Abstract
The function of vascular endothelial growth factor (VEGF) in cancer is not limited to angiogenesis and vascular permeability. VEGF-mediated signalling occurs in tumour cells, and this signalling contributes to key aspects of tumorigenesis, including the function of cancer stem cells and tumour initiation. In addition to VEGF receptor tyrosine kinases, the neuropilins are crucial for mediating the effects of VEGF on tumour cells, primarily because of their ability to regulate the function and the trafficking of growth factor receptors and integrins. This has important implications for our understanding of tumour biology and for the development of more effective therapeutic approaches.
Vascular endothelial growth factor (VEGF) was identified and isolated as an endothelial cell-specific mitogen that has the capacity to induce physiological and pathological angiogenesis1,2. In a separate context, a factor that promotes vascular hyperpermeability, vascular permeability factor, was isolated and later shown to be identical to VEGF3,4. This VEGF is now known as VEGFA and is a member of a larger family of growth factors that also includes VEGFB, VEGFC, VEGFD and placental growth factor (PLGF). These family members differ in their expression pattern, receptor specificity and biological functions5. VEGFA, which is often referred to as VEGF, has been studied more than the other members of this family and it has several distinct variants (VEGF121, VEGF145, VEGF148, VEGF165, VEGF183, VEGF189 and VEGF206). These variants occur because of alternative splicing, and they also differ in receptor specificity and function5. Unsurprisingly, the role of VEGFs in angiogenesis and lymphangiogenesis has dominated the VEGF research field since the initial discovery of VEGFs, and these studies have provided considerable insights into the mechanisms that underlie the complex process of angiogenesis6. Importantly, these studies provided the foundation for the development of anti-angiogenic therapies that target VEGF and VEGF receptors7,8.
It has become apparent that the function of VEGF is not limited to angiogenesis and vascular permeability9. VEGF, for example, can affect the function of immune cells that are present in the tumour microenvironment and, consequently, it can affect the host response to tumours (see, for example, REF. 10). In addition, VEGF receptors may regulate the function of fibroblasts in the tumour stroma11 (BOX 1; FIG. 1). One of the most interesting developments is the discovery that autocrine and paracrine VEGF signalling occur in tumour cells and that this signalling contributes to key aspects of tumorigenesis, especially the function of cancer stem cells, independently of angiogenesis (FIG. 1). Signalling downstream of VEGF in tumour cells is mediated by VEGF receptor tyrosine kinases (RTKs) and neuropilins (NRPs). The NRPs have a major role in this signalling because of their ability to interact with and to affect the function of multiple RTKs and integrins. This Review focuses on VEGF signalling in tumour cells and its implications for tumour biology and therapy.
Box 1. Other functions of VEGF in the tumour microenvironment.
In addition to affecting endothelial and tumour cells, vascular endothelial growth factor (VEGF) influences tumour function by targeting other cell types in the tumour microenvironment. Notably, immune cells can express VEGF receptors, and the functions of these cells can be regulated by VEGF signalling; for example, CD4+ forkhead box protein P3 (FOXP3)+ regulatory T cells, which suppress an antitumour immune response, express neuropilin 1 (NRP1) and are ‘guided’ into tumours by VEGF, which functions as a chemoattractant10. Ablation of NRP1 in this population of T cells increases the activation of CD8+ T cells and there is a concomitant reduction in tumour growth. Macrophages in the hypoxic tumour microenvironment secrete VEGF, which contributes to the many functions of VEGF in tumours123. In addition to their many other functions, fibroblasts in the tumour stroma secrete VEGF. These cells express NRP1 and use it to increase fibronectin fibril assembly, which augments tumour growth; however, whether this process involves VEGF is not known11.
Figure 1. VEGF functions in tumours.
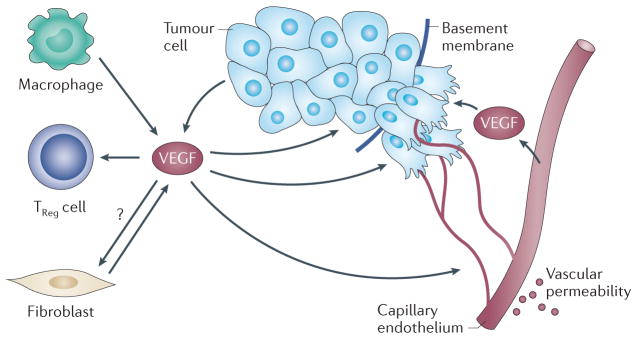
Vascular endothelial growth factor (VEGF) that is secreted by tumour and stromal cells, including macrophages, endothelial cells and fibroblasts, has multiple functions in the tumour microenvironment, which involve the ability of VEGF to interact with VEGF receptors that are expressed on different cell types. VEGF functions as a primary stimulus for angiogenesis, which is a process that involves the ability of VEGF receptors to stimulate signalling pathways that induce the proliferation and the migration of endothelial cells, and the ability of these cells to degrade and to remodel the extracellular matrix. These processes culminate in sprouting angiogenesis and the formation of new blood vessels. VEGF can also increase vascular permeability, which results in the deposition of a provisional fibrin matrix that triggers the formation of desmoplastic stroma. By contrast, VEGF secreted by tumour cells functions in an autocrine manner and promotes dedifferentiation and an epithelial–mesenchymal transition phenotype, with a consequent enhancement of tumour invasion and survival, and it can facilitate the function of cancer stem cells (FIG. 3). VEGF can also function as a chemoattractant to recruit regulatory T (TReg) cells that inhibit an antitumour immune response. Tumour fibroblasts also secrete VEGF. Neuropilin 1 that is expressed on tumour fibroblasts may contribute to tumour growth by nucleating fibronectin fibril formation, but it is not known whether this process involves VEGF. Arrows indicate the source and the targets of VEGF in tumours.
