Abstract
Dopamine (DA) and N-methyl-D-aspartate receptors (NMDARs) contribute in the neural processes underlying drug-driven behaviors. DA is a potent modulator of NMDAR, but few studies have investigated the functional interaction between DA and NMDAR in the context of substance abuse. We combined the rat model of cocaine self-administration with brain slice electrophysiology to study DA modulation of NMDA currents in the oval bed nucleus of the stria terminalis (ovBNST), a dense DA terminal field involved in maintenance of cocaine self-administration amongst other drug related behaviors. Long-Evans rats self-administered intravenous cocaine (0.75 mg/kg/injection) on a progressive ratio (PR) schedule of reinforcement for 15 days and whole-cell patch-clamp recordings were done on the 16th day. DA reduced NMDA currents in brain-slices from cocaine self-administering rats, but not in those of drug-naïve and sucrose self-administering, or when cocaine exposure was passive (yoked), revealing a mechanism unique to voluntary cocaine intake. DA reduced NMDA currents by activating G-protein-coupled D1- and D2-like receptors that converged on phospholipase C and protein phosphatases. Accordingly, our study reveals a mechanism that may contribute to dysfunctional synaptic plasticity associated with drug-driven behaviors during acute withdrawal.
Keywords: Brain slice electrophysiology, Cocaine, Dopamine, NMDA, Self-administration
1. Introduction
Repeated drug exposure produces long-lasting alterations in the neuromodulatory effects of dopamine (DA) (Beurrier and Malenka, 2002; Bonci and Williams, 1996; Li and Kauer, 2004; Thomas et al., 2000). However, few studies measured DA neuromodulation in animal models of drug self-administration, which have a better predictive value for human addiction (Belin et al., 2008; Deroche-Gamonet et al., 2004; Panlilio, 2010; Vanderschuren and Everitt, 2004). Nonetheless, DA modulation of GABAA-mediated inhibitory synaptic currents reverses (from reduction to enhancement) in rats with a prolonged cocaine self-administration experience due to a change in the respective contribution of D2 and D1-like DA receptors (Krawczyk et al., 2011b). These changes occur in the oval bed nucleus of the stria terminalis (ovBNST), a dense DA terminal field (Freedman and Cassell, 1994; Hasue and Shammah-Lagnado, 2002) involved in drug-driven behaviors including cocaine, ethanol, and heroin self-administration (Eiler et al., 2003; Epping-Jordan et al., 1998; Hyytia and Koob, 1995; Walker et al., 2000) and stress-induced relapse to drug seeking (Erb and Stewart, 1999; Leri et al., 2002). Furthermore, most addictive drugs enhance in vivo DA levels in the ovBNST region (Carboni et al., 2000). In contrast to GABA synaptic transmission, however, cocaine self-administration does not affect DA modulation of AMPA transmission (Krawczyk et al., 2011b).
Yet, DA is also a potent modulator of the N-methyl-D-aspartate receptors (NMDARs) (Kotecha et al., 2002; Lee et al., 2002; Martina and Bergeron, 2008; Seamans et al., 2001; Wang and O’Donnell, 2001; Zheng et al., 1999) which themselves contribute to forms of synaptic plasticity associated with exposure to addictive drugs (Stuber et al., 2008; Ungless et al., 2001; Zweifel et al., 2008). Despite the critical role of both DA and NMDAR in the neural processes underlying drug-driven behaviors, it is currently unknown if their interaction changes through the course of drug self-administration. This is an unfortunate gap given the crucial role of both DA and NMDAR in the neuropathology of drug addiction (Carr and Kalivas, 2008).
Here, we combined behavioral testing of sucrose or cocaine self-administration and brain slice patch-clamp recordings to study DA modulation of NMDA currents in the rat ovBNST. We report that DA reduced NMDA currents in cocaine self-administering, but not in drug-naïve or sucrose self-administering rats, or when cocaine exposure was passive (yoked). DA acted through G-protein coupled D1- and D2-like receptors both activating phospholipase C and protein phosphatases. Thus, the data reveal modulatory effects of DA on NMDA currents uniquely associated with voluntary cocaine intake during acute cocaine withdrawal. We propose that this mechanism could bias bidirectional synaptic plasticity in cocaine-dependent rats.
2. Materials and methods
2.1. Subjects
Forty-three Long Evans male rats (Charles River Laboratories) weighing 250–300 g were used and experiments were conducted in accordance with the CCAC guidelines for use of animals in experiments and approved by the Queen’s University Animal Care Committee.
