Abstract
The bed nucleus of the stria terminalis (BST) is a basal forebrain structure considered to be part of a cortico-striato-pallidal system that coordinates autonomic, neuroendocrine and behavioural physiological responses.
Recent evidence suggests that the BST plays a role in the emotional aspect of pain. The objective of the present study was to further understand the neurophysiological bases underlying the involvement of the BST in the pain experience, in both acute and chronic pain conditions. Using c-Fos as an indicator of neuronal activation, the results demonstrated that a single toe-pinch in rats produced nuclei-and condition-specific neuronal responses within the anterior region of the BST (antBST). Specifically, acute noxious stimulation increased c-Fos in the dorsal medial (dAM) and fusiform (FU) nuclei. Chronic neuropathic pain induced by chronic constriction injury (CCI) of the sciatic nerve decreased the number of c-Fos positive cells following acute mechanical stimulation in the dAM and FU nuclei, and increased c-Fos immunoreactivity in the ventral medial (vAM) aspect of the BST. In addition, the results revealed a nuclei-specific sensitivity to the surgical procedure. Following noxious stimulation to animals that received a sham surgery, c-Fos immunoreactivity was blunted in the FU nucleus while it increased in the oval (OV) nucleus of the BST.
Altogether, this study demonstrates that pain induces nuclei-and condition-specific neuronal activation in the BST revealing an intriguing supraspinal neurobiological substrate that may contribute to the physiology of acute nociception and the pathophysiology of chronic pain.
Keywords: Bed nucleus of the stria terminalis (BST), c-Fos, Mechanical noxious stimulation, Neuropathic pain
1. Introduction
Pain is more than a sensory-discriminative experience: it has emotional and cognitive aspects as well. Emotion-induced increases in nociceptive thresholds are necessary for several critical physiological functions, including childbirth and escape from predators (Jorum, 1988). Similarly, emotion-induced hyperalgesia is adaptive since it promotes protection of injured tissue and hence, allows time for healing (Imbe et al., 2006). Like many other physiological functions, there is a fine line between normal regulation (of nociception) and the development of pathophysiological states. Sustained analgesia makes individuals vulnerable to tissue damage whereas hyperalgesia can, under certain circumstances, contribute to persistent or chronic pain conditions (Urban and Gebhart, 1999; Vanegas and Schaible, 2004). Furthermore, pathological emotional states such as anxiety impair normal nociceptive and healing processes (Imbe et al., 2006).
Evidence suggests that modulation of nociception originates in the brain, although the exact neurobiological bases are still poorly understood. A better understanding of the neurobiological systems involved in the cognitive and emotional aspects of pain will help in developing better approaches to treat and manage chronic pain. In both rodents and humans, supraspinal sites involved in the negative emotional component of pain include the anterior cingulate cortex and the amygdala (Johansen and Fields, 2004; Johansen et al., 2001; Neugebauer and Li, 2002; Neugebauer et al., 2004; Rainville et al., 1997). Evidence also suggests the bed nucleus of the stria terminalis (BST) as a critical brain site contributing to the physiological manifestation of the emotional aspect of pain. The BST is a basal forebrain structure consisting of 14 distinct nuclei that forms, with the amygdaloid nuclei, a complex referred to as the extended amygdala (Alheid et al., 1995). The anterior region of the BST (antBST), comprises 8 distinct nuclei: fusiform (FU), dorsal and ventral anteromedial (dAM and vAM), dorsal and ventral anterolateral (dAL and vAL), oval (OV), rhomboid (RH) and juxtacapsular (JX) nuclei. Efferent and afferent projections of the antBST suggest a role in coordinating neuroendocrine, autonomic, and somatomotor responses that together, could contribute to the peripheral manifestations of emotions (LeDoux, 2000).
Evidence for the contribution of the BST in the emotional aspect of pain is currently two-fold: anatomically, the BST receives afferents from a subpopulation of glutamatergic C-fibers (IB4 positive) that terminate primarily at limbic targets (BST, globus pallidus, hypothalamus) rather than sensory-discriminative regions of the brain (lateral thalamus, somatosensory cortex) (Braz et al., 2005). Physiologically, BST lesions block pain-induced conditioned place aversion, a measure of the emotional aspect of pain in rats (Deyama et al., 2007).