VEGF receptors on tumour cells
VEGF RTKs and NRPs
The hypothesis that VEGF signalling contributes to the functions of tumour cells implies that tumour cells express specific VEGF receptors that mediate this signalling. The classical VEGF receptors are the RTKs VEGFR1 (also known as FLT1), VEGFR2 (also known as FLK1 and KDR) and VEGFR3 (also known as FLT4)12. Although the expression of these receptors was initially thought to be limited to endothelial cells, it is now known that most of these receptors are expressed by many tumour types and that their expression correlates with clinical parameters (TABLE 1). VEGFR2 is the predominant RTK that mediates VEGF signalling in endothelial cells and that drives VEGF-mediated angiogenesis12. Interestingly, some tumour cells express VEGFR2 and it has a prime role in mediating VEGF signalling (see, for example, REFS 13,14), but the response of other tumour cells to VEGF seems to be independent of this RTK (see, for example, REFS 15,16), which indicates that VEGF signalling in these cells is mediated by other receptors.
Table 1.
Expression of VEGFs and VEGF receptors in human cancers*
| VEGF or receptor | Cancer |
|---|---|
| VEGFs | |
| VEGF | Bladder129,130, brain14,131,132, breast‡ (REFS 38,133–135), colon‡ (REFS 87,136), gastric‡ (REF. 137), oral squamous‡ (REFS 138,139), lung‡ (REFS 140–143), mesothelioma‡ (REF. 144), myeloid leukaemia145, ovarian146,147, pancreatic91,148 and prostate§ (REFS 149–151) |
| VEGFB | Breast|| (REFS 134,152) and lung140 |
| VEGFC | Breast153, cervical|| (REFS 153–155), colon‡|| (REFS 153,156,157), gastric158, oral squamous159, lung‡ (REFS 140,153,160) and prostate153 |
| VEGFD | Cervical154, gastric161 and lung140 |
| PLGF | Breast¶ (REF. 162), colon‡ (REF. 136), gastric‡§|| (REF. 163) and hepatocellular¶ (REF. 164) |
| VEGF receptors | |
| VEGFR1 | Bladder130, brain131,132, breast‡ (REFS 133–135,152,165), colon18,92, head and neck166, lung‡ (REFS 140–142), melanoma167, mesothelioma144, myeloid leukaemia145, oesophageal168, ovarian86,146,147, pancreatic (REFS 91,148) and prostate (REF. 169) |
| VEGFR2 | Bladder§ (REF. 129), brain14,131,132,161,170,171, breast‡ (REFS 133,135,172), cervical173, colon§|| (REFS 87,174), endometrial‡ (REF. 175), gastric137, head and neck166,176, hepatocellular‡ (REF. 177), lung‡ (REFS 140–142,178), melanoma167, mesothelioma144, multiple myeloma179, myeloid leukaemia145, oesophageal168, ovarian86,146,147, pancreatic91,148, prostate149,169, renal cell carcinoma180, squamous181 and thyroid|| (REF. 182) |
| VEGFR3 | Breast153, cervical§ (REFS 153,154), colon‡|| (REFS 153,156), gastric‡|| (REFS 158,161), head and neck159,166, lung‡§ (REFS 140,153,160), oesophageal168 and prostate153 |
| NRP1 | Brain|| (REFS 14,63,183,184), breast‡ (REFS 135,185,186), colon§ (REFS 83,187,188), lung143,185,189, melanoma167, ovarian147,190,191, pancreatic84,188,192–194 and prostate§¶ (REFS 150,151,195) |
| NRP2 | Bladder196, breast38,186,197,198, colon113,197, lung143,189,197, melanoma197,199, ovarian190, pancreatic193,194, prostate‡§ (REF. 15) and renal cell§|| (REF. 200) |
NRP, neuropilin; PLGF, placental growth factor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
The data reported are primarily based on immunohistochemical analyses of tumours and indicate expression of VEGFs or VEGF receptors specifically in tumour cells.
Studies that showed a correlation between expression and poor survival or outcome.
Studies that showed a correlation between expression and disease stage or progression.
Studies that showed a correlation between expression and metastasis.
Studies that showed a correlation between expression and recurrence.
VEGFR1 binds to VEGF with a higher affinity than VEGFR2, but the tyrosine phosphorylation of VEGFR1 in response to VEGF is weaker5. This observation, together with the existence of an alternatively spliced soluble form of VEGFR1, indicates that this RTK can function as a decoy receptor by sequestering VEGF from VEGFR2, thus regulating VEGFR2 signalling17. Nonetheless, some tumour cells express VEGFR1 in the absence of VEGFR2 and seem to use this RTK as a signalling receptor to mediate key functions17–19. However, the signalling mechanism of VEGFR1 remains to be elucidated. In contrast to VEGFR1 and VEGFR2, VEGFR3 does not bind VEGFA, and this RTK primarily functions in lymphangiogenesis as a receptor for VEGFC and VEGFD5,17.
Given that some tumour cells seem to lack expression of one or more of the VEGF RTKs but respond to autocrine and paracrine VEGF signals, it can be inferred that other types of receptors mediate or contribute to VEGF signalling in these cells. In this context, during recent years, the NRPs have garnered the most attention as VEGF receptors that function in tumour initiation and progression20,21. The NRPs were initially identified as neuronal receptors for class 3 semaphorins, which are axon guidance factors that function in the developing nervous system22,23. NRPs primarily function as co-receptors because they lack an intrinsic signalling capability; for example, NRPs form a complex with specific plexins in neurons and other cell types to form functional semaphorin receptors24,25. The two NRPs that are expressed in vertebrates (NRP1 and NRP2) are transmembrane glycoproteins that show 44% homology at the amino acid level. They contain four distinct extracellular domains that mediate ligand binding and a short cytoplasmic domain that lacks catalytic activity21,26,27. Alternative splicing of NRP1 and NRP2 can produce multiple isoforms, including secreted, soluble forms and NRP2 variants that have differences in their cytoplasmic domains28. There is also evidence that NRPs are modified by O-linked glycosylation and that this glycosylation can increase ligand binding and receptor expression11,29–31.
The crucial finding in the context of this Review is that NRPs can function as VEGF receptors and that they are expressed on tumour cells32. This seminal finding led to studies aiming to understand the contribution of NRPs to tumour biology. NRPs that are in a complex with specific plexins can also contribute to tumour cell function by functioning as semaphorin receptors33 (BOX 2). Although there is some indication that plexins contribute to VEGF signalling34, more data are needed, especially in tumour cells. The NRPs form complexes with VEGF RTKs (VEGFR1 and VEGFR2) and increase the affinity of these receptors for VEGF35. The NRPs can also affect the activity of many other receptors that are crucial for tumour cell function, and there is evidence that they may signal independently of other receptors. The crucial issue is whether these functions involve VEGF. In addition, the question of whether NRP1 and NRP2 in their capacity as VEGF receptors differ in their ability to affect tumour cells has not been investigated in depth, apart from the few examples that are cited below.
