Abstract
The bed nucleus of the stria terminalis (BST) is a cluster of nuclei within the extended amygdala, a forebrain macrostructure with extensive projection to motor nuclei of the hindbrain. The subnuclei of the BST coordinate autonomic, neuroendocrine, and somatomotor functions and receive robust neuromodulatory monoaminergic afferents, including 5-HT-, noradrenaline (NA)-, and dopamine (DA)-containing terminals. In contrast to 5-HT and NA, little is known about how DA modulates neuronal activity or synaptic transmission in the BST. DA-containing afferents to the BST originate in the ventral tegmental area, the periaqueducal gray, and the retrorubral field. They form a fairly diffuse input to the dorsolateral BST with dense terminal fields in the oval (ovBST) and juxtacapsular (jxBST) nuclei. The efferent-afferent connectivity of the BST suggests that it may play a key role in motivated behaviors, consistent with recent evidence that the dorso-lateral BST is a target for drugs of abuse. This study describes the effects of DA on synaptic transmission in the ovBST. Whole cell voltage clamp recordings were performed on ovBST neurons in brain slices from adult rats in the presence or absence of exogenous DA and receptor-targeted agonists and antagonists. The results showed that DA selectively and exclusively reduced inhibitory synaptic transmission in the ovBST in a dose-dependent and D2-like dopamine receptor-dependent manner. DA also modulated excitatory synaptic transmission in a dose-dependent dependent manner. However, this effect was mediated by α2-noradrenergic receptors. Thus these data reveal a double dissociation in catecholaminergic regulation of excitatory and inhibitory synaptic transmission in the ovBST and may shed light on the mechanisms involved in neuropathological behaviors such as stress-induced relapse to consumption of drugs of abuse.
INTRODUCTION
The bed nucleus of the stria terminalis (BST) receives adrenergic, noradrenergic, serotoninergic, and dopaminergic inputs (Freedman and Cassell 1994; Hasue and Shammah-Lagnado 2002; Meloni et al. 2006; Phelix et al. 1992). The distribution of axons immunoreactive for tyrosine hydroxylase (TH), dopamine (DA)-β-hydroxylase (DβH), phenylethanolamine-N-methyl transferase (PNMT), or 5-HT reveals that monoaminergic inputs to the BST are topographically organized with both overlapping and exclusive distributions, depending on specific BST subregions (Phelix et al. 1992).
5-HT immunoreactive terminals are broadly distributed rostrocaudally but mostly dorsal to the anterior commissure in the BST (Phelix et al. 1992). However, 5-HT immunoreactivity is more robust in the medial than the lateral parts of the BST and largely avoids the oval (ovBST) and juxtacapsular (jxBST) areas, which are located dorsolaterally (Larriva-Sahd 2004, 2006; Phelix et al. 1992). 5-HT modulates neuronal activity and reduces excitatory synaptic transmission in the BST (Guo and Rainnie 2010; Guo et al. 2009; Hammack et al. 2009; Levita et al. 2004). The specific effects of 5-HT on BST neurons activity is dynamically modulated by, and causally related to, stress and anxiety (Hammack et al. 2009; Levita et al. 2004).
DβH [noradrenaline (NA) and/or adrenaline]-containing fibers are mostly concentrated in the ventrolateral BST although there is DβH immunoreactivity medially in the dorsal BST (Freedman and Cassell 1994; Kozicz 2001; Phelix et al. 1992). Although rare, adrenergic terminals are present in the dorso-lateral region of the intermediate (from a rostrocaudal perspective) regions of the BST (Phelix et al. 1992). NA also modulates both neuronal excitability and synaptic transmission in the ventral and dorsal BST (Dumont and Williams 2004; Egli et al. 2005; McElligott and Winder 2008, 2009; McElligott et al. 2010; Shields et al. 2009). Noradrenergic modulation of BST neuron excitability and synaptic transmission is also dynamically altered by various physiological and pathological conditions (Dumont and Williams 2004; McElligott and Winder 2008, 2009; McElligott et al. 2010) including stress, anxiety, pain, reward, and addiction (Cecchi et al. 2002; Delfs et al. 2000; Deyama et al. 2008, 2009; Dumont and Williams 2004; Leri et al. 2002).
In contrast to 5-HT and NA, little is known about the neurophysiology of DA in the BST (Kash et al. 2008). Although dopaminergic fibers form a fairly diffuse input to the dorsolateral BST, dense terminal fields can be observed in the ovBST and jxBST (Deutch et al. 1988; Freedman and Cassell 1994; Hasue and Shammah-Lagnado 2002; Meloni et al. 2006; Phelix et al. 1992). These dopaminergic inputs originate from the ventral tegmental area, the periaqueducal gray region, and the retrorubral field (Hasue and Shammah-Lagnado 2002; Meloni et al. 2006). A recent study on dopaminergic regulation of neuronal activity and synaptic transmission in the dorsolateral BST in the mouse (Kash et al. 2008) reported that DA produced a modest increase in spontaneous excitatory, but not inhibitory, synaptic transmission, an effect that was attributed to a D1/D2-like mediated depolarization of local CRFergic neurons. However, Kash et al. did not specify the subregion of the dorsolateral BST examined in their study.