The objective of the present study was to further understand the neurophysiological bases underlying the involvement of the BST in both acute and chronic pain conditions. Using c-Fos as an indicator of neuronal activation, we found that a single toe pinch, in rats, produced nuclei-and condition-specific neuronal activation within the BST.
2. Methods
2.1. Animals
Adult male Sprague-Dawley rats weighing 180–220 grams at the beginning of the experiments (Charles River Canada, Montréal, Québec) were housed in pairs on a reverse 12-hour light/dark cycle in a temperature-controlled setting with free access to standard rat chow and water. Experiments were conducted in the afternoon and early evening. The rats were acclimatized to the animal facility for no less than 3 days prior to any experimental procedures or surgical manipulation. Animal protocols were approved by the Queen’s University Animal Care Committee in accordance with the guidelines set by the Canadian Council on Animal Care. All efforts were made to ensure that the number of animals used and suffering was kept to a minimum.
On testing day, rats were taken from the animal care facility and brought into the laboratory where they remained in their home cage for a minimum of 1.5 h prior to testing. Lights were turned off in the laboratory as to not affect circadian rhythms established by the reversed light/dark cycle.
2.2. Surgical procedures
Chronic constriction of the sciatic nerve induced neuropathic pain (Bennett and Xie, 1988). Rats were deeply anesthetized with isoflurane (2.5%, by inhalation). Upon absence of a tail flick response, a small incision was made at the mid-thigh of the left hind limb to expose the underlying muscle tissue and a blunt dissection was used to expose the sciatic nerve. Connective tissue was carefully removed from the sciatic and 4 loose ligatures of chromic gut suture (CP Medical, Portland, OR) were knotted around the nerve proximal to its trifurcation point. Sutures were ligated approximately 1 mm apart and care was used to ensure that sutures were not disrupting perineural blood flow. Muscle and skin were sutured shut with monocryl (Ethicon, Somerville, NJ). Sham rats received the same surgical treatment, but the sciatic nerve was not manipulated. Pre-anesthesia, rats were orally administered 0.5 ml of liquid children’s Tylenol (1.7 mg/kg; McNeil, Fort Washington, PA). Pre-operatively, the rats received subcutaneous injection of 5 ml of lactated Ringer’s solution (LRS), 0.013 ml/100 g Tribissen antibiotic and eye gel (Novartis, Mississauga, Ontario, Canada). On the following day, rats were given the same dose of children’s Tylenol and LRS. Eleven days following CCI, all animals displayed ipsilateral hind paw deformities and contralateral paw favouring — two reliable characteristics of a successful CCI. All animals used in this study displayed both these phenotypes and as such, were considered neuropathic. In previous experiments conducted in our laboratory where Von–Frey mechanical allodynia scores were assessed, a very high percentage of CCI animals record significantly reduced thresholds to mechanical stimulation (Holdridge and Cahill, 2007).
2.3. Experimental procedure
A flat-tipped, 1″ fixed-gauge metal alligator clip (from a local electronic shop) applied to the 4th knuckle of the left hind paw was used to evoke an acute noxious injury in this experiment. Prior to the onset of the noxious stimulus, rats were wrapped in a towel with their left hind limb exposed. The stimulus was applied until vocalization (approximately two seconds).
Experiment 1 assessed the effect of acute noxious stimulation on c-Fos-IR in the BST. To determine if acute noxious (toe pinch — TP) or innocuous (light touch — LT) mechanical stimulation (S+) caused neuronal activation in the BST, rats were randomly assigned to one of the following conditions. Condition 1 served as a control group, where rats did not receive the stimulus (S−). Groups were divided based on time elapsed between TP or LTstimulation and euthanasia. Rats in the toe pinch S+1 h group (TP S+1), received a toe pinch, were returned to their home cage and euthanized 1 h following the pinch. TP S+2 and TP S+5 were euthanized 2 and 5 h following toe pinch, respectively. Two additional groups receiving LT rather than TP were included to confirm that neuronal activation evident in the BST was in fact a result of noxious stimulation: LT S+1 and LT S+2. Using the same protocol as the noxious stimulation groups, rats were taken from the home cage, wrapped in a towel and the alligator clip set in the open position was lightly rubbed against the 4th knuckle of the left hind paw for 2 s before returning the animal to their home cage.