2.2. Surgical procedures
Twenty-six rats were implanted with indwelling catheters for intravenous cocaine administration (described in Krawczyk et al., 2011b). Before surgical procedures, the rats were allowed to acclimatize to surroundings for a minimum of 3 days. The rats were weighed and anesthetized with isoflurane (2–3%, 3–5 l/min). We used manufactured indwelling catheters for intra-jugular cannulations (Model IVSA28, Camcath, Inc.). The end of the tubing was inserted 32 mm into the right jugular vein, towards the right atria, and tied with 4.0 suturing silks. The rest of the tubing was fed subcutaneously to a back-mounted 28 Ga cannula. All incisions were closed with 4–0 absorbable suturing silk. Upon surgery completion and recovery from anesthesia, the rats were returned to the colony room. The rats received Anafen (5 mg/kg) injections, subcutaneously, for 3 days post-surgery. I.V. cannulae were flushed daily with a sterile heparin–saline solution (20 IU heparin/ml) to prevent clots and conserve patency.
2.3. Electrophysiology and experimental groups (Fig. 1A)
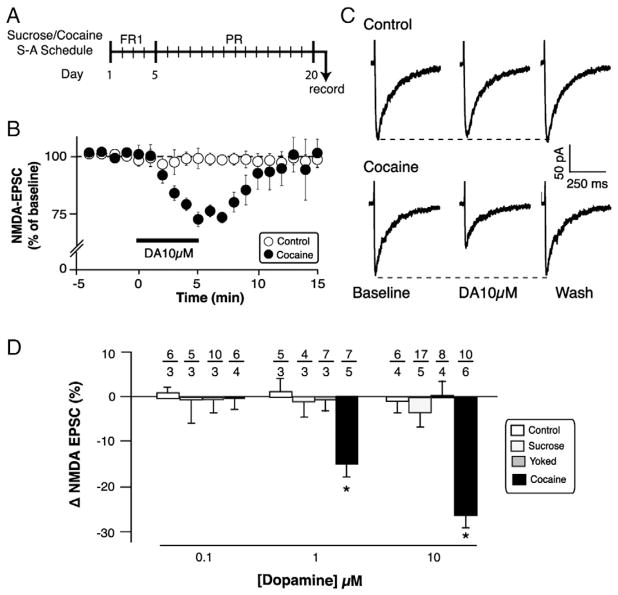
Fig. 1.
DA reduced whole-cell NMDA-EPSCs in ovBST neurons of cocaine self-administering rats. A. Schedule of sucrose and cocaine self-administration training. B. Effect of DA 10 μM on the amplitude of NMDA-EPSCs as a function of time in ovBST neurons. C. Representative NMDA-EPSCs in ovBST neurons of Control (top) and cocaine self-administering (bottom) rats without DA (left), after 5 min exogenous DA (middle), or after a 15-min wash (right). D. Summary of the effects of DA on NMDA-EPSCs in ovBST neurons. DA was significantly more potent at reducing NMDA-EPSCs in the cocaine self-administering group (group × dose interaction: F(6,79) = 4.6, p = 0.0005). *; significantly different from 0 (single sample mean t-tests). Number of neurons/rats indicated above each bar.
Singly housed, age-matched rats were assigned to four groups: control (n = 11), sucrose (n = 6), cocaine self-administering (n = 20), and yoked (n = 6). Upon reaching pre-determined criteria for acquisition, rats in the sucrose and cocaine self-administering groups graduated to a progressive ratio schedule of reinforcement (PR) (Krawczyk et al., 2011b; Richardson and Roberts, 1996). The rats were trained on the PR schedule of reinforcement – instead of fixed ratio – to be consistent with our previous studies (Krawczyk et al., 2011b, 2013). This schedule also reflects at least one aspect of compulsive cocaine intake and thus has better translational value to substance abuse and addiction (Deroche-Gamonet et al., 2004). Final ratio was the dependent variable used to quantify the PR schedule of reinforcement. Final ratio was defined as the last bout of lever pressing required to reach breakpoint (BP). BP is the last reward received within a 60-min time period (Richardson and Roberts, 1996). Trained rats remained on PR for 15 ± 2 (cocaine self-administering) and 15 ± 3 d (sucrose) and reached similar final ratios (cocaine self-administering: 107 ± 16 presses, n = 20; sucrose: 71 ± 30 presses, n = 6 [two-tailed t-test on final ratio pressing: t(24) = −1.03, p = 0.3]). Yoked rats were included in the study to delineate potential mechanisms of voluntary drug intake (Dumont et al., 2005; Krawczyk et al., 2011b, 2013).