In addition to TH-positive axons, the ovBST contains several key components of the brain DA system including DA receptors (although the exact subtypes remains unclear) and DARPP-32, suggesting that DA may be an important neuro-modulator of neuronal activity, synaptic transmission, or both, in this region (Freedman and Cassell 1994; Gustafson and Greengard 1990; Hasue and Shammah-Lagnado 2002; Meloni et al. 2006). The presence of multiple DA inputs as well as the connectivity of the ovBST is consistent with a role in motivated behaviors (Dong et al. 2001a,b; Larriva-Sahd 2006; McDonald et al. 1999). In fact, pharmacological manipulation of DA receptors in the dorsolateral BST alters natural and pharmacological rewards-motivated behaviors (Eiler et al. 2003; Epping-Jordan et al. 1998). Furthermore, the dorsolateral BST is ideally located to integrate stress and reward information and consequently, consistent with its reported role in stress-induced relapse to chronic voluntary drug intake in animal models of drug addiction (Erb et al. 2001; Leri et al. 2002). Because DA (reward) and NA (stress) response pathways co-localize in the dorsolateral BST, interaction between the DA and NA in this brain region might play a critical role in integrating stress- and reward-induced behaviors.
The objective of the present study was to elucidate the neuromodulatory effects of DA on synaptic transmission in the ovBST. Whole cell voltage clamp recordings were done on ovBST neurons in brain slices from adult rats in the presence and absence of exogenous DA and selective receptor-targeted agonists and antagonists. DA reduced the amplitude of evoked GABAA inhibitory postsynaptic currents (IPSC) presynaptically in a D2 dopamine receptor (D2R)- and dose-dependent manner. In contrast, the effect of DA on excitatory transmission was absolutely dependent on functional α2-adrenergic receptors (α2R), a mechanism that overlapped substantially with the neuromodulatory mechanism of NA. DA did not produce measurable effects on passive neurophysiological properties of ovBST also suggesting presynaptic effects of DA. Immunohistochemical studies confirmed that D2R but not D1R are expressed in the ovBST. Together, our results demonstrate how DA modulates synaptic transmission in the ovBST, an important step in understanding the role of ovBST DA in motivated behaviors. Furthermore, the effect of DA on synaptic transmission in the ovBST could be dissociated from those of NA, revealing synaptic mechanisms that could help integrate stress (NA)- and reward (DA)-related stimuli in the ovBST.
METHODS
Subjects
Adult male Long Evans and Sprague-Dawley rats (Charles River Laboratories, www.criver.com) weighing 300 – 650 g, were housed in a climate-controlled colony room. The animals were maintained on a 12 h reversed light/dark cycle (09.00 h lights off – 21.00 h lights on) and fed ad libitum. All the experiments were conducted in accordance with the Canadian Council on Animal Care guidelines for use of animals in experiments and approved by the Queen’s University Animal Care Committee or the French (87– 848, Ministère de l’Agriculture et de la Forêt) and European Economic Community (86 – 6091 EEC) guidelines for the care of laboratory animals and were approved by the Ethical Committee of Centre National de la Recherche Scientifique, Région Aquitaine.
Brain slices preparation and electrophysiology
Rats were anesthetized with isoflurane, and their brains were rapidly removed. Coronal slices (250 μm) containing the BST were prepared in ice-cold physiological solution containing (in mM) 126 NaCl, 2.5 KCl, 1.2 MgCl2, 6 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, and 12.5 D-glucose. Slices were incubated at 34°C for 60 min and transferred to a chamber that was constantly perfused (1.5 ml/min) with physiological solution maintained at 34°C and equilibrated with 95% O2-5% CO2. Whole cell voltage-clamp recordings were made using microelectrodes filled with a solution containing (in mM) 70 K+-gluconate, 80 KCl, 1 EGTA, 10 HEPES, 2 MgATP, 0.3 GTP, and 1 P-creatine.
All recordings were restricted to the ovBST, and more precisely, to the dorsal half of the ovBST (Fig. 1A). The exact anteroposterior coordinates representing the brain slice used varies slightly from one Brain Atlas to the other (−0.26 mm and −0.12 mm in Swanson and Paxinos and Watson, respectively, Fig. 1A). In practice, we used the slices where the posterior part of the anterior commissure (ac) decussates but where the lateral extension of the ac are still present. Recordings were restricted medially to an imaginary vertical line that would run through the lateral ventricle. In addition, recordings were restricted to the area of the dorsolateral BST located dorsal to the halfway point between the tip of the lateral ventricle and the top of the ac (Fig. 1A). Postsynaptic currents were evoked by local fiber stimulation with tungsten bipolar electrodes while ovBST neurons where voltage-clamped at −70 mV. Stimulating electrodes were placed in the ovBST, 100 –500 μm lateral (IPSC) or dorsal (excitatory postsyn-aptic current, EPSC) from the recorded neurons (Fig. 1A), and paired electrical stimuli (0.1 ms duration, 50 ms interval) were applied at 0.1 Hz. GABAA-IPSC and AMPA-EPSC were pharmacologically isolated with 6,7-dinitroquinoxaline-2,3-dione (DNQX; 50 μM) or picrotoxin (100 μM), respectively. Recordings were made using a Multiclamp 700B amplifier and a Digidata 1440A (Molecular Devices Scientific, www.mds.com). Data were acquired and analyzed with Axograph X (www.axographx.com) running on an Apple computer (www.apple.com).
FIG. 1.
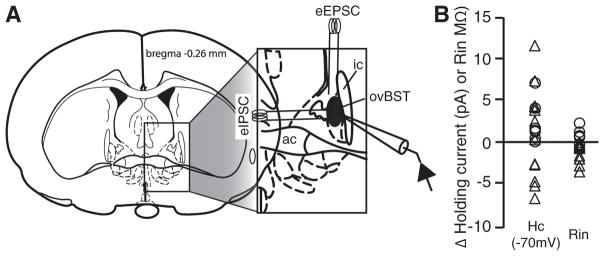
Anatomical localization of recordings and effects of agonists on passive electrophysiological properties of bed nucleus of the stria terminalis (BST) with dense terminal fields in the oval (ovBST) neurons. A: schematic illustrating the anatomical localization of recording pipettes and stimulating electrodes. ac: anterior commissure; ic: internal capsule. B: dot plot showing the effects of dopamine (DA) 30 μM (○) or noradrenaline (NA) 10 μM (△) on membrane holding current (Hc) and membrane input resistance (Rin).