Experiment 2 assessed the effect of CCI on noxious stimulus-induced neuronal activation. Seventeen rats received a CCI of the left common sciatic nerve and were randomly divided into 3 groups following the same protocol of noxious stimulation as described in experiment 1; CCI S-, CCI S+1 and CCI S+2. Additionally, 18 sham operated rats were divided into 3 groups where Sham S-received a sham surgery and no stimulation, Sham S+1 received a toe pinch and were euthanized one hour following S+and Sham S+2 received a toe pinch and were euthanized two hours following mechanical nociceptive stimulation. Since a reliable decline in c-Fos expression was observed in control conditions 5 h following noxious stimulation, a five-hour time period was not included in the CCI experiments in order to keep the number of animals used in this study to a strict minimum.
After the experimental manipulations, rats were deeply anesthetized with sodium pentobarbital (70 mg/kg) and perfused via the aortic arch with 500 ml of 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB), pH 7.4 at 4 °C. Brains were extracted and post-fixed for 24 h at 4 °C in 4% PFA solution. Following post-fixation, brains were cryoprotected in 30% sucrose made in 0.1 M PB overnight or until they sank.
Brains were mounted on a freezing sledge microtome and 30-micron transverse slices were obtained starting at the most rostral point of the BST as outlined in (Swanson, 2005) and concluded when no more BST was evident. Every second slice was collected in 0.1 M TBS (Trizma® base solution, pH 7.4) for immunohistochemical analysis.
2.4. Immunohistochemical detection of c-Fos
Immunohistochemical detection of c-Fos was used as an indirect marker of neuronal activation (Herrera and Robertson, 1996). Following sectioning, free-floating brain slices were incubated in a solution consisting of 0.3% H2O2 and TBS for 10 min to reduce endogenous peroxidase activity. Following 3 five-minute washes with TBS, sections were incubated in solution containing 5% BSA and 0.1% H2O2 in TBS-T (0.1 M TBS with triton X-100) at 4 °C for 2 h s to reduce non-specific immuno-labeling. Following incubation with blocking solution, sections were incubated at 4 °C overnight in a 1:5000 dilution of rabbit anti-c-Fos (Lot 124 K4881, Sigma, St. Louis, MO) prepared in TBS-T and 1% BSA. Sections were then incubated in biotinylated goat anti-rabbit IgG (1:1500; Vector Laboratories, Burlingame, CA) and c-Fos labeling was amplified according to the avidin–biotinylated–horseradish–peroxidase complex (ABC; Vector Laboratories, Burlingame, CA) followed by revelation with 1,3-Diaminobenzidine (DAB) solution (0.15 mg/ml) in TBS.
2.5. Data analysis
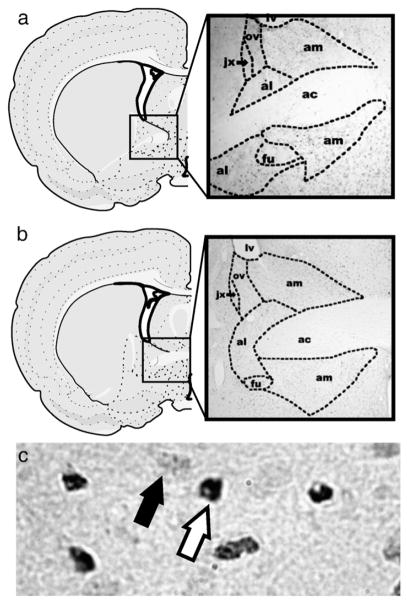
c-Fos immunoreactive labeling was quantified using bright field microscopy examined under a Leica DMRE microscope (Leica Microsystems, Cambridge, ON) equipped with a digital camera (Qimaging Retiga EXi fast 1394, Surrey, BC) and image capturing software (Q Capture, Lukas Microscope Service, Skokie, IL). Three rostral sections of BST were counted for c-Fos-IR. Sections correlated to levels 18 and 19 in the Swanson Atlas (Swanson, 2005) plus one section between levels 18 and 19 not delineated in the atlas which we have named level 18.5 (Fig. 1). All antBST nuclei except the rhomboid nucleus were analyzed: FU, OV, JX, dAM, vAM, dAL and vAL. Nuclei location and perimeter were determined by size and shape of cells in each of the given nuclei, as well as distance from consistent anatomical markers such as fiber tracts (Fig. 1). Quantification was done blind to all experimental conditions.
Fig. 1.