2.4. Slice preparation and electrophysiology
Brain slices were prepared 20 h after the final training session. Brain slice patch clamp recordings were conducted as previously described (Krawczyk et al., 2011a), except external and internal solutions contained (in mM) 151 NaCl, 3 KCl, 3.1 CaCl2, 1.4 NaH2PO4, 25 NaHCO3, 12.5 D-glucose and 130 Cs+MeSO3−, 1 EGTA, 5 HEPES, 2 Mg-ATP, 0.3 GTP, and 1 P-creatine, respectively. NMDA-excitatory post-synaptic currents (EPSCs) were electrically evoked at 0.1 Hz (Vm = −70 mV) in the presence of DNQX (50 μM) and picrotoxin (100 μM). The α2R antagonist yohimbine (5 μM) was present to block DA modulation of glutamate release (Krawczyk et al., 2011a,b).
2.5. Drugs
DA (10 mM), quinpirole (1 mM), SCH-23390 (10 mM), and D(−)-2-amino-5-phosphonopentanoic acid (D-AP5, 100 mM) were prepared in double distilled water. DNQX (100 mM), SKF-81297 (1 mM), sulpiride (1 mM), U-73122 (10 mM), U-73343 (10 mM), okadaic acid (10 mM), sodium orthovanadate (10 mM), yohimbine (1 mM), GTP-β-s (10 mM), H89 (10 mM), and Ro 04-5595 (10 mM) were prepared in DMSO (100%). All drugs were further dissolved in physiological solutions at desired concentrations (final DMSO concentration < 0.1%). Cocaine-HCl was dissolved at 2.5 mg/ml in sterile saline (pH: 7.3). Drugs were obtained from Sigma-Aldrich or Tocris Biosciences except cocaine-HCl (Medisca).
2.6. Statistical analysis
Single sample mean t-tests were used to measure agonist-induced changes in peak NMDA-EPSCs amplitude (mean ± SEM in percent) using a Bonferroni correction. Between-group differences were calculated using ANOVAs with appropriate post-hoc tests. All data analyses were conducted using JMP 9.0 (SAS Institute).
3. Results
3.1. DA reduced NMDA-excitatory post-synaptic currents (EPSCs) in the ovBNST of cocaine self-administering rats (Fig. 1)
DA (0.1 to 10 μM) reversibly reduced the amplitude of evoked NMDA-EPSCs in the cocaine self-administering group but had no effect in control, sucrose, and yoked rats (group × dose interaction: F(6,79) = 4.6, p = 0.0005). Specifically, DA significantly reduced NMDA-EPSCs at 1 (−15.1 ± 2.1%, t(6) = −7.4, p = 0.0003) and 10 μM (−27.1 ± 1.5%, t(9) = −17.9, p < 0.0001) in cocaine self-administering rats. The effect of DA on NMDA-EPSC saturated since DA 30 μM did not produce any further decrease (−27.5 ± 4.3%, n = 6, not shown). To prevent the DA-induced reduction in glutamate release that we previously found to be mediated by pre-synaptic noradrenergic α2R in the ovBST of control, sucrose, and cocaine self-administering rats (Krawczyk et al., 2011a,b), yohimbine (5 μM) was added to the physiological solution. We previously showed that, at this concentration, yohimbine had no effect on the amplitude of evoked EPSCs in the ovBNST of drug-naïve or cocaine-dependent rats (Krawczyk et al., 2011a,b). Furthermore, blocking the pre-synaptic effect of DA in the ovBNST with yohimbine, DA-mediated reduction in NMDA-EPSCs in cocaine self-administering rats observed in the present study was likely due to post-synaptic mechanisms. Furthermore, DA 10 μM did not change the coefficient of variation (baseline: 0.12 ± 0.02 vs. DA; 0.12 ± 0.02, t(8) = 0.87, p = NS) indicative of a post-synaptic location for the effect of DA on NMDA-EPSCs.
3.2. DA preferentially reduced GluN2A-containing NMDA-EPSCs (Fig. 2)
Fig. 2.