Box 2. Semaphorins and plexins in tumour cells.
A discussion of vascular endothelial growth factor (VEGF) signalling in tumour cells must include a mention of semaphorins — especially class 3 semaphorins (SEMA3s) — because they are secreted by tumour cells, they function as neuropilin (NRP) ligands and they have been implicated in tumour-associated functions. A prevailing idea is that SEMA3s and VEGFs carry out antagonistic effects on tumour cells: SEMA3s inhibit tumour growth, migration and invasion, and, by contrast, the VEGFs have pro-tumorigenic functions33,124. This idea is substantiated by the recent report that triple-negative breast tumours, which are poorly differentiated tumours and which have a high frequency of cancer stem cells, are characterized by high VEGFA expression and low SEMA3 expression125. However, there are a few reports indicating that SEMA3s can have a pro-tumorigenic function33,124. SEMA3s function by binding to NRPs in a complex with plexins — primarily type A plexins. Plexins are the only transmembrane receptors that can directly interact with small GTPases126. SEMA3 binding to type A plexins triggers the collapse of the actin cytoskeleton, which results in impaired migration and invasion. Interestingly, other plexin family members such as plexin B1, which binds to SEMA4D, are upregulated or mutated in some cancers, such as prostate carcinomas127. In breast cancer, the ability of the receptor tyrosine kinase ERBB2 to activate the pro-invasive small GTPases RHOA and RHOC is mediated by plexin B1, and this effect is independent of NRPs128. Clearly, there is much more to be learnt about semaphorins and plexins in cancer, and their relationship to VEGF signalling. An important issue that should be investigated in more detail is the role of plexins in VEGF–NRP signalling.
Regulation of VEGF signalling in tumour cells
The majority of studies that have observed VEGF signalling in tumour cells have characterized this signalling as autocrine14,16,36–43, although paracrine signalling does occur (see, for example, REF. 44). Moreover, the existence of autocrine VEGF signalling in human tumours is supported by the observation that VEGF is expressed in tumour cells, as shown by immunohistochemical data (TABLE 1), as well as by in situ hybridization and by analysis of microdissected tumour cells45,46. This reliance on autocrine signalling might reflect the importance of VEGF in sustaining the self-sufficiency or autonomy of tumour cells — a consideration that is highly relevant to aggressive cancers and to the biology of cancer stem cells. Indeed, autocrine VEGF signalling is generally characteristic of more aggressive cancers, including poorly differentiated carcinomas15,16,46. More fundamentally, poorly differentiated carcinomas show an embryonic gene expression pattern and the activation of key developmental pathways47. There are some data that implicate such pathways in the regulation of VEGF and VEGFR expression in tumour cells. Thus, these data provide a causal link between tumour dedifferentiation and the activation of autocrine VEGF signalling, but more investigation is needed (see below). This hypothesis is also supported by the finding that expression of VEGF and VEGFR1 is induced by an epithelial–mesenchymal transition (EMT) of colon carcinoma cells18 — a process that promotes dedifferentiation and progression to more aggressive tumours. Furthermore, VEGF stimulation of normal epithelial cells and differentiated carcinoma cells can induce an EMT46,48.
However, the mechanism by which the signalling pathways that are associated with oncogenic transformation and dedifferentiation regulate the expression of VEGF and VEGF receptors is still unknown. Given that hypoxia-inducible factor (HIF)-mediated transcription is a major driver of VEGF expression in tumours, it is likely that hypoxia helps to establish autocrine signalling networks in tumour cells. An important observation is that aggressive tumour cells sustain HIF-mediated transcription49, and mechanisms that have been implicated in inducing VEGF expression, such as RAS transformation41,50 or EMT18,46, probably directly affect HIF expression or activation. For example, the loss of oestrogen receptor-β (ERβ) expression that occurs in poorly differentiated prostate cancer and that causes an EMT phenotype, stimulates VEGFA transcription in tumour cells by a mechanism that involves transcriptional repression of prolyl hydroxylase 2 (PHD2; also known as EGLN1), which is an enzyme that targets HIF1α for degradation51.
Several recent studies have provided insights into the mechanism of NRP regulation in cancer. Notably, Hedgehog signalling can induce NRP expression38,52, which may be part of a positive feedback loop because NRP-mediated VEGF signalling can also induce the expression of the Hedgehog target gene GLI family zinc finger 1 (GLI1)38,53. The loss of PTEN induces NRP2 transcription in prostate cancer through a mechanism that involves the JUN N-terminal kinase (JNK)–JUN pathway, which provides a direct link between the loss of a tumour suppressor and the induction of NRP2 transcription15. Interestingly, both JUN and GLI1 can bind to the NRP2 promoter and may function together to regulate NRP2 transcription15,38. Expression of the transcription factor COUPTF2 (encoded by NR2F2) correlates with disease recurrence and progression in prostate cancer, and it can directly stimulate the transcription of NRP2 (REFS 54,55). COUPTF2 can also suppress Notch signalling56, which is interesting because there are reports that a Notch ligand — Delta-like 4 (DLL4) — can repress VEGFR2 and NRP1 expression57, but another ligand (DLL1) can stimulate their expression58. Although more work is needed to understand how signalling pathways that contribute to tumour initiation and dedifferentiation regulate the components of VEGF signalling in tumour cells, the fundamental principle of this regulation has been established.
Autocrine VEGF signalling in tumour cells can also be regulated at the level of receptor trafficking, which enables intracellular VEGFR signalling (FIG. 2). Specifically, autocrine NRP1–VEGFR2 signalling in gliomas involves active VEGFR2 that is localized in a cytoplasmic compartment14. This finding is indicative of an increasingly popular view that intracellular VEGFR signalling is important59 and that a key function of NRPs may be to promote the trafficking of VEGFRs and possibly of other growth factor receptors60. This mode of regulation has important implications for therapy (see below).