Schematics (left) and photomicrographs (right) illustrating the anterior region of the BSTat levels 18 (a: AP=−0.11 from bregma) and 19 (b: AP=−0.26 from bregma) (Swanson, 2005). (b) Photomicrograph showing nuclear (white arrow) or cytoplasmic (black arrow) c-Fos immunoreactivity in the antBST.
Only c-Fos-IR cells in which the cell’s nucleus was entirely stained were considered c-Fos positive (Fig. 1). Cells were counted at 200x magnification and questionable cells were verified at 400x magnification. Because of the variability in the number of sections obtained per nucleus, per rat, only the section with the highest number of c-Fos positive cells was used. To obtain the total c-Fos-IR score, values were summed between hemispheres for each BST nucleus. Scores from each rat were then averaged for statistical analysis using Graph Pad Prizm software 4.0 (San Diego, CA). Results were presented as mean± SEM. A series of one-way Analysis of variance (ANOVA) and Newman–Keuls Multiple Comparison Tests were done within each experimental group to generate data for all immunohistochemical experiments. For all experiments, a p<0.05 was considered statistically significant.
3. Results
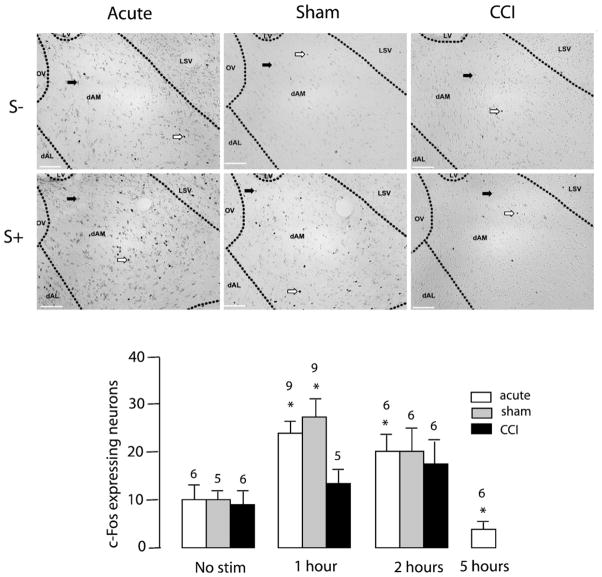
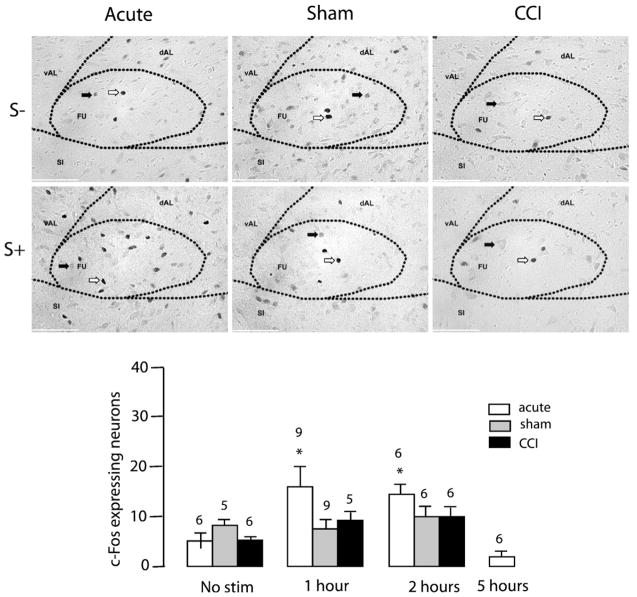
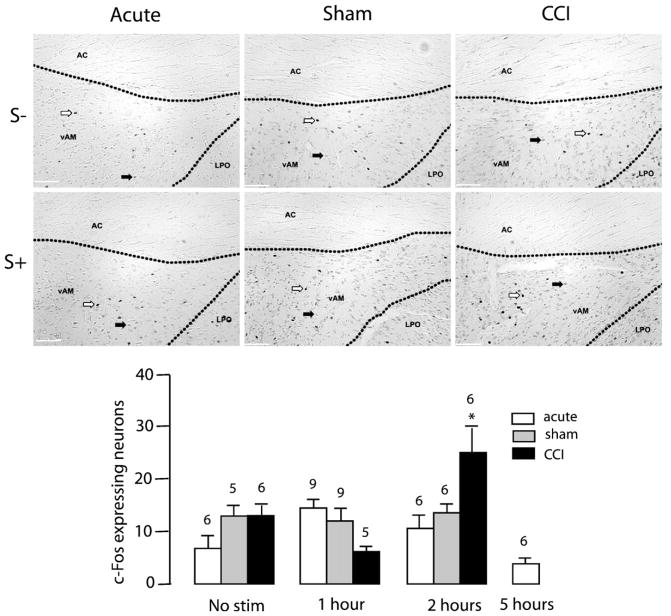
Noxious toe pinch significantly increased c-Fos-IR in the dAM of control and sham-operated animals one hour following noxious stimulation (TP S-: 9.83±2.61, TP S+1: 23.11±2.23 [F (3,23)= 17.91, p<0.0001]) and (Sham S−: 9.80±1.83, Sham S+1: 28.33± 2.77 [F (2,17)=5.856, p<0.05], Fig. 2). This increase in c-Fos-IR in the dAM one hour following noxious insult was blunted in CCI animals (CCI S−: 8.67±3.38, CCI S+1: 13.60±3.86 [F (2,14)=0.803, n.s.], Fig. 2). Noxious toe pinch significantly increased c-Fos-IR in the FU nucleus of control animals one hour following noxious insult (TP S−: 5.66±1.87, TP S+1: 16.50±3.62 [F (3,20)=11.05, p=0.0002], Fig. 3). However, toe-pinch did not increase c-Fos-IR in the FU nucleus following CCI (CCI S-: 4.33± 1.73, CCI S+1: 8.80±2.22 [F (2,14)=2.242, n.s]) or sham surgery (Sham S−: 6.80±1.28, Sham S+1: 6.33±1.84 [F(2,14)= 1.362, n.s], Fig. 3). Noxious toe pinch to CCI rats caused an increase in c-Fos-IR in the vAM nucleus of the BST two hours following stimulation (CCI S−: 6.83±2.85, CCI S+2: 24.00±5.88 [F (2,14)=6.705, p=0.009], Fig. 4). A phenomenon not observed in either sham (Sham S−: 13.00±2.35, Sham S+2:13.33±2.78 [F (2,14)=0.1434, n.s]) or control conditions (TP S−: 7.17±1.78, TP S+2: 9.83±2.74 [F(1,15)=1.347, n.s], Fig. 4). c-Fos-IR returned to basal levels across all nuclei 5 h following the onset of noxious stimulation (data not shown). Light touch stimulation did not cause an increase in c-Fos-IR in any of the 7 nuclei examined (data not shown). These findings confirm that increased levels of c-Fos-IR in the dAM, FU and vAM are a direct result of noxious insult. c-Fos-IR increased significantly in the OV nucleus of sham rats (Sham S−: 2.80±0.97, Sham S+1: 8.00±1.65, Sham S+2: 8.17±1.49 [F (2,14)=4.117, p=0.0392], Fig. 5) following toe-pinch when compared to controls (TP S−: 4.00±1.24, TP S+1: 4.67±1.11, TP S+2: 2.00±0.68 [F (2,14)=1.781, n.s]) and CCI (CCI S−: 2.00±0.68, CCI S+1: 3.40±0,98, CCI S+2: 5.33±2.54 [F (2,14)=1.038, n.s], Fig. 5). There were no increases in c-Fos expression in the vAL, dAL or JX nuclei to any of the non-noxious or noxious-stimuli conditions (Fig. 5).
Fig. 2.
Effect of mechanical noxious stimulation on c-Fos-IR in the dAM nucleus of the BST. Top and middle panels: photomicrographs representing c-Fos immunoreactivity in no-stimulation (S−) or one-hour after (S+) toe-pinch in acute, sham, and CCI rats (from left to right). White arrows indicate specific nuclear expression of c-Fos. Black arrows indicate non-specific cytoplasmic labeling. Scale bar=500 μm. Bar histogram representing the number of c-Fos expressing neurons as a function of time after toe-pinch in acute, sham, and CCI animals. Numbers above bars indicate number of rats. Error bars represent SEM.
Fig. 3.
Effect of mechanical noxious stimulation on c-Fos-IR in the FU nucleus of the BST. Top and middle panels: photomicrographs representing c-Fos immunoreactivity in no-stimulation (S−) or one-hour after (S+) toe-pinch in acute, sham, and CCI rats (from left to right). White arrows indicate specific nuclear expression of c-Fos. Black arrows indicate non-specific cytoplasmic labeling. Scale bar=250 μm. Bottom panel: Bar histogram representing the number of c-Fos expressing neurons as a function of time after toe-pinch in acute, sham, and CCI animals. Numbers above bars indicate number of rats. Error bars represent SEM.
