Abstract
Understanding the neurobiology of motivation might help in reducing compulsive behaviors such as drug addiction or eating disorders. This study shows that excitatory synaptic transmission was enhanced in the bed nucleus of the stria terminalis of rats that performed an operant task to obtain cocaine or palatable food. There was no effect when cocaine or food was delivered passively, suggesting that synaptic plasticity in this area is involved in reward-seeking behaviors.
Unearthing the neural mechanisms that underlie compulsive behaviors is a major goal in addiction research, and animal models of self-administration are particularly useful1,2. The present study addresses two questions about the neurobiological roots of drug addiction. First, do mechanisms that underlie drug-seeking behaviors overlap with those affected by passively administered drugs? Second, are drugs more potent at inducing synaptic adaptations than natural rewards such as food? To address these issues, we recorded excitatory post-synaptic currents (EPSCs) from neurons in the ventral lateral bed nucleus of the stria terminalis (vlBNST) in brain slices from rats that self-administered either cocaine or palatable food pellets for 11 consecutive days. The results were compared with those obtained from rats that received cocaine or food passively (Supplementary Methods online). All procedures were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University.
Rapid synaptic transmission mediated by glutamate results from activation of both AMPA and NMDA receptors. A change in the ratio of AMPA to NMDA receptor–dependent currents is a measure of synaptic plasticity at excitatory synapses in the brain3. Stimulation in the vlBNST evoked EPSCs with both AMPA and NMDA components (Fig. 1a). The AMPA/NMDA ratio was measured in brain slices from rats that received cocaine acutely or repeatedly, either passively or by self-administration. One group of rats self-administered cocaine for 11 consecutive days and a second group received the same amount of cocaine in a response-independent manner. Passive administration of cocaine was computer controlled to match the amount and pattern of cocaine delivery of the self-administration group. The AMPA/NMDA ratio was also determined in slices from rats that lever-pressed for food or that received food passively in the same amount and manner as the self-administration group. Brain slices were obtained 24 h after the last exposure to drug or food. In slices from rats that self-administered cocaine, the AMPA/NMDA ratio was greater than in animals that received cocaine passively (self-administration: 1.8 ± 0.1, n = 7 rats, 14 neurons; passive: 1.0 ± 0.1, n = 5 rats, 10 neurons; F7,80 = 11.2, P < 0.0001; Fig. 1b). The AMPA/NMDA ratio was the same in slices from rats that received cocaine passively as it was in slices from rats that received saline (1.0 ± 0.1, n = 4 rats, 10 neurons) and in untreated slices (1.0 ±0.1, n = 10 rats, 18 neurons). In addition, single injections of cocaine (20 mg kg−1 intraperitoneally) or saline did not increase the AMPA/NMDA ratio (cocaine: 1.1 ±0.2, n = 3 rats, 6 neurons; saline: 1.1 ±0.1, n = 3 rats, 6 neurons). Self-administration of food also increased the AMPA/NMDA ratio compared with passive administration of food pellets (self-administration: 1.3 ± 0.1, n = 7 rats, 13 neurons; passive: 0.8 ± 0.1, n = 7 rats, 11 neurons, P < 0.0001).
Figure 1.
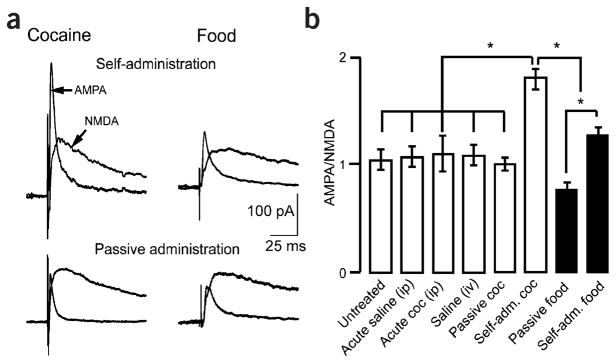
Self-administration of cocaine or palatable food increased the AMPA/NMDA ratio in the vlBNST. (a) Representative traces showing recordings of electrically evoked excitatory postsynaptic currents (EPSC). AMPA EPSCs were isolated through the addition of the NMDA receptor antagonist AP-5 (50 μM). Traces on the left show evoked EPSCs after self-administration (top) and passive administration (bottom). Traces on the right show evoked EPSCs after self-administration (top) or passive administration (bottom) of palatable food. (b) The bar chart shows AMPA/NMDA ratios (average ± s.e.m.) as a function of the treatment that each group of rats received. *P < 0.0001. i.p, intraperitoneal; i.v., intravenous.
These observations demonstrate a role for the vlBNST in the performance of an operant task to obtain a reward. A key component in generating plasticity in vlBNST synapses seems to be the control afforded by self-administration. Self-administration of cocaine produced the most robust increase of the ratio of AMPA receptor– to NMDA receptor–dependent currents, suggesting that the magnitude of changes in excitatory transmission in the vlBNST might also be related to the type of reward as well as to the manner in which it is experienced. There was, however, no correlation between the change in AMPA/NMDA ratio and the amount of cocaine or the number of food pellets that the rats self-administered (Fig. 2). This result shows that the amount of cocaine or food did not influence the increase in synaptic strength. This finding also implies that central mechanisms affecting individual differences in consumption (that is, variations in drive) are downstream from the vlBNST.