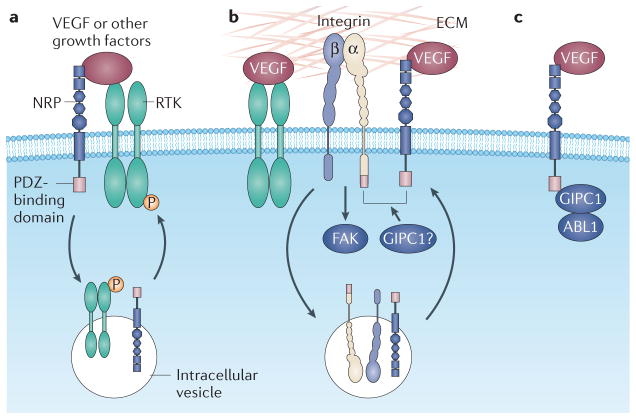
Figure 2. Receptor interactions that promote VEGF signalling in tumour cells, and the central role of NRPs.
a | Neuropilins (NRPs) interact with and potentiate the signalling function of growth factor receptor tyrosine kinases (RTKs), including vascular endothelial growth factor (VEGF) RTKs. This mode of regulation may be associated with internalization of the RTK and signalling from an intracellular compartment. Several growth factors, including hepatocyte growth factor, basic fibroblast growth factor, platelet-derived growth factor and transforming growth factor-β, directly interact with NRPs, but whether this binding is sufficient by itself to induce a signalling response is not known. b | NRPs also interact with specific integrins and activate their ability to bind to extracellular matrix (ECM) ligands, which results in the stimulation of integrin-mediated signalling through focal adhesion kinase (FAK). The RTK VEGF receptor 2 (VEGFR2) can also function in a similar capacity. In addition, NRPs may regulate integrin function by promoting their endocytic recycling. Both NRPs and specific integrin α-subunits (α5 and α6) contain a PDZ (PSD95, DLG and ZO1)-binding domain (Ser-Glu-Ala) at their carboxyl terminus, and PDZ proteins, such as the neuropilin-interacting protein GIPC1 might promote the association of these two classes of receptors. c | NRPs may also signal independently of other receptors, possibly by using their PDZ-binding domains to associate with signalling molecules such as ABL1. Note that these proposed mechanisms are not mutually exclusive, and there is the possibility that VEGF signalling in tumour cells involves the formation of macromolecular complexes that integrate components of these mechanisms.
Functional interactions between VEGF receptors and other receptors
An emerging theme in the literature is that VEGF receptors interact with and affect the function of other growth factor receptors, which is a manifestation of growth factor receptor crosstalk (FIG. 2). In addition to the regulation of VEGF RTKs by NRPs, there are numerous reports that VEGF RTKs and NRPs interact with other growth factor receptors. VEGFR2, for example, forms a complex with MET — the receptor for hepatocyte growth factor (HGF) — in response to VEGF stimulation of glioblastoma cells, and VEGFR2 thereby regulates MET signalling61. As co-receptors, the NRPs are promiscuous and have numerous interactions with other receptors. NRP1 interacts with the MET receptor and enhances the ability of HGF to stimulate the invasion of pancreatic carcinoma cells62 as well as the proliferation and survival of gliomas63. A direct interaction between the extracellular domain of NRP1 and the epidermal growth factor receptor (EGFR) has been shown, which augments the response of tumour cells to EGF and transforming growth factor-α (TGFα)64. This mechanism may contribute to the sustained activation of EGFR that occurs in some advanced cancers. Both NRP1 and NRP2 can interact with TGFβ receptors and can potentiate TGFβ signalling65,66. This finding has implications for EMT, which can be induced by either TGFβ signalling or by NRP-mediated VEGF signalling46,65. There is also evidence that NRPs can bind to specific growth factors in addition to VEGF and PLGF67, including HGF68, basic fibroblast growth factor (bFGF; also known as FGF2)68, TGFβ69 and platelet-derived growth factor (PDGF)70. However, whether this binding involves VEGF or whether it initiates a signalling response remains to be determined.
VEGF receptors also interact with specific integrins and activate or enhance integrin signalling in tumour cells (FIG. 2). This concept was established using the observation that VEGF signalling that is mediated by VEGFR2 can activate the ligand-binding function of multiple integrins in both endothelial cells and tumour cells by a mechanism that involves the PI3K–AKT pathway71. This mode of regulation may be bidirectional because there is also evidence that the αvβ3 integrin can form a complex with VEGFR2 and can increase the level of phosphorylation of this RTK in response to VEGF72. NRPs can also associate with specific integrins and can enhance their function in tumour cells73,74. A salient example of this interaction occurs between VEGF-bound NRP2 and the α6β1 integrin in breast carcinoma cells. NRP2, but not NRP1, co-immunopurifies with this integrin, and NRP2-mediated VEGF signalling enables α6β1 integrin to bind to its matrix ligand laminin, to engage filamentous actin, to form focal adhesions and to activate focal adhesion kinase (FAK)74. Interestingly, NRP2 is localized in focal adhesions that form on laminin, and this observation provides a direct connection between VEGF signalling and focal adhesion dynamics and signalling; this connection is corroborated by data from endothelial cells75,76.
Collectively, the data that are currently available highlight a crucial role for the NRPs in regulating growth factor receptor and integrin signalling (FIG. 2), and this aspect of NRP function may underlie the contribution of NRPs to tumour biology. However, much remains to be learnt about the mechanisms by which NRPs interact with growth factor receptors and integrins, and how they potentiate their function77; for example, does the ability of NRPs to co-immunopurify and to colocalize with RTKs and integrins reflect their association in macromolecular complexes that contain other signalling molecules and endocytic components? Such complexes could regulate and facilitate VEGF signalling and receptor trafficking. The presence of such complexes could be an explanation for most of the data on VEGF signalling in tumour cells, such as the regulation of RTK activity by NRPs and the role of NRPs in receptor trafficking.