Drugs
Stock solution of DA (10 mM), SFK-81297 (1 mM), quinpirole (1 mM), SCH-23390 (10 mM), methysergide (10 mM), 5-HT (10 mM), and propranolol (10 mM) were prepared in double distilled water. Stock solution of DNQX (100 mM), sulpiride (1 mM), yohimbine (1 mM), and, prazosin (1 mM) were prepared in DMSO (100%). Every drug was further dissolved in the physiological solutions at the desired concentration and the final DMSO concentration never exceeded 0.1%. All the drugs were obtained from Sigma-Aldrich (www.sigmaaldrich.com) or Tocris (Tocris, www.tocris.com).
Statistical analyses
We measured drug-induced change in PSC peak amplitude from baseline in percentage {[(peak amplitudedrug − peak amplitudebaseline)/peak amplitudebaseline]*100}. Paired-pulse ratios (PPRs) were calculated by dividing the second (S2) by the first (S1) peak amplitude that we normalized to baseline. Peak amplitudes for S1 and S2 were calculated from a baseline value measured 10 ms after the end of a 1 mV test pulse. Coefficient of variation (CV) analyses was done by plotting r [(1/CV2drug)/(1/CV2baseline)] against π (peak amplitudedrug/peak amplitudebaseline). We computed bivariate linear fits of r by π. Data were all expressed as means ± SE. Drug effects were assessed using one-tailed t-test with hypothesized values of 0 (changes in amplitude) and 1 (changes in PPR). When multiple t-test were done on a single set of data, we adjusted the significance level accordingly (0.05/ number of t-test). When comparing multiple means, one-way ANOVAs where used with Dunnett’s test for multiple comparison when ANOVA deemed significance. All statistical analyses were done with JMP 8.0.
Immunohistochemical detection of D1R and D2R
The rats were deeply anesthetized with sodium chloral hydrate (400 mg/kg ip) and perfused transcardiacally with 50 –100 ml of 0.9% NaCl followed by 300 ml of fixative consisting of 2% paraformaldehyde (PFA) with 0.2% glutaraldehyde in 0.1 M phosphate buffer (PB), pH 7.4. Brains were quickly removed, left overnight in 2% PFA at 4°C, and cut into 60 μm-thick frontal sections with a vibrating blade microtome (Leica, VT1000S, www.Leica.com). To enhance the penetration of the immunoreagents, the sections were equilibrated in a cryoprotectant solution (0.05 M PB, pH 7.4, containing 25% sucrose and 10% glycerol), freeze-thawed, and stored in PBS with 0.03% sodium azide until needed. D1R was detected by immunohistochemistry according to previously described and validated procedures (Berthet et al. 2009; Caille et al. 1996) using a monoclonal antibody raised in rat against a 97 amino acid sequence corresponding to the C terminus of the human D1R (Sigma-Aldrich) (Hersch et al. 1995; Levey et al. 1993). D2R was detected with an affinity-purified rabbit polyclonal antiserum directed against a 28 amino acid sequence within the third cytoplasmic loop from the human D2R corresponding to anti D2-284 peptide (Millipore) that recognizes both short and long isoforms (Ahmed et al. 2010). The sections containing the ovBST were incubated in 4% normal goat serum (NGS) or in normal donkey serum (NDS) in PBS for 1h at room temperature (RT) and then in the antibodies against D1R (1:1,000) or D2R (1:500) supplemented with 1% NGS or NDS overnight at RT. After washing (3 × 10 min) in PBS, for D1R detection, the sections were incubated in goat anti-rat IgG coupled to biotin (Amersham; 1:200 in PBS for 1.5 h) For D2R detection, the sections were incubated in Dako EnVision+ system-horseradish peroxydase (HRP) labeled polymer anti-rabbit for 40 min at RT. After three rinses (3 × 10 min) in PBS for D1-DA receptor detection, the sections were then incubated in avidin-biotin complex (Vector ABC Elite, Vector Laboratories, www.vectorlabs.com; 1:200) in PBS for l.5 h at RT and again rinsed (3 × 10 min) in PBS. The immunoreactive sites were revealed by a combination of the glucose oxidase-diaminobenzidine (DAB; Sigma-Aldritch, 0.05% in TB) method and the DAB-nickel method (Shu et al. 1988). The reaction was stopped by several washes in H2O. The sections were mounted on glass slides, dehydrated, and mounted in Eukitt (Electron Microscopy Sciences, www.emsdiasum.com). Negative immunohistological control demonstrated the absence of signal when omitting the first antibody.
RESULTS
Passive properties of ovBST neurons
No agonist tested in the present study significantly changed passive properties [membrane input resistance (Rin) and membrane holding currents (Hc)] of ovBST neurons voltage-clamped at −70 mV. In particular, DA (30 μM) and NA (10 μM), which produced the largest effects on synaptic transmission, had virtually no effect on Hc and Rin of ovBST neurons (Fig. 1B).
DA decreased inhibitory transmission in the ovBST by activating presynaptic D2R
Exogenously applied DA (0.1 to 100 μM) dose-dependently decreased the amplitude of evoked and pharmacologically isolated GABAA-IPSC in ovBST neurons (Fig. 2). GABAA -IPSC rapidly returned to baseline on when DA was washed out of the perfusion chamber. At concentrations where DA decreased GABAA-IPSC, we observed statistically significant increases in PPR (PPR50 ms) suggesting it acts presynaptically (Fig. 2B). Coefficient of variation (CV) analyses revealed a significant positive correlation, also suggesting a presynaptic effect of DA (Fig. 2C). The D2-like agonist quinpirole dose-dependently and presynaptically mimicked the effects of DA on GABAA-IPSC (Fig. 2B). In contrast, the D1R agonist SKF-81297 did not alter the amplitude of GABAA-IPSC (Fig. 2B).
