |
65 |
4.40% |
8.33 (1.77, 39.27) |
| Wu J [33] |
2011 |
II, III |
P (175 mg/m2) or D (75 mg/m2) + doxorubicin (60 mg/m2) or E (90 mg/m2) every 21 days for a total of 4 cycles |
14 |
40 |
25.90% |
24 |
171 |
12.30% |
2.49 (1.19, 5.25) |
| Le Tourneau C [34] |
2012 |
II, III |
4 cycles of intensified FAC (A 70 mg/m2 d1, C 700 mg/m2 d1 + d8, and F 700 mg/m2 d1 to d5) every 3 weeks |
9 |
10 |
47.40% |
3 |
30 |
9.10% |
9.00 (2.03, 39.93) |
| Ono M [35] | 2012 | II, III | Anthracycline-based regimen (AC: A 60 mg/m2 + C 600 mg/m2 or CEF: C 600 mg/m2 + E 100 mg/m2 + F 600 mg/m2) Taxane-based regimen (weekly P 80 mg/m2 or triweekly D 75 mg/m2) Anthracycline and taxane sequentially or concurrently (A 50 mg/m2 + D 60 mg/m2, AC or CEF followed by weekly P or triweekly D) | 26 | 66 | 28.30% | 9 | 70 | 11.40% | 3.06 (1.34, 7.02) |
A, adriamycin; C, cyclophosphamide; Cb, carboplatin; CI, confidence interval; Cis, cisplatin; d, day; D, docetaxel; E, epirubicin; F, 5-fluorouracil; Gem, gemcitabine; HER2, human epidermal growth factor receptor 2; OR, odds ratio; P, paclitaxel; pCR, pathologic complete response; THP, pirarubicin; TNBC, triple-negative breast cancer; V, vinorelbine; T, Taxane/Taxotere; AUC, area under the curve.
Pathologic complete response and breast cancer subtypes (triple-negative breast cancer and non-triple-negative breast cancer)
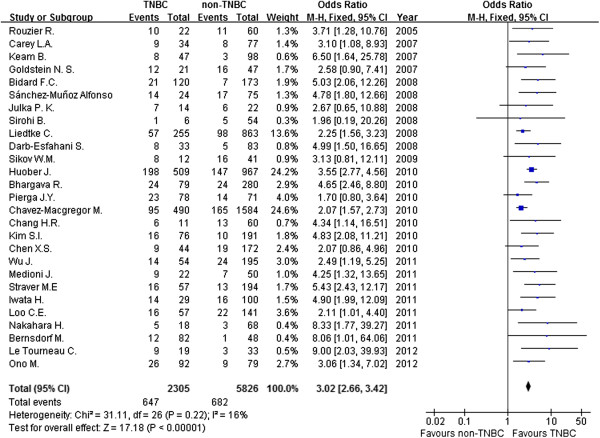
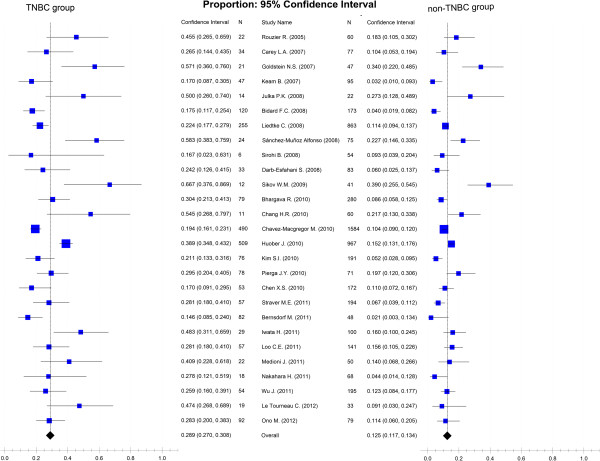
Figure 2 shows the association between pCR and breast cancer subtypes (TNBC and non-TNBC) after NAC. In a fixed-effects meta-analysis of all 27 studies, TNBC has a better pCR rate than non-TNBC (the overall summary estimate OR was 3.02; 95% CI, 2.66 to 3.42) with no obvious evidence of heterogeneity (I2 = 16%, P = 0.22). Figure 3 summarizes the percentage of patients achieving pCR after NAC in TNBC and non-TNBC groups. In a single-group fixed-effects meta-analysis of all 27 studies, the overall summary estimated pCR rate was 28.9% (95% CI, 27.0 to 30.8%) in TNBC and 12.5% (95% CI, 11.7 to 13.4%) in non-TNBC. There was no obvious evidence of heterogeneity (I2 = 44.1% and I2 = 43.8%, respectively).
Figure 2.