Fig. 4.
Effect of mechanical noxious stimulation on c-Fos-IR in the vAM nucleus of the BST. Top and middle panels: Photomicrographs representing c-Fos immunoreactivity in no-stimulation (S−) or one-hour after (S+) toe-pinch in acute, sham, and CCI rats (from left to right). White arrows indicate specific nuclear expression of c-Fos. Black arrows indicate non-specific cytoplasmic labeling. Scale bar=500 μm. Bottom panel: Bar histogram representing the number of c-Fos expressing neurons as a function of time after toe-pinch in acute, sham, and CCI animals. Numbers above bars indicate number of rats. Error bars represent SEM.
Fig. 5.
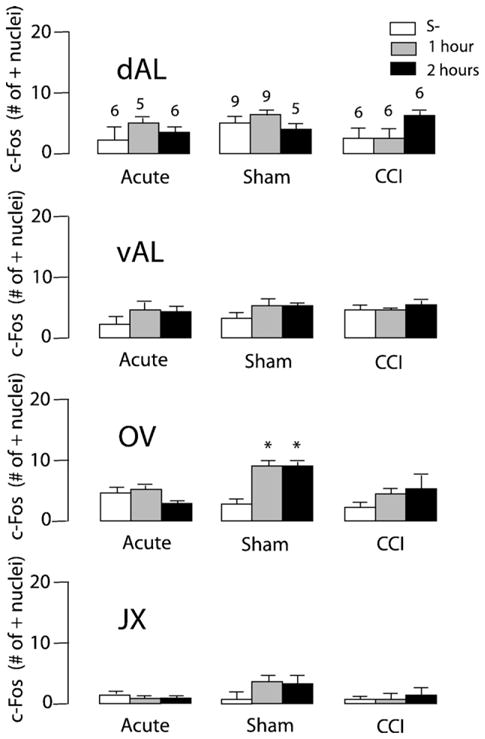
Effect of mechanical noxious stimulation on c-Fos-IR in the other nuclei of the antBST. Number of c-Fos expressing neurons as a function of time after toe-pinch in acute, sham, and CCI animals. Number of animals per group is the same as displayed in dAL. Error bars represent SEM.
4. Discussion
The present study reveals that acute mechanical noxious stimulation increases nuclear c-Fos immunoreactivity in the anterior region of the BST. Since c-Fos is a widely used indicator of neuronal activation in the central nervous system, we suggest that noxious stimulation induces neuronal activation in the BST. More specifically, noxious stimulation-induced c-Fos expression was restricted to specific sub-regions of the antBST and was modified by chronic neuropathic pain or in certain cases, by sham surgical procedures. Given that the BST is thought to contribute to the emotional rather than the sensory-discriminative aspect of pain, our results may provide new insights into the neuronal bases of the emotional aspect of pain.
4.1. Noxious stimulation-induced neuronal activation in the BST
We measured c-Fos immunoreactivity in response to acute noxious mechanical stimulation in normal Sprague-Dawley rats, or in rats that developed peripheral neuropathy following chronic constriction injury of the sciatic nerve. We observed that acute mechanical noxious stimulation increased c-Fos immunoreactiv-ity in the BST and that the response returned to baseline a few hours after the stimulus, which is consistent with the transient expression of c-Fos (Herrera and Robertson, 1996). This is the first study to demonstrate that noxious stimulation triggers nuclei-specific c-Fos expression in the BST. Since this study evaluated each nuclei of the anterior BST specifically, we observed c-Fos expression in response to acute noxious stimulation in only two (FU and dAM) of the eight antBST nuclei.