Another timely issue is how NRPs mediate VEGF signalling independently of VEGF RTKs. Several studies have shown VEGF signalling in cells that lack detectable VEGF RTK expression or involvement; for example, NRP-mediated VEGF signalling was reported to promote the initiation of renal cell carcinoma in the absence of detectable VEGF RTK expression16. In addition, the ability of NRP2-mediated VEGF signalling to affect prostate cancer is not inhibited by bevacizumab, which blocks VEGF interactions with VEGFRs but not with NRPs15. A probable mechanism to explain these phenomena is that NRPs signal by affecting the function of other RTKs and integrins, as discussed above. However, NRPs may signal independently of RTKs (FIG. 2). Specifically, the cytoplasmic domains of NRP1 and NRP2 contain a PDZ-binding domain that can bind to PDZ-containing proteins, especially GIPC1 (also known as NRP-interacting protein (NIP)). GIPC1 is a cytoplasmic scaffolding protein that interacts with a wide range of receptors and that contributes to receptor trafficking and signal transduction78,79, and it has been implicated in tumorigenesis79. A recent study concluded that the NRP1 PDZ-binding domain is required to mediate the PLGF-stimulated growth of medulloblastoma, and that this is independent of VEGFR1 activity80. The PDZ-binding domain is thought to function by forming scaffolding complexes that transduce NRP signals; this hypothesis is corroborated by the finding that GIPC1 mediates the interaction of NRP1 with ABL1, which is a tyrosine kinase that could mediate NRP1 signalling11. GIPC1 can also function as a ‘bridge’ to promote the association of receptors that contain PDZ-binding domains such as NRPs and integrins81. Interestingly, the NRP2 cytoplasmic domain contains a motif that has a partial consensus sequence to an immunoreceptor tyrosine-based activation motif (ITAM)31, although there is no evidence yet that this motif is functional.
VEGF-mediated functions in tumour cells
VEGF regulates key functions of established tumour cells
The overarching theme in this Review is that VEGF signalling in tumour cells markedly affects tumour function and that this is independent of VEGF-mediated angiogenesis and vascular permeability. The initial reports that described the effects of VEGF on tumour cells showed that autocrine VEGF signalling — particularly signalling that is mediated by VEGF RTKs and NRPs — can promote the growth, survival, migration and invasion of cancer cells18,36,62,73,82–91. Most of these studies implicated dominant signalling pathways (for example, the PI3K–AKT and MAPK pathways) as the mechanism by which VEGF influences these processes; for example, VEGFR1 promotes the migration and the invasion of colorectal carcinoma cells by stimulating the activation of ERK1 or ERK2 as well as the activation of JNK and the consequent translocation of the p65 (also known as RELA) subunit of nuclear factor-κB (NF-κB) into the nucleus92. VEGFR1 can also sustain the survival of colorectal carcinoma cells that have undergone an EMT18. Several studies have described the ability of NRP-mediated VEGF signalling to affect the survival of tumour cells by activating the PI3K–AKT pathway; for example, NRP1-mediated VEGF signalling is able to sustain the survival of breast carcinoma cells36,82.
Recent studies have shown that the role of VEGF signalling in tumours might be more complex than initially thought; for example, the above-mentioned VEGF-induced VEGFR2–MET complex in glioblastoma cells contains a tyrosine phosphatase, which inhibits HGF-mediated invasion and mesenchymal transition61. These findings need to be reconciled with other reports that implicate VEGF and VEGFR2 in the function of glioma stem cells14 (see below). In addition, it has been reported that NRP1 on tumour myofibroblasts nucleates fibronectin fibril assembly by a mechanism that involves the α5β1 integrin and that NRP-mediated fibril assembly contributes to tumour growth11, which improves our understanding of the importance of NRP signalling in the tumour microenvironment. However, this study did not investigate the contribution of specific NRP ligands and it is therefore not known if the mechanism is VEGF dependent. VEGF may also regulate autophagy because it has been shown that NRP2-mediated VEGFC signalling mediated by mTOR complex 1 activates an autophagic mechanism that combats chemotherapy-induced stress, which has implications for the role of VEGF signalling in therapy resistance93.
VEGF, cancer stem cells and tumour formation
The importance of VEGF and VEGF receptor functions in cancer has been highlighted by the recent reports that autocrine VEGF signalling has a causal role in tumour formation and in the function of cancer stem cells, and these reports have distinguished this growth factor from many others (FIG. 3). Despite some controversy regarding the existence and the nature of such cells, it is evident that many tumours harbour a small population of cells that have self-renewal potential and the ability to initiate the growth of new tumours94. An initial study using a transgenic mouse model showed that autocrine VEGF signalling synergizes with EGFR signalling to promote the development of squamous carcinoma41. Importantly, this study concluded that the effect of VEGF is cell autonomous and angiogenesis independent. Mechanistically, VEGF was shown to mediate an autocrine proliferation loop that involves VEGFR1 and NRP1. A rigorous analysis of the early stages of squamous carcinoma formation in the skin resulted in several seminal findings that suggest that autocrine VEGF signalling is directly involved in the function of cancer stem cells40. In early-stage tumours or papillomas, cancer stem cells are localized in a perivascular niche that occurs adjacent to endothelial cells. Blocking VEGFR2 reduced the size of the cancer stem cell pool and their self-renewal potential. Conditional deletion of VEGFA in the tumour cells of established tumours caused tumour regression by decreasing both microvascular density and the proliferation and renewal of cancer stem cells. Moreover, genetic deletion of NRP1 prevented the ability of VEGF to promote stem cell-like properties and self-renewal. These findings, which were observed both in vivo and in vitro, clearly establish the importance of autocrine VEGFA signalling that is mediated by NRP1 and VEGFR2 in cancer stem cells. The localization of cancer stem cells adjacent to endothelial cells infers that tumour cell-derived VEGFA functions both as a paracrine factor to stimulate angiogenesis and as an autocrine factor to regulate cancer stem cells (FIG. 3). However, the localization of cancer stem cells adjacent to the endothelium needs to be reconciled with other reports showing that hypoxia drives the self-renewal of cancer stem cells95.
Figure 3. Role of autocrine VEGF signalling in the function of cancer stem cells and tumour formation.