Forest plot of odds ratio for achieving pCR after NAC between TNBC and non-TNBC. CI, confidence interval; TNBC, triple-negative breast cancer; M-H, Mantel–Haenszel method.
Figure 3.
Forest plots of pooled percentage of achieving pCR after NAC for TNBC and non-TNBC groups. TNBC, triple-negative breast cancer.
The subgroup analysis outcomes are shown in Table 2. The initially planned subgroup of chemotherapy intermission was not used due to a lack of similar data in these studies. Instead, we used subgroups for NAC treatment duration, which was defined as the period of time that patients were treated with NAC. These subgroup analyses involve treatment cycle (<4 cycles, 4 cycles, 6 cycles or >6 cycles), types of chemotherapy regimen (anthracycline-based, taxane-containing, platinum-containing, gemcitabine-containing), the number of chemotherapy drugs (two kinds of drugs, three kinds of drugs or four kinds of drugs), treatment duration (<12 weeks, 12 weeks, 16 weeks or >16 weeks) and chemotherapy schedule (conventional vs sequential chemotherapy). We discovered that the pCR rate was higher with TNBC than with non-TNBC for all subgroups. A single-group meta-analysis of all 27 studies [1,8,9,12-35] identified the subgroups (four kinds of chemotherapy drugs, >6 cycles, platinum-containing chemotherapy, 16 weeks’ treatment duration, sequential chemotherapy) with the highest pCR rate for both TNBC and non-TNBC patients.
Table 2.
Subgroup analyses of various factors related to achieving pathologic complete response
| Category | Number of studies (references) | Summary estimate odds ratio (95% CI) | Heterogeneity, I 2 (%) |
pCR rate (95% CI) |
|||
|---|---|---|---|---|---|---|---|
| TNBC (%) | Heterogeneity, I 2 (%) | non-TNBC (%) | Heterogeneity, I 2 (%) | ||||
|
Cycles of NAC |
|
|
|
|
|
|
|
| <4 cycles |
2 [13,25] |
5.27 (2.57, 10.79) |
0 |
19.6 (13.5, 27.6) |
0 |
4.6 (2.7, 7.8) |
0 |
| 4 cycles |
6 [15,21,28,33,34,45] |
3.51 (2.21, 5.57) |
0 |
29.2 (23.0, 26.3) |
41.4 |
15.0 (11.8, 18.8) |
35.1 |
| 6 cycles |
3 [17,27,30] |
3.10 (1.84, 5.22) |
35 |
27.6 (20.3, 36.3) |
0 |
11.0 (8.2, 14.7) |
41.3 |
| >6 cycles |
3 [8,26,29] |
2.77 (1.66, 4.61) |
41 |
36.8 (28.8, 45.6) |
33.8 |
17.8 (13.4, 23.3) |
0 |
|
Types of NAC regimen |
|
|
|
|
|
|
|
| Anthracycline-based |
19 [1,8,9,12-14,16-18,25-34] |
3.19 (2.63, 3.88) |
7 |
26.8 (24.1, 29.6) |
39.8 |
12.1 (10.8, 13.5) |
43.2 |
| Taxane-containing |
10 [8,13,18,19,21,25,26,29],[33,45] |
3.29 (2.41, 4.48) |
0 |
30.5 (25.9, 35.5) |
38.2 |
14.9 (12.6, 17.5) |
44.8 |
| Platinum-containing |
4 [17,19,21,45] |
3.10 (1.59, 6.03) |
0 |
44.2 (30.8, 58.5) |
31.1 |
21.3 (16.3, 27.3) |
43.6 |
| Gemcitabine-containing |
2 [15,31] |
3.49 (1.42, 8.57) |
0 |
44.5 (29.3, 60.8) |
0 |
18.8 (11.2, 29.8) |
30.2 |
|
The number of drug in NAC |
|
|
|
|
|
|
|
| Two kinds of drugs |
9 [13,15,19,21,25,27,32,33],[45] |
3.89 (2.75, 5.49) |
0 |
28.7 (23.8, 34.2) |
36.3 |
12.9 (10.7, 15.5) |
46.1 |
| Three kinds of drugs |
4 [14,17,26,34] |
2.39 (1.77, 3.23) |
16 |
22.5 (18.8, 26.5) |
19.8 |
11.2 (9.5, 13.3) |
43.6 |
| Four kinds of drugs |
4 [8,9,29,31] |