Figure 2.
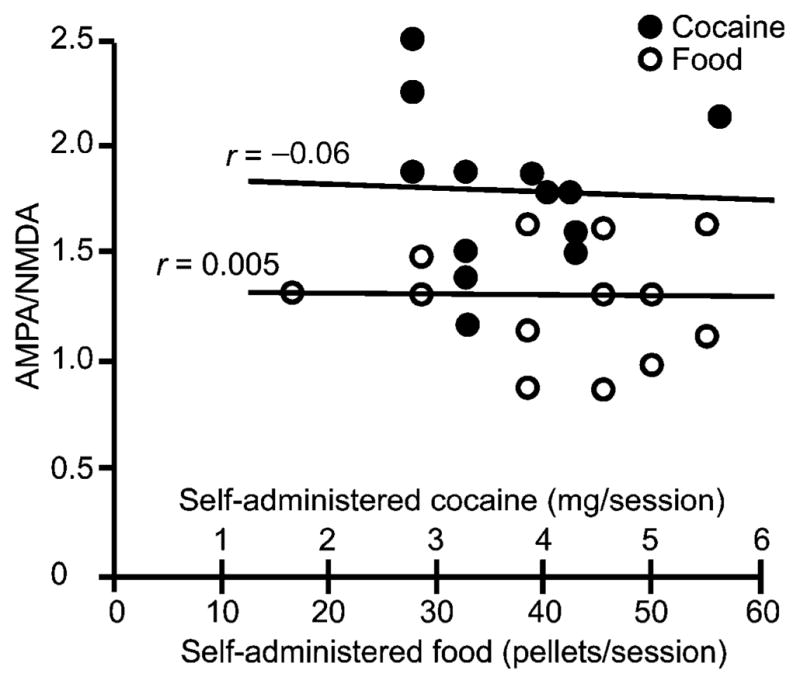
The increase in AMPA/NMDA ratio was independent of the amount of cocaine self-administered or food self-delivered. The dot chart represents the AMPA/NMDA ratio measured in single neurons as a function of the average daily amount of self-administered cocaine or food pellets.
Only self-administration of drug or food reward (not passive administration) caused an increase in excitatory transmission, suggesting that the change in AMPA/NMDA ratio could not have resulted from direct actions of these stimuli on the synapse. Cocaine or amphetamine had no effect on excitatory transmission or a post-synaptic effect on vlBNST neurons (data not shown). Thus, the increase in AMPA/NMDA ratio was probably driven by changes in afferent circuitry to the vlBNST, perhaps from the hippocampus, amygdala or prefrontal cortex4.
The BNST is thought to be part of the reward circuitry because it contributes to relapse of cocaine use, aversion induced by opioid withdrawal, and the reinforcing effects of opioids5–7. Neurons from the vlBNST project to areas involved in food and drug reward4. Projections to lateral hypothalamus, retrorubral areas and the ventral tegmental area (VTA) carry information relevant to drug and feeding motivation and initiation5,8,9. Whereas a single passive exposure to cocaine has been reported to increase excitatory synaptic strength on dopamine neurons in the VTA10,11, the BNSTseems to respond more selectively in that active drug intake is required to increase synaptic strength.
The self-administration of cocaine or food leads to a robust increase in AMPA EPSCs that is predicted to result in more efficient activation of neurons in the vlBNST. The facilitation of an excitatory pathway from the vlBNST to midbrain dopamine neurons and potentially other projection areas would promote reward-seeking behaviors12,13. It is probable that the AMPA/NMDA ratio increased in all animals during the initial food-training period and declined to control levels only in animals that received cocaine or food passively during the subsequent 17 d. The mechanism of this decline is not known, but whether the ratio passively declines or is actively suppressed, the change may contribute to the extinction of the self-administration behavior. We suggest that the BNST could be part of a specific pathway that underlies reward reinforcement, and that events associated with rewarding behaviors modify activity within this circuit.
Supplementary Material
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Markou A, et al. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- 2.Deroche-Gamonet V, Belin D, Piazza PV. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 3.Malenka RC, Nicoll RA. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 4.Dong HW, Swanson LW. J Comp Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- 5.Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 6.Erb S, Salmaso N, Rodaros D, Stewart J. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- 7.Walker JR, Ahmed SH, Gracy KN, Koob GF. Brain Res. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]
- 8.Kelley AE. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Phillipson OT. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- 10.Ungless MA, Whistler JL, Malenka RC, Bonci A. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 11.Borgland SL, Malenka RC, Bonci A. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumont EC, Williams JT. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georges F, Aston-Jones G. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