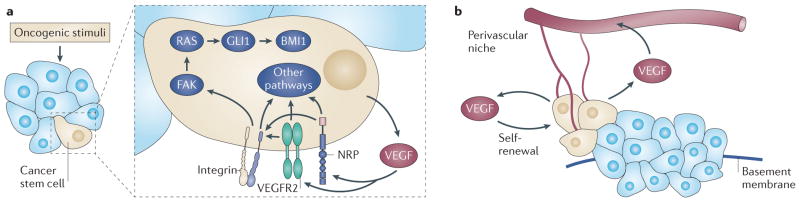
a | The expression of vascular endothelial growth factor (VEGF) and VEGF receptors is induced concomitantly with oncogenic transformation; this facilitates the establishment of autocrine VEGF signalling. This signalling, which is mediated by the receptor tyrosine kinase VEGF receptor 2 (VEGFR2) and by neuropilins (NRPs), could be necessary for the function of cancer stem cells (beige cells) because it seems to maintain the size of the stem cell pool and to sustain self-renewal. The ability of autocrine VEGF signalling that is mediated by NRPs and integrins to regulate the expression of the Hedgehog target GLI family zinc finger 1 (GLI1) and the Polycomb group repressor BMI1 provides one mechanism to account for the contribution of autocrine VEGF signalling to the function of cancer stem cells, but other mechanisms probably exist. b | Cancer stem cells can be localized in a perivascular niche, which enables VEGF that is secreted by these cells to function in a paracrine manner to stimulate angiogenesis in nascent tumours. Autocrine VEGF signalling can also promote dedifferentiation and an epithelial–mesenchymal transition (EMT) phenotype that results in increased migration and invasion into the stroma. FAK, focal adhesion kinase.
An interesting theme that emerges from the above-described studies and other studies is that distinct VEGF family members and VEGF receptors can be used to facilitate tumour initiation and growth; for example, the two studies of squamous carcinoma formation that are discussed above implicated different VEGF RTKs. An explanation for this may be that the initial study identified a VEGFR1-mediated proliferation loop that contributes to tumour growth41 and the second study suggested that VEGFR2 is directly involved in the function of cancer stem cells and their self-renewal40. It is worth noting that VEGFR1 has yet to be implicated in the function of cancer stem cells or in tumour initiation. Other studies have suggested that the contribution of autocrine VEGF signalling to tumour initiation is independent of VEGF RTKs and is driven by NRP signalling. Such a mechanism has been proposed for renal cell carcinoma16. In addition, a PLGF–NRP1 signalling axis that is independent of VEGFR1 contributes to the growth and to the spread of medulloblastomas80. Although it is possible that NRP signalling alone mediates autocrine VEGF signalling in this context, a more probable scenario (discussed above) is that NRPs potentiate the function of other non-VEGF receptors that are crucial for the function of cancer stem cells and tumour initiation. This idea is exemplified by our work on the role of VEGF–NRP2 signalling in the initiation of breast cancer. As mentioned above, VEGF–NRP2 signalling can activate the α6β1 integrin38,74, which is noteworthy because this integrin is necessary for the function of some cancer stem cells96,97. Another important aspect of autocrine VEGF signalling in cancer stem cells is that this signalling can occur in an intracellular compartment. VEGFR2 and NRP1 are preferentially expressed on glioma stem cells that are positive for CD133, and ablation of either VEGFR2 or NRP1 in glioma cells in vivo increases apoptosis and reduces tumour formation14. Importantly, VEGF signalling that is mediated by NRP1 and VEGFR2 maintains a cytosolic pool of active VEGFR2 that may be the source of cell survival signalling and that is resistant to VEGF-specific antibody (bevacizumab) therapy.
Although there are data that clearly implicate VEGF in the function of cancer stem cells and tumour initiation, much less is known about the signalling mechanisms responsible for this function. An example of such a mechanism derives from our work on VEGF–NRP2-mediated activation of the α6β1 integrin. This activation induces activation of the FAK–RAS signalling pathway that culminates in non-canonical Hedgehog signalling, which in turn activates GLI1. GLI1 then induces the expression of BMI1 (REF.38) (FIG. 3), which is a Polycomb group transcriptional repressor that has been implicated in self-renewal and tumour initiation98,99. As mentioned above, GLI1 can stimulate the transcription of NRP2 (REFS 38,52), which thereby creates a positive feedback loop that has the potential to sustain the self-renewal properties of cancer stem cells. Hedgehog signalling also has a crucial role in tumour cell–stromal cell interactions in this context, and this is shown by the finding that tumour-derived sonic hedgehog stimulates PLGF expression in stromal cells, which promotes the growth of medulloblastomas80.
However, a fundamental issue is whether VEGF signalling alone can cause oncogenic transformation. Although there is a report that VEGFR1 can cause transformation100, a more feasible hypothesis is that autocrine VEGF signalling is induced at the same time as other oncogenic events that drive tumour initiation, but that it can be an important (if not essential) component of the initiation process, as described above. In this context, a thought-provoking finding is that chronic inflammation causes upregulation of VEGFR2 in intestinal epithelial cells and that VEGFR2 signalling in these cells is required for tumour growth; this finding provides a causal link between inflammation and cancer that involves VEGF signalling13. However, this discussion of inflammation focuses on the role that autocrine VEGF signalling has in maintaining the function of cancer stem cells. Moreover, the data that are currently available clearly implicate autocrine VEGF signalling in sustaining self-renewal38,40, which is consistent with the more general hypothesis that autocrine signalling is required to maintain a stem cell state101. Autocrine VEGF signalling is also closely associated with tumour dedifferentiation and with EMT46, which are processes that may be involved in the genesis of cancer stem cells101,102. Moreover, autocrine VEGF signalling has been implicated in the metastatic cascade36,80, and this is consistent with the recently identified role of cancer stem cells in tumour dissemination102.
Although other growth factors may be involved in the autocrine signalling pathways that contribute to the function of cancer stem cells, VEGF is increasingly becoming recognized as a crucial factor. A potential explanation for this phenomenon is that the mechanisms that regulate VEGF expression — especially HIF-mediated transcription — are essential components of a stem cell phenotype95,103. The argument can be made that autocrine VEGF expression is a manifestation of HIF activation that is associated with the genesis of cancer stem cells and that is characteristic of poorly differentiated tumours49,95. For example, in high-grade prostate carcinoma, VEGF, HIF1α and NRP expression is higher in poorly differentiated tumour cells compared with more differentiated tumour cells46.