4.83 (2.80, 8.35) |
0 |
45.7 (35.8, 55.9) |
0 |
15.5 (11.4, 20.6) |
0 |
|
Total treatment duration of NAC |
|
|
|
|
|
|
|
| <12 weeks |
2 [13,25] |
5.27 (2.57, 10.79) |
0 |
19.6 (13.5, 27.6) |
35.4 |
4.6 (2.7, 7.8) |
0 |
| 12 weeks |
7 [14,15,21,28,30,33,34] |
3.42 (2.34, 4.99) |
0 |
24.9 (20.5, 29.9) |
42 |
13.3 (10.7, 16.3) |
42.6 |
| 16 weeks |
2 [19,45] |
2.88 (1.27, 6.56) |
0 |
44.2 (28.4, 61.3) |
41.4 |
24.8 (17.7, 33.7) |
46.7 |
| >16 weeks |
6 [8,17,24,26,27,29] |
3.47 (2.80, 4.30) |
7 |
37.6 (34.0, 41.2) |
25.8 |
14.7 (12.9, 16.6) |
38 |
|
NAC schedules |
|
|
|
|
|
|
|
| Conventional chemotherapy |
8 [13-15,17,21,25,27,45] |
4.40 (3.02, 6.42) |
0 |
24.1 (19.8, 29.0) |
35.6 |
9.7 (7.7, 12.1) |
44.5 |
| Sequential chemotherapy | 4 [8,26,29,31] | 2.96 (1.85, 4.72) | 20 | 37.4 (30.0, 45.5) | 22.5 | 17.2 (13.2, 22.1) | 0 |
CI, confidence interval; NAC, neoadjuvant chemotherapy; pCR, pathologic complete response; TNBC, triple-negative breast cancer.
A sensitivity analysis shown that excluding small cases studies and changing the effect model had little effect on estimated OR and pCR rate and did not change the strength of the association between NAC and pCR for TNBC and non-TNBC. The ORs were 3.13 (95% CI, 2.66 to 3.68) for excluding small cases studies and 2.92 (95% CI, 2.56 to 3.34) for changing the effect model. For TNBC patients, the odds of pCR were 27.2% (95% CI, 25.3 to 29.2%) for excluding small cases studies and 30.5% (95% CI, 25.9 to 35.5%) for changing the effect model. For non-TNBC patients, the odds of pCR were 11.5% (95% CI, 10.7 to 12.5%) for excluding small case studies and 12.5% (95% CI, 10.4 to 14.9%) for changing the effect model. Funnel plots were generated to test for potential publication bias (Figures 4 and 5). Potential publication biases were found in these funnel plots.
Figure 4.
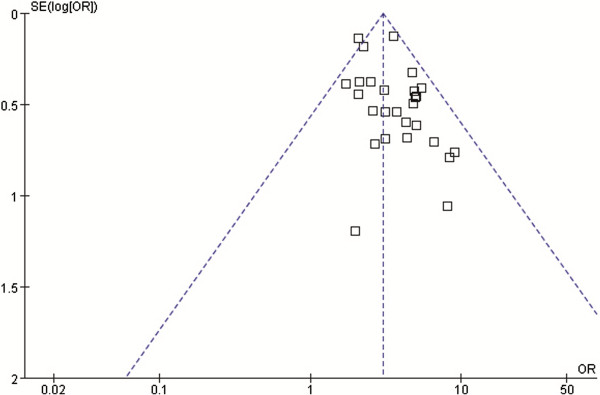
Funnel plot for identifying publication bias in the relationship for achieving pCR between TNBC and non-TNBC. OR, odds ratio.
Figure 5.
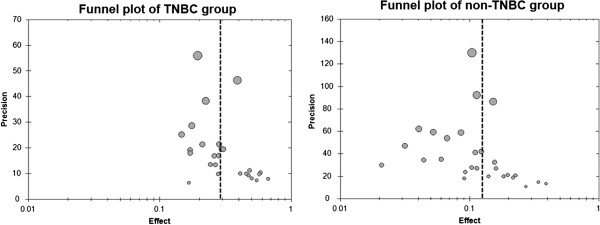
Funnel plot for identifying publication bias for the pooled pCR rates in TNBC and non-TNBC. TNBC, triple-negative breast cancer.
Discussion
TNBC is a subtype of breast cancer that has particular biological features such as high pathologic grade, poor prognosis, short survival, strong tumor invasion, and a high incidence of local relapse and distant metastasis [46]. In addition, a high pCR rate after NAC is also a significant characteristic of TNBC, and pCR has been proved to be a typical marker predictive of clinical response and survival in TNBC patients [11,47]; however, diverse pCR rates have been reported in various studies. In this meta-analysis of 27 studies containing 9,460 cases, pCR rates were 28.9% (95% CI, 27.0 to 30.8%) for 2,952 cases of TNBC and 12.5% (95% CI, 11.7 to 13.4%) for 6,508 cases of non-TNBC. Patients with TNBC have a higher probability of achieving pCR than those with non-TNBC (OR, 3.02; 95% CI, 2.66 to 3.42); that is, the TNBC pCR rate is about two times that of non-TNBC and TNBC exhibits a better response to NAC than non-TNBC.