We observed altered acute noxious stimulation-induced c-Fos expression in chronic neuropathic pain conditions. Chronic nerve constriction completely blunted mechanical pain-induced expression of c-Fos in some BST regions (dAM, FU) whilst stimulating expression in previously silent regions (vAM). Similarly, we saw that the sham surgical procedure altered acute noxious stimulation-induced c-Fos expression in certain regions of the antBST (increased in OV and decreased in FU). It is unclear how these modifications in c-Fos expression alter antBST activity, however, given the strong correlation between noxious stimuli-induced c-Fos expression and neuronal activation, at least in the dorsal horn of the spinal cord, the most plausible hypothesis is that the change in c-Fos expression is due to modifications in neuronal responding, habituation or sensitization in the BST (Bullitt, 1990; Coggeshall, 2005). Alternatively, noxious information from the periphery to the BST might be modified after chronic pain or sham surgery. Alteration in signaling pathways leading to c-Fos expression could have also affected c-Fos immunoreactivity. There is, however, little evidence from the literature suggesting alterations in the signaling pathways leading to c-Fos protein expression whereas abundant evidence supports underlying modifications in neuronal activity with associated changes in behaviours (Bullitt, 1990; Coggeshall, 2005). It is unclear why the pain-induced c-Fos-IR was blunted in the FU in both CCI and shams animals, although both inflammation and simple surgical incisions alter c-Fos expression in the spinal cord (Prewitt and Herman, 1998). It is plausible, then, that sham surgery alone is sufficient to produce lasting changes to c-Fos expression in supraspinal areas, including the BST.
The consequences of altered BST c-Fos expression, neuronal activation, or both on the pain experience are currently unknown. However, a better understanding of how the BST contributes to the pain experience might shed light on the potential impact of these modifications.
4.2. Significance of noxious stimulation-induced neuronal activation in the BST
We hypothesized that noxious stimulation would trigger neuronal activity and hence c-Fos expression in the BST since it receives direct (and potentially indirect) afferents from the dorsal horn of the spinal cord (Braz et al., 2005; Burstein and Giesler, 1989; Cliffer et al., 1991). Our observations confirm that these afferents carry noxious (but not innocuous) information from the periphery to the BST. Noxious stimulation-induced c-Fos expression was sub-region-specific, which reinforces the idea that the BST is a cluster of nuclei with specific functions rather than a homogeneous brain structure. Given the small size of each BST nuclei, it is currently difficult to lesion a single BST nucleus and thus, assessing the behavioural or physiological functions of individual BST nuclei is currently not attainable. Nonetheless, our study demonstrates that the dAM and the FU are regions of the antBST that respond to acute mechanical noxious stimulation and might contribute to the pain experience. Conversely, several antBST nuclei (AL, JX) showed no apparent response to acute noxious insult.
There is evidence from the literature that the BST contributes to the emotional rather than the sensory-discriminative or cognitive components of pain. Lesions to the antBST block pain-induced conditioned place aversion in rodents, a measure of the emotional component of pain in rats (Deyama et al., 2007). Furthermore, we saw that noxious stimulus-induced c-Fos expression was bilateral in the BST, an observation consistent with brain systems that mediate emotional aspects of pain (Bernard and Besson, 1990; Chudler and Dong, 1995; Chudler et al., 1993). In contrast, sensory-discriminative brain regions such as the lateral thalamus or the sensory cortex display lateralization (usually contralateral to the stimulus).
The projection pattern of the AM aspect suggests that it contributes in coordinating neuroendocrine, autonomic, and behavioural or somatic responses associated with maintaining energy balance (Dong and Swanson, 2006a). Similarly, the FU projects along approximately 4 distinct pathways that terminate in the central amygdala, hypothalamus (PVH), midbrain (ventral tegmental area) and lower brainstem (lateral periaque-ducal gray, raphe) (Dong et al., 2001b). Each of these descending pathways corresponds to important physiological functions triggered by nociceptive stimuli such as defensive behaviours, descending analgesia, autonomic control of breathings, cardiovascular responses, arousal, and activation of HPA axis (Choi et al., 2007; Dick and Coles, 2000; Rossi et al., 1994; Satoh and Fibiger, 1986; Vertes, 1991). Given this broad projection pattern of each BST sub-nuclei, noxious stimuli-induced activation of the BST should result in generalized physiological responses consistent with an emotional response.
5. Conclusions
The neurobiological construct of the emotional aspect of pain is at the supraspinal level and more specifically involves brain regions such as the anterior cingulate cortex, the amygdala, and the BST (Borszcz, 2006; Deyama et al., 2007; Johansen et al., 2001; Neugebauer et al., 2004; Rainville et al., 1997). The BST is ideally located anatomically and functionally to receive incoming noxious information, process this information along with additional incoming neocortical (prefrontal cortex) and other limbic structure (amygdala, hippocampus) information, and relay this message to the periphery (midbrain and brainstem) (Dong et al., 2000; Dong et al., 2001a; Dong et al., 2001b; Dong and Swanson, 2003, 2004a, b, 2006a, b, 6c).