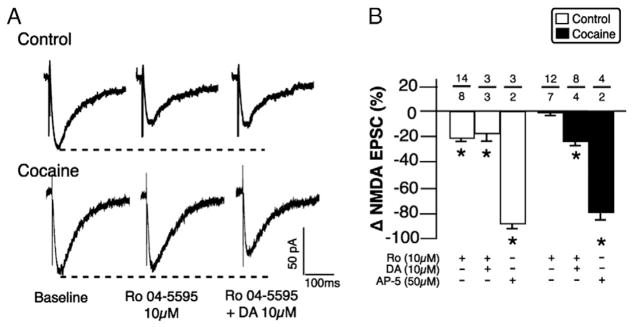
DA reduced GluN2A-containing NMDA-EPSCs in cocaine self-administering rats. A. NMDA-EPSCs in ovBST neurons of control (top) and cocaine self-administering (bottom) rats before drug application (left), after a 10-min bath application of the selective GluN2B antagonist Ro 04-5595 (middle), and after 5 min DA in the presence of Ro (right). B. Effect of Ro, of DA in the presence of Ro, and of the competitive NMDAR antagonist AP-5 on evoked NMDA-EPSCs in ovBST neurons from control and cocaine self-administering rats. *; p < 0.001 from 0 (single sample mean t-tests); (group × drug; F = 27.7, p < 0.0001). Number of neurons/rats indicated above each bar.
NMDAR subunit substitution is a key mechanism in controlling bidirectional plasticity of excitatory synapses in several areas of the brain including the BNST (Morishita et al., 2007; Philpot et al., 2007; Weitlauf et al., 2005). We therefore investigated whether DA displayed NMDAR subunit selectivity in its capacity of reducing ovBNST NMDA currents. The GluN2B-selective antagonist Ro 04-5595 (10 μM) reduced NMDA-EPSC amplitude in control (−21.4 ± 1.9%, n = 14) but not in cocaine self-administering rats (−0.21 ± 3.3%, n = 12), suggesting a preferential expression of synaptic GluN2A-containing NMDAR after cocaine self-administration. Ifenprodil (3 μM), another GluN2B-selective antagonist, produced similar effects (−21.6 ± 5.8%, n = 3), supporting a distinctive NMDAR subunit composition in control and cocaine self-administering rats. GluN2B blockade did not alter DA-mediated reduction in NMDA-EPSC (−23.8 ± 1.9%, n = 8) in slices prepared from cocaine self-administering rats, suggesting that DA preferentially acts on GluN2A-containing NMDARs. Consistent with the results in Fig. 1, DA was similarly ineffective at reducing NMDA currents in control rats (Ro alone: −21.7 ± 5.8 and DA in the presence of Ro: 0.7 ± 2.5%, n = 3) when GluN2B receptors were blocked. Bath application of the competitive NMDA antagonist AP-5 (50 μM) completely abolished evoked NMDA-EPSCs in both control and cocaine self-administering rats, confirming that those events were indeed carried by NMDA receptors.
3.3. Cooperative activation of D1- and D2-like DA receptors reduced NMDA-EPSCs in cocaine self-administering rats (Fig. 3)
Fig. 3.
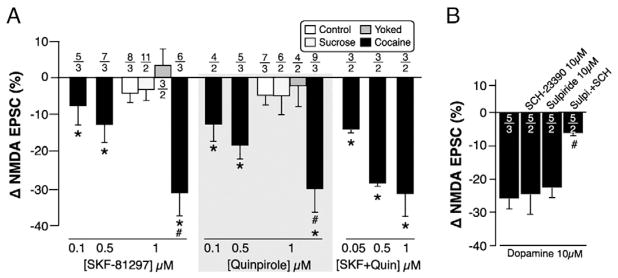
Cooperation between D1 and D2 DA receptors in cocaine self-administering rats. A. Effect of the D1-like agonist SKF-81297 (left), the D2-like agonist quinpirole (middle), or both agonists (right) on NMDA-EPSCs in ovBST neurons of cocaine self-administering rats. B. Effect of the D1 antagonist SCH-23390 and the D2 antagonist sulpiride on DA-mediated reduction in NMDA-EPSCs. #; p < 0.05 (one-way ANOVA–Tukey–Kramer HSD). *; p < 0.008 from 0 (single mean t-tests). Number of neurons/rats indicated above each bar.