With the rapid development of molecular and genetic diagnosis techniques, the heterogeneity of breast cancer has been discovered. Based on the analysis of RNA expression profiles, four distinct molecular subtypes of breast cancer (luminal subgroup, basal-like subgroup, HER2 subgroup and normal-like breast tumors) were identified and reported by Perou et al. [48]. The basal-like breast cancer is IHC characterized by overexpression of cytokeratin 5/6/14 and epidermal growth factor receptor and lack of expression of ER, PR and HER2 [49,50]. There is intrinsic homology but incomplete overlap between IHC-defined TNBC and molecular-defined basal-like breast cancer. Nearly 80% of TNBC cases have a basal-like molecular profile [51,52]. In addition to the basal-like profile, TNBC encompasses other molecular subtypes, particularly normal-like and claudin-low [53]. In this meta-analysis, we found four studies of participants with basal-like breast cancer and an estimated pCR rate of 42.5% (95% CI, 32.4 to 53.2%). There was no obvious evidence of heterogeneity (I2 = 31.2%). Basal-like breast cancer has a higher pCR rate than TNBC. Thus, there is evidence that the subtype of triple-negative cancers is heterogeneous and we cannot simply consider them a single group.
Both anthracyclines and taxanes are usually used in the neoadjuvant treatment of breast cancer, and patients respond well to them. Of the 27 studies in this meta-analysis, 19 used anthracycline-based NAC and 11 used taxane-containing regimens. The pCR rates for TNBC were 26.8% (95% CI, 24.1 to 29.6%) for the anthracycline-based group and 30.5% (95% CI, 25.9 to 35.5%) for the taxane-containing group, a non-significant difference. Interestingly, the platinum-containing group had a higher pCR rate than either the anthracycline-based or taxane-containing groups. It is believed that most TNBC cells are expected to have a BRCA1 mutation or absence [54,55], which is useful for the treatment of TNBC since loss of BRCA1 function in TNBC is related to the sensitivity of DNA-damaging chemotherapy agents (platinum, alkylating agents, etc.) and may also be related to the resistance of spindle poisons (taxanes and vinblastines) [56]. TNBC is strongly related to germ-line mutations in the BRCA1 gene, and 90% of BRCA1-mutated cancers are TNBC [57]. Some researchers have demonstrated that the addition of platinum agents to anthracycline and/or taxane regimens in NAC has promise for outcomes [58]. Although the gemcitabine-containing group included two studies with 108 cases [15,31], we should not ignore this group, which achieved the highest pCR rate. Due to lack of sufficient cases to support gemcitabine use in NAC for TNBC, more clinical trials should be implemented.
A hypothesis-generating study indicated that TNBC/basal-like breast cancer had a poorer response to anthracycline-based therapy compared with other breast cancer subtypes [59]. The results of this study were laterally validated through this meta-analysis, which indicated that the anthracycline-based group had the lowest pCR. Although some new drugs have been used in NAC for TNBC (such as EGFR inhibitors (NCT00491816), epothilones (NCT01097642) and ixabepilone (NCT01097642)), the platinum-containing strategy was still the first choice in most clinical trials of TNBC and NAC (NCT00887575, NCT01194869 and NCT00813956). It is a pity that the final reports of these clinical trials have not been submitted; however, these reports were very valuable for providing informative references for the clinical practice. Based on the platinum-containing subgroup analysis of 292 cases from 4 studies [17,19,21,45] and some cell biology research [54-57], we recommend the platinum-containing strategy should be used in NAC for TNBC.
From the subgroup analyses of cycles, drug types, treatment duration and chemotherapy schedules (Table 2), we observed that groups of more than six cycles, four kinds of drugs, 16 weeks treatment duration and sequential chemotherapy obtained the highest pCR rate in the respective subgroups for TNBC (36.8%: 95% CI, 28.8 to 45.6%; 45.7%: 95% CI, 35.8 to 55.9%; 37.6%: 95% CI, 34.0 to 41.2%; 37.4%: 95% CI, 30.0 to 45.5%, respectively). We found that the NAC scheme of FAC/TEC-T or T-FAC/TEC had greater weight in the subgroups for four kinds of drugs [8,9,29] and sequential chemotherapy for TNBC [8,26,29] (the weight was 76.6% and 84.5%, respectively). This chemotherapy scheme may be a good choice of NAC for TNBC.