This study reveals that the BST is responsive to pain stimuli in a nuclei-and condition-specific manner and that not all aspects of the BST contribute to the pain response. Given its afferents, the BST could be critically involved in the resulting autonomic, neuroendocrine, and behavioural responses observed after noxious insults and thus could contribute to the peripheral manifestation of emotions (LeDoux, 2000). Our observation that chronic conditions such as peripheral neuropathy or in some cases, a simple surgical procedure can modify neuronal activity in the BST is intriguing. These findings could lead to a better understanding of the role of supraspinal sites in the pathophysiology of chronic pain.
Acknowledgments
Canadian Institute of Health Research, Premier’s excellence research award (CMC) and Queen’s University supported this work.
Abbreviations
- antBST
anterior region of the BST
- BST
bed nucleus of the stria terminalis
- CCI
chronic constriction injury
- CeA
central nucleus of the amygdala
- c-Fos-IR
c-Fos immunoreactivity
- DAB
1,3-Diaminobenzidine
- dAL
dorsal anterolateral
- dAM
dorsal anteromedial
- FU
fusiform
- HPA
hypothalamic-pituitary-adrenal axis
- JX
juxtacapsular
- LRS
lactated Ringer’s solution
- LT
light touch
- OV
oval
- PB
phosphate buffer
- PFA
paraformalde-hyde
- PVH
paraventricular nucleus of the hypothalamus
- RH
rhomboid
- S−
no stimulation
- S+
noxious mechanical stimulation
- S+1
noxious mechanical stimulation plus one hour
- S+2
noxious mechanical stimulation plus two hours
- S+5
noxious mechanical stimulation plus five hours
- TBS
Trizma® base solution
- TP
toe pinch
- vAL
ventral anterolateral
- vAM
ventral anteromedial
References
- Alheid GF, de Olmos JS, Baltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. San Diego: Academic; 1995. pp. 495–578. [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–90. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Borszcz GS. Contribution of the ventromedial hypothalamus to generation of the affective dimension of pain. Pain. 2006;123:155–68. doi: 10.1016/j.pain.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–93. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–30. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Burstein R, Giesler GJ., Jr Retrograde labeling of neurons in spinal cord that project directly to nucleus accumbens or the septal nuclei in the rat. Brain Res. 1989;497:149–54. doi: 10.1016/0006-8993(89)90981-5. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–34. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60:3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Sugiyama K, Dong WK. Nociceptive responses in the neostriatum and globus pallidus of the anesthetized rat. J Neurophysiol. 1993;69:1890–903. doi: 10.1152/jn.1993.69.6.1890. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci. 1991;11:852–68. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Deyama S, Nakagawa T, Kaneko S, Uehara T, Minami M. Involvement of the bed nucleus of the stria terminalis in the negative affective component of visceral and somatic pain in rats. Behav Brain Res. 2007;176:367–71. doi: 10.1016/j.bbr.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respiration physiology. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Dong H, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J Comp Neurol. 2003;463:434–72. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004a;468:277–98. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004b;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006a;494:142–78. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006b;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J Comp Neurol. 2006c;494:108–41. doi: 10.1002/cne.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Holdridge SV, Cahill CM. Spinal administration of a delta opioid receptor agonist attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Eur J Pain. 2007;11:685–93. doi: 10.1016/j.ejpain.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–92. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98:8077–82. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorum E. Analgesia or hyperalgesia following stress correlates with emotional behavior in rats. Pain. 1988;32:341–8. doi: 10.1016/0304-3959(88)90046-2. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol. 2002;87:103–12. doi: 10.1152/jn.00264.2001. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–34. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chemical Neuroanat. 1998;15:173–85. doi: 10.1016/s0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–71. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rossi F, Maione S, Berrino L. Periaqueductal gray area and cardiovascular function. Pharmacol Res. 1994;29:27–36. doi: 10.1016/1043-6618(94)80095-2. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fibiger HC. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol. 1986;253:277–302. doi: 10.1002/cne.902530302. [DOI] [PubMed] [Google Scholar]
- Swanson LW, editor. Brain Maps: Structure of the rat brain. San Diego: Elsevier; 2005. [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7687–92. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–68. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]