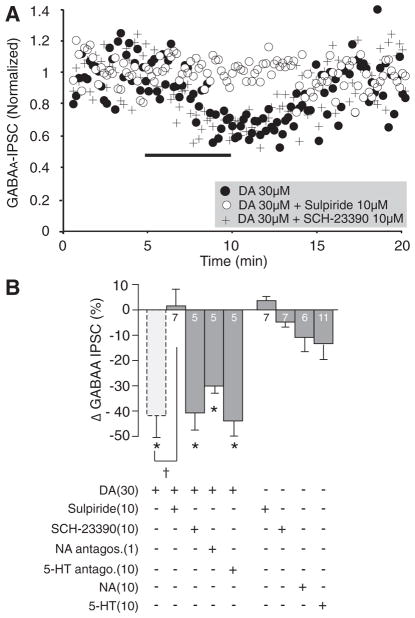
FIG. 2.
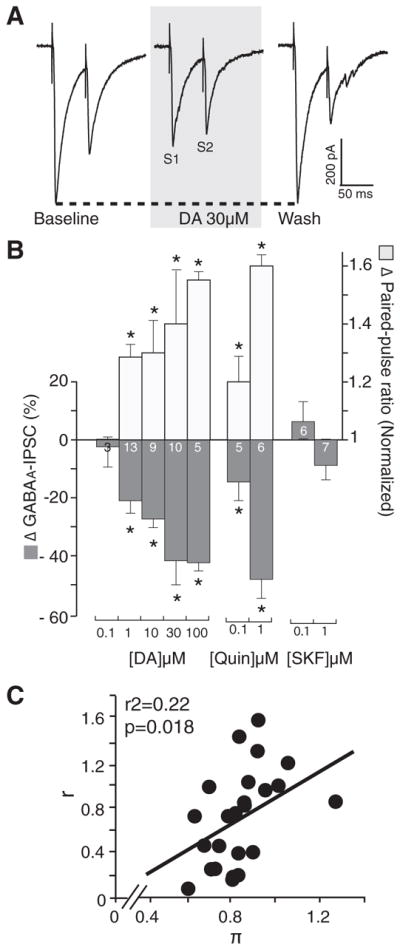
Effects of DA, D1R, and D2R agonists on the amplitude of evoked GABAA-inhibitory postsynaptic current (IPSC) in the ovBST. A: representative traces showing the effects of bath application of DA on electrically evoked GABAA-IPSC in the ovBST. Each trace is the average of 5 consecutive events. B: bar graph summarizing the effects of DA agonists on the peak amplitude and paired-pulse ratios of evoked GABAA-IPSC in the ovBST. C: coefficient of variation analysis of the effects of DA (0.1–30 μM) on evoked GABAA-IPSC in the ovBST. S1, stimulus 1; S2, stimulus 2. r, [(1/CV2drug)/(1/CV2baseline)]; Π, peak amplitudedrug/Peak amplitudebaseline. *, significantly different from 0; P < 0.01.
DA 30 μM was co-applied with either the D1R antagonist SCH-23390 (10 μM), the D2R antagonist sulpiride (10 μM), a cocktail of noradrenergic antagonists (prazosin 0.1 μM, yohimbine 1 μM, and propranolol 1 μM) or the 5-HT1,2,7 receptor antagonist (methysergide 10 μM). Only D2R blockade with sulpiride altered DA-induced reduction in GABAA-IPSC [F(4,19) = 7.6, P = 0.0008; Fig. 3]. As a result, DA produced significant decreases in GABAA-IPSC when co-applied with SCH-23390, noradrenergic antagonists, or methysergide (Fig. 3B). Likewise, NA (10 μM) modestly reduced GABAA-IPSC amplitude (Fig. 3B). On average, 5-HT (10 μM) had no significant effect on GABAA-IPSC amplitude in the ovBST (Fig. 3B). However, 5-HT decreased (−26.0 ± 6.8, n = 8) or slightly increased (11.0 ± 6.1, n = 3) GABAA-IPSC in the ovBST. Sulpiride and SCH-23390 did not produce any effects on their own (Fig. 3B).
FIG. 3.
Pharmacological characterization of the effects of DA on the amplitude of evoked GABAA-IPSC in the ovBST. A: representative dot plot showing the time course of the effects of DA on evoked GABAA-IPSC in the ovBST in the absence and the presence of the D1 dopamine receptor (D1R) antagonist SCH-23390 or the D2 dopamine receptor (D2R) antagonist sulpiride. B: bar chart summarizing the effect of monoaminergic agonists and antagonists on evoked GABAA-IPSC in the ovBST. *, significantly different from 0; P < 0.01. †, significantly different from DA 30 μM; P < 0.05.