Three meta-analyses were published recently on breast cancer and pCR. Von Minckwitz et al. [11] presented a meta-analysis of 6,377 operable and non-metastatic breast cancer patients, who received neoadjuvant anthracyclines or taxanes. They discerned various definitions of pCR and evaluated the prognostic impact of pCR on disease-free survival and overall survival in various breast cancer subgroups. The authors concluded that pCR should be conservatively defined as ypT0 ypN0 excluding ductal carcinoma in situ and that pCR is an effective mark of survival for TNBC, luminal B and non-luminal (HER2-positive). Kong et al. [10] completed a meta-analysis that included 16 studies with 3,776 patients with breast cancer to determine whether pathologic response after NAC predicts outcomes. The authors concluded that the pathologic response is prognostic for relapse-free survival, disease-free survival and overall survival. Houssami et al. [60] reported a meta-analysis with two analysis models to provide evidence of the association between various factors for breast cancer and the rates of achieving pCR. Our meta-analysis included 27 studies with 9,460 non-metastatic breast cancer patients, and we aimed to evaluate the association between pCR and breast cancer subtypes (TNBC and non-TNBC) after NAC, and originally tried to identify factors related to achieving pCR for TNBC.
There are some potential limitations in this meta-analysis. Hormone receptor assessment varies across different studies, and different IHC standards are used to define positivity. Most studies define ER/PR-negative IHC using the threshold of <10% immunoreactive cells. The American Society of Clinical Oncology and the College of American Pathologists guidelines for IHC dictate that a threshold of <1% of cells should be used to define ER/PR-negative so that more patients with breast cancer will receive endocrine therapy [53,61]. Moreover, it is unfortunate that sufficient detailed survival data for performing survival analysis are lacking.
Conclusions
In summary, this meta-analysis provides strong evidence that TNBC has a higher pCR rate than non-TNBC. In the NAC setting, these factors of platinum-containing, more than six cycles, four kinds of drugs, 16 weeks’ treatment duration and sequential chemotherapy may result in a higher pCR rate. This information provides valuable direction for clinicians performing relevant clinical studies in the future.
Abbreviations
CI: confidence interval; ER: estrogen receptor; HER2: human epidermal growth factor receptor 2; IHC: immunohistochemistry; NAC: neoadjuvant chemotherapy; OR: odds ratio; pCR: pathologic complete response; PR: progesterone receptor; TNBC: triple-negative breast cancer.
Competing interests
No potential competing interests were disclosed.
Authors’ contributions
KW and QY performed statistical analysis and wrote the manuscript; KW, YL, AW and QY performed literature search and stratified the data; AW and ZY provided meaningful discussion key points; KW and AW revised and edited the manuscript. All authors read and approved the final manuscript.
Contributor Information
Kunpeng Wu, Email: 67104234@qq.com.
Qiaozhu Yang, Email: 18463284@qq.com.
Yi Liu, Email: plliu78@sina.com.
Aibing Wu, Email: wab801016@163.com.
Zhixiong Yang, Email: yangzhixiong068@126.com.
Acknowledgements
This work was supported by funding from the National Natural Science Foundation of China (81201672).
References
- Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- Tischkowitz M, Brunet JS, Begin LR, Huntsman DG, Cheang MC, Akslen LA, Nielsen TO, Foulkes WD. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23(Suppl 6):vi7–vi12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- Harris LN, Broadwater G, Lin NU, Miron A, Schnitt SJ, Cowan D, Lara J, Bleiweiss I, Berry D, Ellis M, Hayes DF, Winer EP, Dressler L. Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: results from CALGB 9342. Breast Cancer Res. 2006;8(6):R66. doi: 10.1186/bcr1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;2:CD005002. doi: 10.1002/14651858.CD005002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer. 2011;47(14):2084–2090. doi: 10.1016/j.ejca.2011.06.014. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- Goldstein NS, Decker D, Severson D, Schell S, Vicini F, Margolis J, Dekhne NS. Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer. 2007;110(8):1687–1696. doi: 10.1002/cncr.22981. [DOI] [PubMed] [Google Scholar]
- Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, Chie EK, Han W, Kim DW, Moon WK, Kim TY, Park IA, Noh DY, Heo DS, Ha SW, Bang YJ. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer. 2007;7:203. doi: 10.1186/1471-2407-7-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidard FC, Matthieu MC, Chollet P, Raoefils I, Abrial C, Domont J, Spielmann M, Delaloge S, Andre F, Penault-Llorca F. p53 status and efficacy of primary anthracyclines/alkylating agent-based regimen according to breast cancer molecular classes. Ann Oncol. 2008;19(7):1261–1265. doi: 10.1093/annonc/mdn039. [DOI] [PubMed] [Google Scholar]
- Julka PK, Chacko RT, Nag S, Parshad R, Nair A, Oh DS, Hu Z, Koppiker CB, Nair S, Dawar R, Dhindsa N, Miller ID, Ma D, Lin B, Awasthy B, Perou CM. A phase II study of sequential neoadjuvant gemcitabine plus doxorubicin followed by gemcitabine plus cisplatin in patients with operable breast cancer: prediction of response using molecular profiling. Br J Cancer. 2008;98(8):1327–1335. doi: 10.1038/sj.bjc.6604322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Muñoz A, García-Tapiador AM, Martínez-Ortega E, Dueñas-García R, Jaén-Morago A, Ortega-Granados AL, Fernández-Navarro M, Torre-Cabrera C, Dueñas B, Rueda AI, Morales F, Ramírez-Torosa C, Martín-Salvago MD, Sánchez-Rovira P. Tumour molecular subtyping according to hormone receptors and HER2 status defines different pathological complete response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Clin Transl Oncol. 2008;10(10):646–653. doi: 10.1007/s12094-008-0265-y. [DOI] [PubMed] [Google Scholar]
- Sirohi B, Arnedos M, Popat S, Ashley S, Nerurkar A, Walsh G, Johnston S, Smith IE. Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol. 2008;19(11):1847–1852. doi: 10.1093/annonc/mdn395. [DOI] [PubMed] [Google Scholar]
- Darb-Esfahani S, Loibl S, Muller BM, Roller M, Denkert C, Komor M, Schluns K, Blohmer JU, Budczies J, Gerber B, Noske A, du Bois A, Weichert W, Jackisch C, Dietel M, Richter K, Kaufmann M, von Minckwitz G. Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res. 2009;11(5):R69. doi: 10.1186/bcr2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikov WM, Dizon DS, Strenger R, Legare RD, Theall KP, Graves TA, Gass JS, Kennedy TA, Fenton MA. Frequent pathologic complete responses in aggressive stages II to III breast cancers with every-4-week carboplatin and weekly paclitaxel with or without trastuzumab: a Brown University Oncology Group Study. J Clin Oncol. 2009;27(28):4693–4700. doi: 10.1200/JCO.2008.21.4163. [DOI] [PubMed] [Google Scholar]
- Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, Brufsky AM, Lembersky BC, Ahrendt GM. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. 2010;116(6):1431–1439. doi: 10.1002/cncr.24876. [DOI] [PubMed] [Google Scholar]
- Chang HR, Glaspy J, Allison MA, Kass FC, Elashoff R, Chung DU, Gornbein J. Differential response of triple-negative breast cancer to a docetaxel and carboplatin-based neoadjuvant treatment. Cancer. 2010;116(18):4227–4237. doi: 10.1002/cncr.25309. [DOI] [PubMed] [Google Scholar]
- Chavez-Macgregor M, Litton J, Chen H, Giordano SH, Hudis CA, Wolff AC, Valero V, Hortobagyi GN, Bondy ML, Gonzalez-Angulo AM. Pathologic complete response in breast cancer patients receiving anthracycline- and taxane-based neoadjuvant chemotherapy: evaluating the effect of race/ethnicity. Cancer. 2010;116(17):4168–4177. doi: 10.1002/cncr.25296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XS, Wu JY, Huang O, Chen CM. Molecular subtype can predict the response and outcome of Chinese locally advanced breast cancer patients treated with preoperative therapy. Oncol Rep. 2010;23:1213–1220. doi: 10.3892/or_00000752. [DOI] [PubMed] [Google Scholar]
- Huober J, von Minckwitz G, Denkert C, Tesch H, Weiss E, Zahm DM, Belau A, Khandan F, Hauschild M, Thomssen C, Hogel B, Darb-Esfahani S, Mehta K, Loibl S. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat. 2010;124(1):133–140. doi: 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- Kim SI, Sohn J, Koo JS, Park SH, Park HS, Park BW. Molecular subtypes and tumor response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Oncology. 2010;79(5–6):324–330. doi: 10.1159/000322192. [DOI] [PubMed] [Google Scholar]