Therapy
VEGF-targeted therapy — either alone or in combination with chemotherapy — is used for the treatment of many cancers8. Antibody-mediated inhibition of VEGF using bevacizumab is currently the predominant mode of VEGF-targeted therapy, although drugs that inhibit VEGF RTK activity (such as sunitinib and sorafenib) are also used8. The prevailing idea is that such therapy targets angiogenesis and other endothelial cell functions, and this aspect of VEGF-targeted therapy has been extensively studied and reviewed8,104,105. In this Review, we are interested in the possibility that targeting VEGF and VEGF receptors specifically on tumour cells could be effective in light of our increasing understanding of the importance of autocrine VEGF signalling in tumour initiation and in the biology of aggressive cancers. However, a potential caveat could be that although VEGF-targeted therapy (primarily bevacizumab) has reduced tumour burden and improved survival in some cancers, it has not been as successful as initially anticipated106. If we assume that bevacizumab has the potential to inhibit autocrine VEGF signalling in tumour cells, the modest effect of this drug that has been observed so far would diminish the importance and therapeutic potential of targeting VEGF signalling in tumour cells. However, a crucial observation is that bevacizumab does not inhibit the interaction of VEGF with NRPs107. Given the importance of NRPs to cancer stem cells and to VEGF signalling in tumour cells, which has been established in preclinical studies, this observation has widespread therapeutic implications and indicates that therapies that target NRPs or VEGF–NRP interactions could be very effective, especially when they are used in combination with antibodies against VEGF108 (FIG. 4). Interestingly, clinical trials involving patients with advanced gastric and breast cancer assessed the efficacy of bevacizumab and concluded that high NRP1 expression is prognostic of a poor response to bevacizumab109,110, reinforcing the importance of targeting NRP and the need for combination therapy.
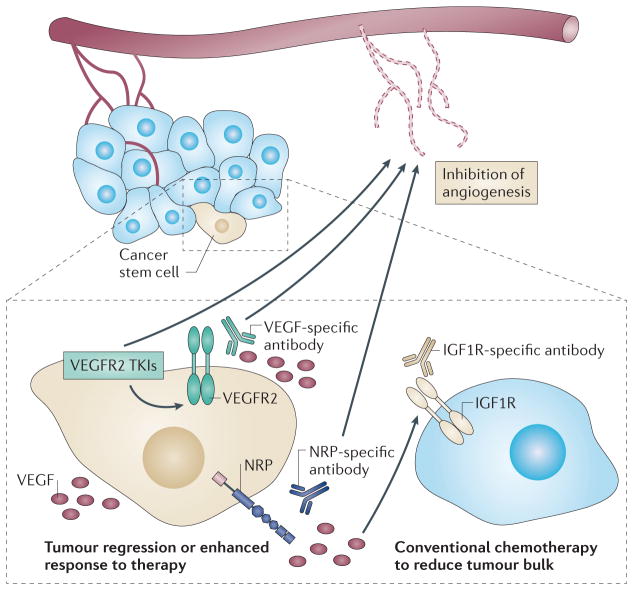
Figure 4. Therapeutic targeting of VEGF signalling in tumour cells.
The functional importance of vascular endothelial growth factors (VEGFs) and VEGF receptors — that is, neuropilins (NRPs) and VEGF receptor tyrosine kinases (RTKs) — that are expressed by tumour cells, in particular those that are expressed by cancer stem cells (beige cells), provides an important opportunity for the development of new therapeutic approaches, especially for highly aggressive tumours. These approaches have the potential to promote tumour regression and to improve the response to standard chemotherapy and radiation therapy. So far, strategies that inhibit VEGF signalling have primarily focused on targeting angiogenesis using either bevacizumab to inhibit VEGF or tyrosine kinase inhibitors (TKIs) that target VEGF RTKs such as VEGF receptor 2 (VEGFR2). NRPs are becoming recognized as crucial effectors of autocrine VEGF signalling in tumours, and more emphasis should be placed on targeting them therapeutically. Although some side effects were observed during the initial clinical use of a humanized NRP1 antibody, targeting NRPs is still a potentially effective strategy, and approaches need to be developed that minimize these side effects. It is also important to note that bevacizumab does not inhibit the binding of VEGF to NRPs, which highlights the importance of targeting NRPs directly and developing VEGF-specific reagents (such as placental growth factor (PLGF)-specific antibodies) that inhibit NRP binding. Targeting NRPs can result in compensatory signalling by other growth factor receptors, which indicates the potential importance of using a combination therapy. This possibility is shown by the compensatory insulin-like growth factor 1 receptor (IGF1R) signalling that occurs in prostate cancer, and it is likely that other mechanisms of compensation in response to VEGF pathway inhibition will be discovered for other tumour cells. These approaches may benefit from the use of conventional chemotherapy to reduce overall tumour burden.
Targeting NRPs and VEGF RTKs
Preclinical data suggests that targeting NRPs could potentially be used as a mode of cancer therapy. NRP expression is minimal in most adult tissues, which reduces the possibility that NRP-based therapies would perturb normal tissue function, and mouse studies using therapeutic NRP-specific antibodies have reported minimal side effects108,111. In addition, function-blocking NRP1- and NRP2-specific antibodies have been shown to inhibit tumour growth and to cause stasis in mice38,80,108,112. Similar results have been achieved using RNA interference (RNAi) to deplete NRP expression113. Other therapeutic approaches include the use of small peptides that prevent NRP oligomerization114 or soluble forms of NRPs that function as decoy receptors107. Importantly, most of these studies concluded that targeting NRPs had a direct effect on tumour cells in vivo, and some studies showed that there was little effect on tumour angiogenesis38.