Similar to the effects of DA, the D1R and D2R agonists SKF-81297 and quinpirole (0.05–1 μM) reduced the amplitude of NMDA-EPSC in cocaine self-administering rats, but not in control, sucrose or, yoked rats. At 1 μM, SFK-81297 (F(3,24) = 10.16, p = 0.0002) and quinpirole (F(3,22) = 5.35, p = 0.006) significantly reduced NMDA-EPSCs in cocaine self-administering rats, compared to all other groups. SKF, quinpirole, and DA were equally efficacious at reducing NMDA-EPSCs, producing a maximal reduction of approximately 30% (Figs. 1D, 2B). Neither the D1R antagonist SCH-23390 (10 μM) nor the D2R antagonist sulpiride (10 μM) alone blocked the effect of DA on NMDA-EPSCs in cocaine self-administering rats. However, co-application of both antagonists significantly block the effects of DA, suggesting two distinct receptors but perhaps downstream convergence resulting in a similar effect on NMDA receptors (one-way ANOVA–Tukey–Kramer; F(2,15) = 9.99, p = 0.0002).
3.4. D1R and D2R converged on a G-protein/PLC/protein phosphatase signaling pathway (Fig. 4)
Fig. 4.
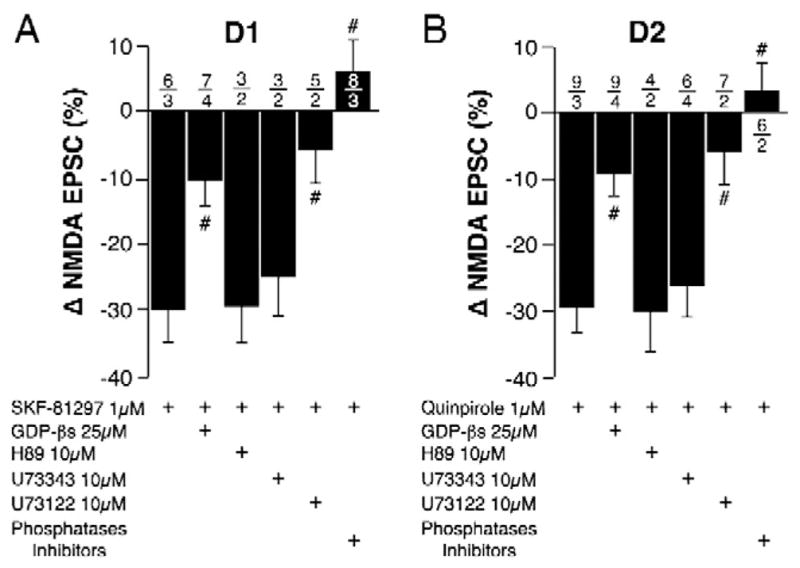
D1 and D2 converged on a G/PLC/protein phosphatases signaling pathway. Effects of intracellular applications of G-protein, PLC, PKA, and phosphatase inhibitors on SFK-81297- (left) and quinpirole- (right) effects in cocaine self-administering rats. #; p < 0.05 compared to SKF-81297 or quinpirole alone (one-way ANOVA–Dunnett’s method). Number of neurons/rats indicated above each bar.
Our data suggest a post-synaptic effect of DA mediated through D1R and D2R (see Fig. 1 and Krawczyk et al., 2011a,b). In support of this hypothesis, the intracellular addition of various pharmacological blockers of signaling molecules interfered with the effects of both SKF and quinpirole in cocaine self-administering rats (F(5,26) = 10.1, p < 0.0001 and F(5,35) = 7.6, p < 0.0001, respectively). Specifically, the non-hydrolyzable GTP analog GTP-β-s (25 μM), prevented SKF-81297-(p = 0.01) and quinpirole- (p = 0.006) mediated reduction of NMDA-EPSC. Further downstream, the broad-spectrum PLC inhibitor U-73122 also completely blocked the effects of both SKF-81297- (p = 0.005) and quinpirole- (p = 0.003). However, U-73343, the inactive analog of U-73122, was ineffective at blocking the effects of SKF (p = 0.96) or quinpirole (p = 0.98). Likewise, the PKA inhibitor H89 (10 μM) did not block the effects of SKF-81297- (p = 0.99) or quinpirole- (p = 0.99). Finally, co-application of okadaic acid (10 μM) and sodium orthovanadate (10 μM), which respectively inhibit PP1/2A and tyrosine phosphatases, abolished the effects of SKF-81297- and quinpirole (p < 0.0001 in both cases).
4. Discussion
Supported by its predictive validity for several aspects of human addictions, the self-administration rat model is an important tool to identify neural mechanisms underlying substance abuse (Deroche-Gamonet et al., 2004; Panlilio, 2010; Vanderschuren and Everitt, 2004). Here, dopamine (DA) reduced currents carried by GluN2A-containing NMDAR in the oval bed nucleus of the stria terminalis (ovBNST) of cocaine self-administering rats. Mechanistically, DA acted through de novo functional post-synaptic D1R and D2R converging onto a G-proteins/PLC/protein phosphatases signaling pathway.