DA decreased fast excitatory transmission in the ovBST by activating presynaptic α2R
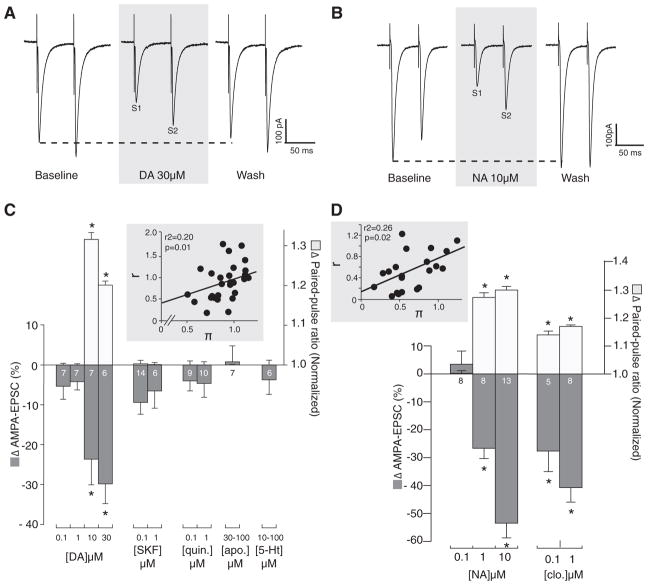
Exogenously applied DA (0.1–30 μM) dose-dependently decreased the amplitude of evoked and pharmacologically isolated AMPA-EPSC in ovBST neurons (Fig. 4A). AMPA-EPSC rapidly returned to baseline when DA was washed out of the perfusion chamber. At concentrations where DA decreased AMPA-EPSC, we observed statistically significant increases in PPR50 ms, suggesting it acted presynaptically (Fig. 4A1). CV analyses revealed a significant positive correlation, also suggesting a presynaptic effect of DA (Fig. 4A1, inset). In contrast to the effects of DA on GABAA-IPSC, bath application of D1R or D2R agonists did not mimic the effects of DA on AMPA-EPSC (Fig. 4A1). Bath application of the D2R agonist quinpi-role (0.1–1 μM), of the D1/D5 full agonist SKF-81297 (0.1–1 μM), or of the broad-spectrum DAR agonist apomorphine (30 –100 μM) did not significantly change the amplitude of evoked AMPA-EPSC (Fig. 4A1). Likewise, 5-HT (10 –100 μM) did not affect the amplitude of AMPA-EPSC in any tested ovBST neurons (Fig. 4A1).
FIG. 4.
Catecholaminergic modulation of evoked AMPA-excitatory PSC (EPSC) in the ovBST. A: representative traces showing the effects of bath application of DA on electrically evoked AMPA-EPSC in the ovBST. Each trace is the average of 5 consecutive events. A1: bar graph summarizing the effects of DA and 5-HT agonists on the peak amplitude and paired pulse ratio (PPR50 ms) of evoked AMPA-EPSC in the ovBST. Inset: coefficient of variation (CV) analyses. B: representative traces showing the effects of bath application of noradrenergic agonists on electrically evoked AMPA-EPSC in the ovBST. Each trace is the average of 5 consecutive events. B1: bar graph summarizing the effects of noradrenergic agonists on the peak amplitude and PPR50 ms of evoked AMPA-EPSC in the ovBST. Inset: CV analyses. *, significantly different from 0; P < 0.01.
However, exogenously applied NA (0.1–10 μM) dose-dependently reduced the amplitude of evoked AMPA-EPSC by acting presynaptically as shown by significant changes in PPR50 ms (Fig. 4B1). CV analyses revealed a significant positive correlation, also suggesting a presynaptic effect of NA (Fig. 4B1, inset). Furthermore, the α2R agonist clonidine dose-dependently reduced the amplitude of evoked AMPA-EPSC (Fig. 4B1). Clonidine also decreased AMPA-EPSC presynaptically because we measured a significant increase in PPR50 ms (Fig. 4B1). Furthermore, the α2R antagonist yohimbine (5 μM) completely abolished NA (t(15) = −7.7, P = 0.0001)- and DA (t(13) = 4.9, P = 0.0001)-induced reduction in AMPA-EPSC (Fig. 5). In contrast, the effect of NA was unaffected by α1- and β-adrenergic receptors blockade with prazosin (5 μM) and propranolol (5 μM; Fig. 5C).
FIG. 5.
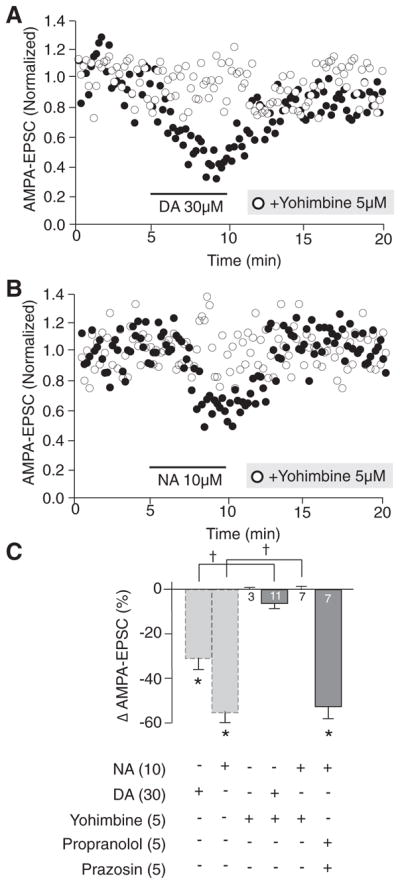
Pharmacological characterization of the effects of DA and NA on evoked AMPA-EPSC in the ovBST. A: representative dot plot showing the time course of the effects of DA on evoked AMPA-EPSC in the ovBST in the absence and the presence of the α2R antagonist yohimbine. B: representative dot plot showing the time course of the effects of NA on evoked AMPA-EPSC in the ovBST in the absence and the presence of the α2R antagonist yohimbine. C: bar chart summarizing the effects of noradrenergic antagonists on dopaminergic and noradrenergic modulation of evoked AMPA-EPSC in the ovBST. *, significantly different from 0; P < 0.01. †, P < 0.05.
Localization of D1R and D2R in the ovBST
We observed immunoreactivity for both D1R and D2R in the anterior BST (Fig. 6). However, the distribution of immunoreactivity for both receptors was not homogeneous. Notably, the ovBST was completely devoid of D1R immunostaining (Fig. 6A). In contrast, the ovBST contained immunoreactivity for D2R, which, at the light microscopy level, appeared to be in the neuropil and cytoplasm of some cells bodies (Fig. 6B).