- Pierga JY, Delaloge S, Espie M, Brain E, Sigal-Zafrani B, Mathieu MC, Bertheau P, Guinebretiere JM, Spielmann M, Savignoni A, Marty M. A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients. Breast Cancer Res Treat. 2010;122(2):429–437. doi: 10.1007/s10549-010-0939-3. [DOI] [PubMed] [Google Scholar]
- Straver ME, Rutgers EJ, Rodenhuis S, Linn SC, Loo CE, Wesseling J, Russell NS, Oldenburg HS, Antonini N, Vrancken Peeters MT. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17(9):2411–2418. doi: 10.1245/s10434-010-1008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernsdorf M, Ingvar C, Jorgensen L, Tuxen MK, Jakobsen EH, Saetersdal A, Kimper-Karl ML, Kroman N, Balslev E, Ejlertsen B. Effect of adding gefitinib to neoadjuvant chemotherapy in estrogen receptor negative early breast cancer in a randomized phase II trial. Breast Cancer Res Treat. 2011;126(2):463–470. doi: 10.1007/s10549-011-1352-2. [DOI] [PubMed] [Google Scholar]
- Iwata H, Sato N, Masuda N, Nakamura S, Yamamoto N, Kuroi K, Kurosumi M, Tsuda H, Akiyama F, Ohashi Y, Toi M. Docetaxel followed by fluorouracil/epirubicin/cyclophosphamide as neoadjuvant chemotherapy for patients with primary breast cancer. Jpn J Clin Oncol. 2011;41(7):867–875. doi: 10.1093/jjco/hyr081. [DOI] [PubMed] [Google Scholar]
- Loo CE, Straver ME, Rodenhuis S, Muller SH, Wesseling J, Vrancken Peeters MJ, Gilhuijs KG. Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. J Clin Oncol. 2011;29(6):660–666. doi: 10.1200/JCO.2010.31.1258. [DOI] [PubMed] [Google Scholar]
- Medioni J, Huchon C, Le Frere-Belda MA, Hofmann H, Bats AS, Eme D, Andrieu JM, Oudard S, Lecuru F, Levy E. Neoadjuvant dose-dense gemcitabine plus docetaxel and vinorelbine plus epirubicin for operable breast cancer: improved prognosis in triple-negative tumors. Drugs R D. 2011;11(2):147–157. doi: 10.2165/11591210-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H, Yasuda Y, Machida E, Maeda Y, Furusawa H, Komaki K, Funagayama M, Nakahara M, Tamura S, Akiyama F. MR and US imaging for breast cancer patients who underwent conservation surgery after neoadjuvant chemotherapy: comparison of triple negative breast cancer and other intrinsic subtypes. Breast Cancer. 2011;18(3):152–160. doi: 10.1007/s12282-010-0235-4. [DOI] [PubMed] [Google Scholar]
- Wu J, Li S, Jia W, Su F. Response and prognosis of taxanes and anthracyclines neoadjuvant chemotherapy in patients with triple-negative breast cancer. J Cancer Res Clin Oncol. 2011;137(10):1505–1510. doi: 10.1007/s00432-011-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tourneau C, Dettwiler S, Beuzeboc P, Alran S, Laurence V, Pierga JY, Freneaux P, Sigal-Zafrani B, Dieras V, Vincent-Salomon A. Pathologic response to short intensified taxane-free neoadjuvant chemotherapy in patients with highly proliferative operable breast cancer. Am J Clin Oncol. 2012;35(3):242–246. doi: 10.1097/COC.0b013e318209d34c. [DOI] [PubMed] [Google Scholar]
- Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, Katsumata N, Kinoshita T, Takiguchi Y, Tanzawa H, Fujiwara Y. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132(3):793–805. doi: 10.1007/s10549-011-1554-7. [DOI] [PubMed] [Google Scholar]
- Chavez-Macgregor M, Brown E, Lei X, Litton J, Meric-Bernstram F, Mettendorf E, Hernandez L, Valero V, Hortobagyi GN, Gonzalez-Angulo AM. Bisphosphonates and pathologic complete response to taxane- and anthracycline-based neoadjuvant chemotherapy in patients with breast cancer. Cancer. 2012;118(2):326–332. doi: 10.1002/cncr.26144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, Fatima A, Gelman RS, Ryan PD, Tung NM, De Nicolo A, Ganesan S, Miron A, Colin C, Sgroi DC, Ellisen LW, Winer EP, Garber JE. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28(7):1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy N, Quispe D, Shi R, Mansour R, Burton GV. Cisplatin-gemcitabine therapy in metastatic breast cancer: improved outcome in triple negative breast cancer patients compared to non-triple negative patients. Breast. 2010;19(3):246–248. doi: 10.1016/j.breast.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Chan D, Yeo WL, Tiemsim Cordero M, Wong CI, Chuah B, Soo R, Tan SH, Lim SE, Goh BC, Lee SC. Phase II study of gemcitabine and carboplatin in metastatic breast cancers with prior exposure to anthracyclines and taxanes. Investig New Drugs. 2010;28(6):859–865. doi: 10.1007/s10637-009-9305-x. [DOI] [PubMed] [Google Scholar]