4.1. Loss of GluN2B-mediated NMDA-EPSC in cocaine self-administering rats
Dysfunctional regulation of NMDAR by DA may contribute in several neurological disorders, including substance abuse (Argilli et al., 2008; Kelley, 2004; Schilstrom et al., 2006). NMDARs are tetrameric complexes formed by two obligatory GluN1 subunits and heteromeric or homomeric dimers of four possible GluN2 subunits (Cull-Candy and Leszkiewicz, 2004). Previous studies showed that GluN2 composition significantly affects bidirectional plasticity of excitatory synapses (Barria and Malinow, 2005; Morishita et al., 2007; Philpot et al., 2007; Schotanus and Chergui, 2008; Weitlauf et al., 2005). Therefore, it was important to determine whether DA acted on NMDAR of specific composition. Kash et al. (2008a, 2009) showed that chronic passive (experimenter-controlled) exposure to alcohol alters the subunit composition of NMDAR throughout the dorsolateral BNST, which includes the ovBNST. We observed that NMDA-EPSC became insensitive to non-competitive GluN2B antagonists, suggesting a loss of synaptic diheteromeric GluN1/GluN2B2 NMDAR (GluN2B non-competitive antagonists being ineffective at triheteromeric GluN1/GluN2A/GluN2B (Hatton and Paoletti, 2005)). Thus, these results show that DA reduced EPSCs from GluN2A-containing NMDAR in the ovBNST of cocaine self-administering rats.
This selectivity might have significant consequences on NMDAR-dependent plasticity in the ovBNST. Although still under debate, evidence suggests that an increase in GluN2B/2A ratio promotes LTP at excitatory synapses, while a decrease in GluN2B/2A ratio promotes LTD (Morishita et al., 2007; Philpot et al., 2007; Weitlauf et al., 2005). In the dorsal BNST, GluN2A subunits are not involved in LTP, suggesting a role for GluN2A-containing NMDAR in LTD in the BNST (Weitlauf et al., 2004, 2005). Thus, with cocaine self-administration, DA may bias bidirectional synaptic plasticity towards LTP, thereby ‘locking’ excitatory synapses in the ovBNST. Although speculative, this might contribute to exaggerated AMPA-mediated currents in the BNST of cocaine self-administering rats (Dumont et al., 2005).
4.2. De novo D1R and D2R in the ovBNST of cocaine self-administering rats
The rat ovBNST is largely devoid of D1R, as we previously showed functionally and anatomically (Krawczyk et al., 2011a but see Kash et al., 2008b). Here, however, D1R activation contributed to the effects of DA on NMDA-EPSC in cocaine self-administering rats. This is consistent with our previous observation of de novo D1-mediated regulation of GABA transmission in ovBNST neurons of cocaine self-administering rats (Krawczyk et al., 2011b, 2013). This phenomenon is not unique to the BNST: increase in D1R expression also occurs in the shell of the nucleus accumbens following chronic cocaine self-administration (Nader et al., 2002). Thus, cocaine self-administration may be accompanied by DRD1 gene expression or receptor trafficking to the membrane surface, mechanisms that will require further study.
D2R is typically located on inhibitory synapses in the ovBNST of drug-naïve rats (Krawczyk et al., 2011a). However, D2R is absent in the ovBNST of rats self-administering cocaine (Krawczyk et al., 2011b), an observation consistent with drug-related D2R dysfunction in the brain reward system (Dalley et al., 2007; Nader et al., 2002; Volkow et al., 1990). With respect to DA regulation of NMDA-EPSC however, we observed de novo D2R function, an intriguing observation in the context of the neural basis of substance abuse.
D1R activation often potentiates, whereas D2R usually depresses NMDA currents in the brain (Kotecha et al., 2002; Seamans et al., 2001; Tseng and O’Donnell, 2004; Zheng et al., 1999). However, D1R and D2R can also cooperate at cellular and behavioral levels (Hopf et al., 2003; Phillips et al., 1994). Specifically, both D1R and D2-like (D4) receptors negatively regulate NMDA currents in lateral amygdala pyramidal neurons (Martina and Bergeron, 2008). Here, we demonstrate that the D1R agonist SKF-81297 and D2R agonist quinpirole both contributed, in a dose-dependent and summative fashion, to NMDA-EPSC reduction. While D1R and D2R can form functional hetero-dimers (Lee et al., 2004), our results suggest otherwise. First, each agonist produced a response independently — a behavior that differs from heterodimers, which require co-stimulation of both of constituent receptors (Lee et al., 2004). Second, both D1R and D2R antagonists were required to block the effects of DA in the ovBNST, whereas either D1R or D2R blockade is sufficient to inhibit heterodimer signaling (Lee et al., 2004). Our data revealed that simultaneous and individual applications of both agonists at 1 μM produced effects of identical magnitude, suggesting converging D1- and D2-like effects on NMDA currents.