FIG. 6.
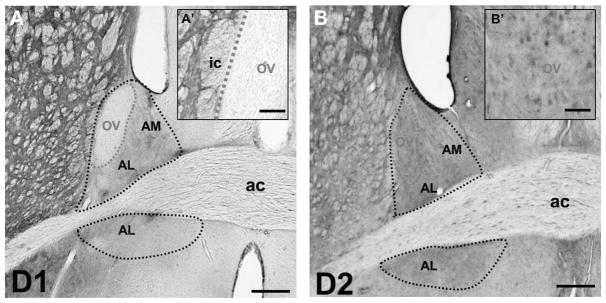
Immunohistochemical localization of D1R and D2R in the ovBST. Light micrographs of the rat brain immunostained to reveal immunoreactivity for D1R (A and A′) and D2R (B and B′). The black doted line delineates the anterior BST, whereas the red dotted line delineates the ovBST. ac, anterior commissure; ic, internal capsula; ov, oval nucleus. Scale bars: A and B, 0.3 mm; A′, 0.1 mm; B′, 50 μm.
DISCUSSION
This study examines the effects of DA in the ovBST of the rat and demonstrates the involvement of differential mechanisms for its effects on inhibitory and excitatory synaptic transmission. DA reduced the amplitude of whole cell evoked GABAA-IPSC presynaptically in an exclusively D2R-dependent manner, while it reduced fast excitatory transmission, also presynaptically, but through α2R. This conclusion is strongly supported by the use of specific D2R and α2R agonists and antagonists, and immunohistochemical evidence for expression of α2R (Shields et al. 2009) and D2R but not D1R in the ovBST (this study).
Although, the neurophysiological D2-like effect of DA on inhibitory synaptic transmission was clearly presynaptic, immunohistochemical data do not unequivocally demonstrate exclusive presynaptic localization of D2R. In fact, we detected perikarya-like immunolabeling of D2R in the ovBST. However, previous studies showed the absence of D2R mRNA in the dorsolateral BST (Bouthenet et al. 1991). As such, these perikarya-like structures could also be axonal envelopes of ovBST perikarya or local ovBST interneurons expressing D2R. In a 2006 study, Larriva-Sahd clearly demonstrated that there are several subtypes (11) of neurons in the ovBST among which subpopulations of short axon neurons that do not exit the nucleus and could thus be considered interneurons (Larriva-Sahd 2006). However, our electrophysiological data did not reveal any D2-like postsynaptic responses ruling out a possible reduction in lateral inhibition in the ovBST through hyperpolarization of GABA interneurons. Accordingly, the most parsimonious interpretation of our results would be that DA, acting on D2-containing GABAergic axons from extra-ovBST origin, reduces GABA release. To resolve this question, higher resolution studies of the expression of D2R mRNA in BST subnuclei or single-cell PCR experiments would be needed to confirm or infirm the single previous study regarding D2R mRNA absence.
Both our neurophysiological and immunohistochemical data suggest that the expression of D1R is low in the ovBST. This result is consistent with previous studies showing low expression of D1R mRNA and protein in the dorsolateral BST (Fremeau et al. 1991; Scibilia et al. 1992). Furthermore, no D1-like mediated effect on synaptic transmission in the ovBST was detected in this study, which contrasts with a previous report by Kash et al. (2008). Kash et al. suggested that DA, through D1/D2-like receptors, depolarizes corticotropin releasing hormones (CRH)-containing neurons and facilitates glutamate release, which would explain the modest increase in the frequency of spontaneous EPSC observed in their study. This discrepancy could be explained by several differences in the experimental systems used in the present study and by Kash et al., including the use of 6 – 8 wk old mice versus 12 wk and older rats. Furthermore because Kash et al. did not restrict their study to the ovBST, their results may reflect synaptic activity in the dorsomedial or the more ventral part of the dorsal BST, where we detected low-level expression of D1R (see Fig. 6). It should also be noted that there was virtually no detectable spontaneous EPSC in our experimental system. Accordingly, the effects of DA on evoked EPSC were measured by electrically stimulating the stria terminalis, the main route of excitatory entry to the ovBST (Larriva-Sahd 2006). As such, our study extends Kash et al. by revealing regional D1 effects within the dorsolateral BST.
In our system, the effect of DA on the amplitude of evoked EPSC was not mimicked or reproduced by D1R or D2R agonists but was dependent on presynaptic α2R as described previously for NA (Dumont and Williams 2004; Egli et al. 2005; McElligott and Winder 2008, 2009; McElligott et al. 2010; Shields et al. 2009). These results are consistent with the widespread distribution of α2R immunoreactivity in the BST, including the ovBST (Shields et al. 2009). Furthermore, α2R are ideally located to modulate excitatory inputs entering the BST because stria terminalis fibers, including those innervating the ovBST, co-express α2R, the vesicular glutamate transporter VGLUT1, and TH (which could contain either DA or NA) (Shields et al. 2009). Together, this evidence demonstrates that if DA had the ability to activate α2R in the ovBST, it would very likely modulate excitatory transmission, which is consistent with our observations. In contrast, our results do not support a role for α2R in modulating GABAergic transmission in the BST, which is also consistent with previous studies (Dumont and Williams 2004; Forray et al. 1999; Shields et al. 2009).