- Fan Y, Xu BH, Yuan P, Ma F, Wang JY, Ding XY, Zhang P, Li Q, Cai RG. Docetaxel-cisplatin might be superior to docetaxel-capecitabine in the first-line treatment of metastatic triple-negative breast cancer. Ann Oncol. 2013;24(5):1219–1225. doi: 10.1093/annonc/mds603. [DOI] [PubMed] [Google Scholar]
- Staudacher L, Cottu PH, Dieras V, Vincent-Salomon A, Guilhaume MN, Escalup L, Dorval T, Beuzeboc P, Mignot L, Pierga JY. Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Ann Oncol. 2011;22(4):848–856. doi: 10.1093/annonc/mdq461. [DOI] [PubMed] [Google Scholar]
- Uhm JE, Park YH, Yi SY, Cho EY, Choi YL, Lee SJ, Park MJ, Lee SH, Jun HJ, Ahn JS, Kang WK, Park K, Im YH. Treatment outcomes and clinicopathologic characteristics of triple-negative breast cancer patients who received platinum-containing chemotherapy. Int J Cancer. 2009;124(6):1457–1462. doi: 10.1002/ijc.24090. [DOI] [PubMed] [Google Scholar]
- Nogi H, Kobayashi T, Suzuki M, Tabei I, Kawase K, Toriumi Y, Fukushima H, Uchida K. EGFR as paradoxical predictor of chemosensitivity and outcome among triple-negative breast cancer. Oncol Rep. 2009;21(2):413–417. [PubMed] [Google Scholar]
- Jones RL, Rojo F, A’Hern R, Villena N, Salter J, Corominas JM, Servitja S, Smith IE, Rovira A, Reis-Filho JS, Dowsett M, Albanell J. Nuclear NF-kappaB/p65 expression and response to neoadjuvant chemotherapy in breast cancer. J Clin Pathol. 2011;64(2):130–135. doi: 10.1136/jcp.2010.082966. [DOI] [PubMed] [Google Scholar]
- Chen XS, Nie XQ, Chen CM, Wu JY, Wu J, Lu JS, Shao ZM, Shen ZZ, Shen KW. Weekly paclitaxel plus carboplatin is an effective nonanthracycline-containing regimen as neoadjuvant chemotherapy for breast cancer. Ann Oncol. 2010;21(5):961–967. doi: 10.1093/annonc/mdq041. [DOI] [PubMed] [Google Scholar]
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- Bayraktar S, Gluck S. Molecularly targeted therapies for metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2013;138(1):21–35. doi: 10.1007/s10549-013-2421-5. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Metzger-Filho O, Tutt A, de Azambuja E, Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA Jr, Ellis P, Di Leo A, Baselga J, Sotiriou C, Piccart-Gebhart M. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012;30(15):1879–1887. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109(1):25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Baehner FL, Reis-Filho JS. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. J Pathol. 2010;220(2):263–280. doi: 10.1002/path.2648. [DOI] [PubMed] [Google Scholar]
- Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- Penault-Llorca F, Viale G. Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann Oncol. 2012;23(Suppl 6):vi19–vi22. doi: 10.1093/annonc/mds190. [DOI] [PubMed] [Google Scholar]
- Comen E, Davids M, Kirchhoff T, Hudis C, Offit K, Robson M. Relative contributions of BRCA1 and BRCA2 mutations to ‘triple-negative’ breast cancer in Ashkenazi women. Breast Cancer Res Treat. 2011;129(1):185–190. doi: 10.1007/s10549-011-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, McKean-Cowdin R, Ma H, Spicer DV, Van Den Berg D, Bernstein L, Ursin G. Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: results from a population-based study of young women. J Clin Oncol. 2011;29(33):4373–4380. doi: 10.1200/JCO.2010.33.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96(22):1659–1668. doi: 10.1093/jnci/djh312. [DOI] [PubMed] [Google Scholar]
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- Gelmon K, Dent R, Mackey JR, Laing K, McLeod D, Verma S. Targeting triple-negative breast cancer: optimising therapeutic outcomes. Ann Oncol. 2012;23(9):2223–2234. doi: 10.1093/annonc/mds067. [DOI] [PubMed] [Google Scholar]
- Martin M, Romero A, Cheang MC, Lopez Garcia-Asenjo JA, Garcia-Saenz JA, Oliva B, Roman JM, He X, Casado A, de la Torre J, Furio V, Puente J, Caldés T, Vidart JA, Lopez-Tarruella S, Diaz-Rubio E, Perou CM. Genomic predictors of response to doxorubicin versus docetaxel in primary breast cancer. Breast Cancer Res Treat. 2011;128(1):127–136. doi: 10.1007/s10549-011-1461-y. [DOI] [PubMed] [Google Scholar]
- Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48(18):3342–3354. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134(7):e48–e72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]