4.3. Signaling pathways
D1R are predominantly Gαs-coupled and activate adenylate cyclase (AC) and cyclic AMP-dependent protein kinase (PKA), but alternative signaling, including through the Gq/phospholipase C (PLC) pathway, is also common (Jin et al., 2001; Neve et al., 2004). D2R generally recruits heterotrimeric Gαi/0 proteins to negatively control AC activity and also regulate effectors such as ion channels, phospholipases, protein kinases, and receptor tyrosine kinases by Gβγ-dependent processes (reviewed by Neve et al., 2004). D1- and D2-like DA receptors modulate NMDA currents, positively and negatively, through both G-protein-dependent and independent pathways (Gonzalez-Islas and Hablitz, 2003; Kotecha et al., 2002; Lee et al., 2002; Martina and Bergeron, 2008). Our study indicates that both D1R and D2R activated G-protein-dependent pathways converge on PLC, suggesting a novel PLC-dependent negative modulation of NMDAR in the brain.
We sought to determine the intracellular link between DA receptor activation of PLC and the reduction in NMDA currents. Phosphorylation of NMDAR positively correlates with NMDA-mediated currents or expression at the membrane surface (Dunah et al., 2004; Wang et al., 1994). Consistent with these observations, post-synaptic inhibition of PP1, PP2A, and tyrosine phosphatases completely abolished SKF-81297- and quinpirole-mediated reduction in NMDA-EPSCs.
4.4. Potential functional and behavioral implications
Further to our previous studies, the data suggest that cocaine self-administration profoundly changes the rules by which DA regulates synaptic transmission and consequently, neuronal activity in the ovBNST. In control rats, DA reduces inhibitory transmission carried by GABAA channels, which may result in an increase in ovBNST neuronal activity (Krawczyk et al., 2011a, 2013). In cocaine self-administering rats, however, DA may reduce neuronal activity by both increasing GABA release (Krawczyk et al., 2011b, 2013) and reducing NMDA currents (this study). In addition, we recently measured significant increases in AMPA-mediated excitatory synaptic transmission in the ovBNST of cocaine self-administering rats (unpublished results). Accordingly, DA may gate and reinforce cocaine-related cues carried by strong AMPA signals by reducing overall ovBNST network excitability during acute cocaine withdrawal. Future studies may reveal that DA-mediated regulation of NMDA currents in the ovBNST contributes in the motivation to self-administer cocaine, one index of compulsive cocaine use in rats trained on a PR schedule of reinforcement (Belin et al., 2008; Deroche-Gamonet et al., 2004).
In summary, few studies have investigated the functional interaction between DA and NMDAR in animals with a history of drug (of abuse) intake (Argilli et al., 2008; Schilstrom et al., 2006). This is an important gap given the crucial role of both DA and NMDAR in the neuropathology of drug addiction (Carr and Kalivas, 2008). Here we demonstrated that cocaine self-administration profoundly affects how DA modulates NMDAR in the ovBNST, a robust target of midbrain DA neurons (Hasue and Shammah-Lagnado, 2002; Krawczyk et al., 2011a). These results may help elucidate the mechanisms of dysfunctional synaptic plasticity produced by drugs-of-abuse during the acute withdrawal phase (Jones and Bonci, 2005; Thomas et al., 2008).
Acknowledgments
This project was supported by a Canadian Institutes of Health Research operating grant MOP-79277, the J.P. Bickell Foundation, the Canadian Foundation for Innovation, and Queen’s University.
Abbreviations
- DA
Dopamine
- BNST
Bed Nucleus of the Stria Terminalis
- ov
oval
- NMDARs
N-methyl-d-aspartate receptors
- BP
Breakpoint
- PR
Progressive ratio
- FR1
Fixed ratio-1
- EPSCs
Excitatory post-synaptic currents
Footnotes
Author contributions: É.C.D. designed research with M.K., X.M., J.D.; all authors conducted experiments and analyzed data; É.C.D. wrote paper with M.K., X.M., J.D., and A.A.J.