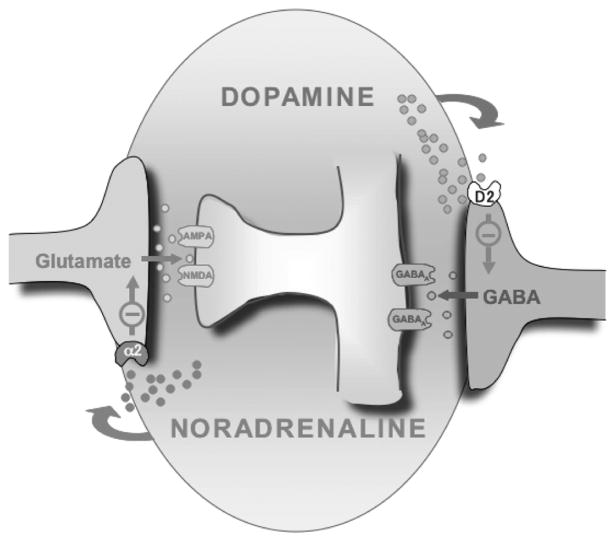
Cross-talk between cathecolaminergic subpathways, including activation of α2R by DA, is not uncommon and occurs in other brain regions and species (Cornil and Ball 2008; Cornil et al. 2002; Guiard et al. 2008). Interestingly, the affinity of DA for α2R is approximately threefold lower than that of NA (Boyajian and Leslie 1987), and in fact, 10 μM NA and DA 30 μM were equipotent at reducing AMPA-EPSC in the ovBST (see Fig. 4, A1 and B1). However, it is yet unclear whether α2R-mediated regulation of excitatory transmission by DA is physiologically, behaviorally, or pathophysiologically significant. In vivo DA can, at best, be measured in the nM range in the BST (Carboni et al. 2000). In brain slices, exogenous application of DA in the nM range does not produce significant effects (see Figs. 2 and 4). We thus suggest a double dissociation between the effects of DA and NA on inhibitory and excitatory synaptic transmission in the ovBST (Fig. 7). This double dissociation is appreciable at lower concentration of both DA and NA (e.g., at 1 μM). It is thus likely that in physiological conditions, the effect of DA is restricted to inhibitory transmission whereas NA should preferentially modulate excitatory synaptic transmission.
FIG. 7.
Double-dissociation of the catecholaminergic modulation of synaptic transmission in the ovBST. The ovBST receives robust glutamatergic projection from insular cortex, piriform cortex, ventral subiculum, and basolateral amygdala as well as DA inputs from the midbrain and NA inputs from the brain stem. GABAergic inputs to ovBST are from local short-axon GABAergic neurons or from the central nucleus of the amygdala. Functional evidences from this study demonstrate that release of DA in the ovBST could activate D2R and selectively reduce inhibitory influence to promote neuronal activation and release of NA could activate α2R and selectively inactivate excitatory drive to the ovBST. This functional double dissociation of the effects of DA and NA in the ovBST may be involved in processing both stress-and reward-related stimuli.
In contrast to DA and NA, 5-HT does not modulate excitatory transmission and is largely undetected immunohistochemically in the ovBST (Phelix et al. 1992). This observation contrasts with 5-HT-induced reduction in evoked EPSC reported by Guo et al. in the anterolateral BST (Guo and Rainnie 2010), and suggests that 5-HT plays little or no neuromodulatory role of excitatory transmission in the ovBST. Nonetheless we observed bidirectional modulation of inhibitory transmission by 5-HT in the ovBST (see RESULTS). It is, however, unlikely that DA mediated its effect through 5-HT receptors because methysergide did not interfere with the DA-mediated reduction in IPSC. 5-HT modulation of inhibitory transmission in the ovBST or in the BST altogether has not been done and will require a thorough investigation such as those done for excitatory transmission (Guo and Rainnie 2010).
Anatomical evidence suggests that the ovBST plays a role in neurocircuits that influence ingestive behavior including its motivational component (Dong et al. 2001b). Accordingly, the BST, and in particular its oval region, could be key within the neural circuits sensitive to drugs of abuse. The ovBST receives ascending inputs from the cerebrospinal trunk, horizontal inputs from basal forebrain-related regions, and top-down cortical inputs. Cortical projections, likely conveying excitatory inputs, mostly originate in the viscerosensory portion of the dysgranular insular cortex and the olfactory amygdalopiriform transitional area (McDonald et al. 1999), which could, respectively, convey internal and external cues triggering operant behaviors toward reward. How excitatory inputs into the ovBST affect intrinsic neural network function is currently unknown. However, Larriva-Sahd extensively described the local neuroanatomy of the ovBST, which is compartmentalized into a shell and a core (Larriva-Sahd 2006). The shell seems to receive and transmit incoming information to the core, which is home of projections neurons. ovBST projection is quite complex and widespread but should result in coordinated autonomic, neuroendocrine, and somatomotor responses (Dong et al. 2001b) that may drive foraging behavior when body nutrients (or drugs) levels become low and external cues predict substance availability. Consistent with this proposition, pharmacological manipulations of the dorsal BST influence motivated behaviors toward natural or pharmacological rewards (Eiler et al. 2003; Epping-Jordan et al. 1998; Erb et al. 2001; Hyytia and Koob 1995; Leri et al. 2002; Walker et al. 2000). However, the specific contribution of the ovBST in such pathways has not been reproduced and/or analyzed experimentally because of technical difficulties associated with selectively targeting this small brain region. Consequently, the exact contribution of DA-dependent effects in the ovBST to motivated behaviors is not yet understood.
Reward prediction correlates with release of DA from midbrain neurons (Fiorillo et al. 2003; Phillips et al. 2003). Therefore salience-induced release of DA in the ovBST could activate D2R, selectively reduce inhibitory influence to promote neuronal activation and, perhaps, reward-seeking behaviors. In fact, microinjection of the D2R antagonist eticlopride in the dorsolateral BST reduced sucrose self-administration (Eiler et al. 2003). In contrast, NA, which is released in the BST in response to stress (Pacak et al. 1995), should inactivate excitatory drive to the ovBST without affecting inhibitory transmission. This functional double dissociation of the effects of DA and NA in the ovBST may help fine tune the neurological integration of stress and goal-directed behaviors (Bowers et al. 1999) (Fig. 7).
Acknowledgments
GRANTS
This project was supported by Canadian Institutes of Health Research Operating Grant MOP-79277, the J. P. Bickell Foundation, and Queen’s University.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- Ahmed MR, Berthet A, Bychkov E, Porras G, Li Q, Bioulac BH, Carl YT, Bloch B, Kook S, Aubert I, Dovero S, Doudnikoff E, Gurevich VV, Gurevich EV, Bezard E. Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson’s disease. Sci Transl Med. 2010;2:28ra28. doi: 10.1126/scitranslmed.3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, Bloch B. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J Neurosci. 2009;29:4829– 4835. doi: 10.1523/JNEUROSCI.5884-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Attiast E, Amit Z. Stress enhances the response to reward reduction but not food-motivated responding. Physiol Behav. 1999;67:777–782. doi: 10.1016/s0031-9384(99)00129-8. [DOI] [PubMed] [Google Scholar]
- Boyajian CL, Leslie FM. Pharmacological evidence for alpha-2 adrenoceptor heterogeneity: differential binding properties of [3H]rauwolscine and [3H]idazoxan in rat brain. J Pharmacol Exp Ther. 1987;241:1092–1098. [PubMed] [Google Scholar]
- Caille I, Dumartin B, Bloch B. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 1996;730:17–31. doi: 10.1016/0006-8993(96)00424-6. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Rolando MT, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci. 2000;20:RC102. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF. Interplay among catecholamine systems: dopamine binds to alpha2-adrenergic receptors in birds and mammals. J Comp Neurol. 2008;511:610– 627. doi: 10.1002/cne.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430– 434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Goldstein M, Baldino F, Jr, Roth RH. Telencephalic projections of the A8 dopamine cell group. Ann NY Acad Sci. 1988;537:27–50. doi: 10.1111/j.1749-6632.1988.tb42095.x. [DOI] [PubMed] [Google Scholar]
- Deyama S, Katayama T, Kondoh N, Nakagawa T, Kaneko S, Yamaguchi T, Yoshioka M, Minami M. Role of enhanced noradrenergic transmission within the ventral bed nucleus of the stria terminalis in visceral pain-induced aversion in rats. Behav Brain Res. 2009;197:279–283. doi: 10.1016/j.bbr.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Deyama S, Katayama T, Ohno A, Nakagawa T, Kaneko S, Yamaguchi T, Yoshioka M, Minami M. Activation of the beta-adrenoceptor-protein kinase A signaling pathway within the ventral bed nucleus of the stria terminalis mediates the negative affective component of pain in rats. J Neurosci. 2008;28:7728–7736. doi: 10.1523/JNEUROSCI.1480-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430– 455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198– 8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657– 668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse. 2003;48:45–56. doi: 10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Markou A, Koob GF. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 1998;784:105–115. doi: 10.1016/s0006-8993(97)01190-6. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Noradrenaline inhibits glutamate release in the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res. 1999;55:311–320. doi: 10.1002/(SICI)1097-4547(19990201)55:3<311::AID-JNR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–252. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci USA. 1991;88:3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier P. Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus ceruleus, and dorsal hippocampus. Mol Pharmacol. 2008;74:1463–1475. doi: 10.1124/mol.108.048033. [DOI] [PubMed] [Google Scholar]
- Guo JD, Hammack SE, Hazra R, Levita L, Rainnie DG. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JD, Rainnie DG. Presynaptic 5-HT(1B) receptor-mediated serotonergic inhibition of glutamate transmission in the bed nucleus of the stria terminalis. Neuroscience. 2010;165:1390–1401. doi: 10.1016/j.neuroscience.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson EL, Greengard P. Localization of DARPP-32 immunoreactive neurons in the bed nucleus of the stria terminalis and central nucleus of the amygdala: co-distribution with axons containing tyrosine hydroxylase, vasoactive intestinal polypeptide, and calcitonin gene-related peptide. Exp Brain Res. 1990;79:447– 458. doi: 10.1007/BF00229315. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T. Axon terminals containing tyrosine hydroxylase- and dopamine-beta-hydroxylase immunoreactivity form synapses with galanin immunore-active neurons in the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res. 2001;914:23–33. doi: 10.1016/s0006-8993(01)02770-6. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. Juxtacapsular nucleus of the stria terminalis of the adult rat: extrinsic inputs, cell types, and neuronal modules: a combined Golgi and electron microscopic study. J Comp Neurol. 2004;475:220–237. doi: 10.1002/cne.20185. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. Histological and cytological study of the bed nuclei of the stria terminalis in adult rat. II. Oval nucleus: extrinsic inputs, cell types, neuropil, and neuronal modules. J Comp Neurol. 2006;497:772– 807. doi: 10.1002/cne.21011. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA. 1993;90:8861– 8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG. 5-hydroxytryptamine(1a)-likereceptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128:583–596. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann NY Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, Winder DG. Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc Natl Acad Sci USA. 2010;107:2271–2276. doi: 10.1073/pnas.0905568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology. 2008;33:2313–2323. doi: 10.1038/sj.npp.1301635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, McCarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Res. 1995;688:242–246. doi: 10.1016/0006-8993(95)00566-9. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992;28:949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614– 618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Scibilia RJ, Lachowicz JE, Kilts CD. Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse. 1992;11:146–154. doi: 10.1002/syn.890110208. [DOI] [PubMed] [Google Scholar]
- Shields AD, Wang Q, Winder DG. alpha2A-adrenergic receptors heterosynaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience. 2009;163:339–351. doi: 10.1016/j.neuroscience.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Walker JR, Ahmed SH, Gracy KN, Koob GF. Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]