References
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Malenka RC. Enhanced inhibition of synaptic transmission by dopamine in the nucleus accumbens during behavioral sensitization to cocaine. J Neurosci. 2002;22:5817–22. doi: 10.1523/JNEUROSCI.22-14-05817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–9. doi: 10.1016/s0896-6273(00)80082-3. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Rolando MT, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci. 2000;20:RC102. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Kalivas PW. Confused about NMDA and addiction? Targeted knockouts provide answers and new questions. Neuron. 2008;59:353–5. doi: 10.1016/j.neuron.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–4. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol Pharmacol. 2004;65:121–9. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, II, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse. 2003;48:45–56. doi: 10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Markou A, Koob GF. The dopamine D-1 receptor antagonist Sch 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 1998;784:105–15. doi: 10.1016/s0006-8993(97)01190-6. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–52. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II–III pyramidal neurons in rat prefrontal cortex. J Neurosci. 2003;23:867–75. doi: 10.1523/JNEUROSCI.23-03-00867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–74. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. J Neurosci. 2003;23:5079–87. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–9. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Jin LQ, Wang HY, Friedman E. Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J Neurochem. 2001;78:981–90. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–5. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kash TL, Matthews RT, Winder DG. Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2008a;33:1379–90. doi: 10.1038/sj.npp.1301504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008b;28:13856–65. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, II, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34:2420–9. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–79. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, et al. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–22. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Krawczyk M, Georges F, Sharma R, Mason X, Berthet A, Bezard E, et al. Double-dissociation of the catecholaminergic modulation of synaptic transmission in the oval bed nucleus of the stria terminalis. J Neurophysiol. 2011a;105:145–53. doi: 10.1152/jn.00710.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Sharma R, Mason X, Debacker J, Jones AA, Dumont EC. A switch in the neuromodulatory effects of dopamine in the oval bed nucleus of the stria terminalis associated with cocaine self-administration in rats. J Neurosci. 2011b;31:8928–35. doi: 10.1523/JNEUROSCI.0377-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Mason X, DeBacker J, Sharma R, Normandeau CP, Hawken ER, et al. D1 dopamine receptor-mediated LTP at GABA synapses encodes motivation to self-administer cocaine in rats. J Neurosci. 2013;33:11960–71. doi: 10.1523/JNEUROSCI.1784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, et al. Dual regulation of NMDA receptor functions by direct protein–protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–30. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, et al. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–8. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–8. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kauer JA. Repeated exposure to amphetamine disrupts dopaminergic modulation of excitatory synaptic plasticity and neurotransmission in nucleus accumbens. Synapse. 2004;51:1–10. doi: 10.1002/syn.10270. [DOI] [PubMed] [Google Scholar]
- Martina M, Bergeron R. D1 and D4 dopaminergic receptor interplay mediates coincident G protein-independent and dependent regulation of glutamate NMDA receptors in the lateral amygdala. J Neurochem. 2008;106:2421–35. doi: 10.1111/j.1471-4159.2008.05584.x. [DOI] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2007;52:71–6. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Panlilio LV. Stimulant self-administration. In: Olmstead MC, editor. Animals models of drug addiction. New York: Springer; 2010. pp. 57–81. [Google Scholar]
- Phillips GD, Robbins TW, Everitt BJ. Bilateral intra-accumbens self-administration of d-amphetamine: antagonism with intra-accumbens SCH-23390 and sulpiride. Psychopharmacology (Berl) 1994;114:477–85. doi: 10.1007/BF02249339. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, et al. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006;26:8549–58. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. Long-term potentiation in the nucleus accumbens requires both NR2A- and NR2B-containing N-methyl-D-aspartate receptors. Eur J Neurosci. 2008;27:1957–64. doi: 10.1111/j.1460-9568.2008.06173.x. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001;98:301–6. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–2. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, Bonci A. Modulation of long-term depression by dopamine in the mesolimbic system. J Neurosci. 2000;20:5581–6. doi: 10.1523/JNEUROSCI.20-15-05581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine–glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–9. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–7. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–24. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- Walker JR, Ahmed SH, Gracy KN, Koob GF. Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]
- Wang J, O’Donnell P. D(1) dopamine receptors potentiate NMDA-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex. 2001;11:452–62. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- Wang LY, Orser BA, Brautigan DL, MacDonald JF. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature. 1994;369:230–2. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, Grueter BA, Winder DG. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24:5741–7. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–90. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. Opposite modulation of cortical N-methyl-D-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 1999;91:527–35. doi: 10.1016/s0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–96. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]