Unfortunately, recent Phase I results using MNRP1685A, which is a humanized NRP1-specific monoclonal antibody, have raised concerns about targeting NRPs for therapy. MNRP1685A is cleared from the circulation more rapidly than other humanized antibodies, which suggests that it may have off-target effects115. Another concern is the preliminary report that patients who were dosed with MNRP1685A in combination with bevacizumab showed frequent but transient decreases in platelet levels and clinically significant proteinuria116. Given that NRP1 can affect the function of multiple growth factor receptors, these effects of MNRP1685A may not be surprising. However, these results should not discourage future work on the therapeutic targeting of NRPs, given the biological importance of NRPs in cancer. Strategies that target NRPs on tumour cells or cancer stem cells more specifically may decrease potential toxicity and off-target effects. The discovery that peptides with a carboxy-terminal arginine residue bind to NRP1 and NRP2 on the cell surface has been exploited as a novel approach to deliver cytotoxic peptides to tumour cells, and such tumour-penetrating peptides can be used to facilitate the delivery of co-administered drugs directly to tumour tissue109,117. Another novel NRP-targeting method that has important implications for therapy comes from our work that showed that the inhibition of NRP2 in prostate cancer cells induces the expression of the insulin-like growth factor 1 receptor (IGF1R) and triggers downstream signalling, which increases tumour proliferation15. However, NRP2 and IGF1R combination therapy proved to be effective in reducing tumour burden (FIG. 4). This study also found that NRP2 is a valid biomarker for predicting the response to IGF1R therapy.
Some studies have investigated the effect of blocking VEGF RTKs or their activity in tumour cells14,40,118,119. Of note, antibody-mediated inhibition of VEGFR2 in the mouse model of skin cancer that is discussed above caused tumour regression by reducing the cancer stem cell pool size and by impairing cancer stem cell renewal, as well as by decreasing microvascular density40. In addition, the inhibition of VEGFR2 expression or activity blocked the VEGF–VEGFR2–NRP1 signalling axis. This inhibition also impeded the viability of glioma stem cells in vitro and increased the survival of mice that harboured glioma xenografts14. The interesting result of this study, which has been alluded to above, is that VEGFR2 signalling occurs intracellularly and is blocked by inhibitors of VEGFR2 tyrosine kinase activity but not by bevacizumab. In this context, it is thought-provoking that the inhibition of VEGFR2 expression in ovarian carcinoma cells has been shown to result in increased tumour growth in vivo and was associated with increased VEGF and NRP1 expression119. This finding confirms the importance of NRPs in tumour cells119.
Targeting VEGF
Despite the concerns that relate to bevacizumab, the targeting of VEGF family members has the potential to be a very effective approach for inhibiting tumour cell function. This is supported by the report that bevacizumab treatment of patients with locally advanced breast cancer significantly increased tumour cell apoptosis120. In addition, antibody-mediated inhibition of PLGF in medulloblastomas had a direct antitumour effect in vivo and caused tumour regression80, and it had minimal side effects. Similar results were achieved by blocking NRP1, but VEGFR1 inhibition had no effect. These findings substantiate the feasibility of using antibodies that are specific for other VEGF family members that block binding to NRPs. The potential of targeting VEGF is validated by the suppression of pancreatic carcinoma cell tumorigenesis by using a ‘VEGF-trap’ that sequesters VEGF121.
The above findings are tempered by the report that anti-angiogenic therapy involving inhibition of either VEGF or VEGF RTKs increased tumour invasion and metastasis122. Of particular relevance, conditional deletion of VEGFA in pancreatic carcinoma cells, which should disrupt autocrine VEGF signalling, increased tumour invasiveness. By contrast, conditional deletion of VEGFA in established squamous carcinomas caused tumour regression40. An explanation for these seemingly contradictory findings is that VEGFA deletion inhibits slow-cycling cancer stem cells and selects for cells that proliferate at a higher rate and that may — at least in the short-term — be invasive. This hypothesis is supported by the above-mentioned finding that NRP2 inhibition in prostate cancer, which targets a putative stem cell population, increases IGF1R-mediated cell proliferation15. Although more work is needed to understand these fundamental issues, it is becoming evident that combined modes of therapy will be necessary to target VEGF signalling in tumour cells.
Conclusions and future perspectives
The salient theme of this Review is that VEGF signalling in tumour cells — especially autocrine signalling — can be an essential component of tumour initiation and it can be intimately associated with oncogenic transformation. More specifically, compelling data indicate that VEGF signalling promotes the function of cancer stem cells and sustains their self-renewal. These functions of VEGF are independent of its contribution to angiogenesis and, for this reason, constitute a paradigm shift in our understanding of the role of VEGF in cancer. Continued work to understand the relationship between autocrine VEGF signalling and the biology of cancer stem cells is warranted; in particular, because of the potential of using VEGF signalling as a therapeutic target.
VEGF signalling in tumour cells can involve VEGF RTKs, other RTKs, NRPs and integrins, but NRPs seem to be at the centre of signalling events that enable VEGF to affect tumour cell function, especially tumour initiation and the function of cancer stem cells. Much remains to be learnt about how NRPs function in this context, but it is evident that they have the ability to regulate the function and the trafficking of RTKs and integrins. An important issue in the future will be determining the extent to which NRP regulation of other receptors involves VEGF. Moreover, how this regulation occurs is only beginning to be understood, and the possibility that NRPs are components of macromolecular signalling complexes merits particular attention. NRPs may also have some semi-autonomous signalling potential that derives from their ability to interact with PDZ domain-containing proteins. Both NRP1 and NRP2 have been implicated in the function of cancer stem cells and are thought to have other functions in tumours, but the relative contributions of these two receptors is not yet known. Related issues are whether the NRP2 cytoplasmic domain variants have functional differences and the extent to which NRP glycosylation affects their ability to promote tumour initiation. It is somewhat surprising that the contribution of VEGF RTKs to VEGF signalling in tumour cells has not been consistent among studies — especially given the dominant role of VEGFR2 in driving angiogenesis — and it will be important to understand why their functional importance is diminished in some tumour cells.
The realization that autocrine VEGF signalling can be crucial for tumour initiation and for the characteristics of highly aggressive cancers provides a promising opportunity for the development of new therapeutic approaches. Such approaches are particularly intriguing because NRPs seem to be essential for this VEGF signalling and can be therapeutically targeted using currently available reagents. However, this excitement is tempered by the complexities that are associated with targeting VEGF and VEGF receptors, including potential toxicity, the possibility that cells resistant to such therapy can be highly aggressive and the possibility that compensatory signalling mechanisms may offset potential benefits. The development of more effective strategies will probably involve approaches that target tumour cells more specifically as well as the use of a combination of therapeutic reagents that overcome the resistance caused by targeting single molecules.
Key points.
Tumour cells express vascular endothelial growth factor (VEGF) receptors and respond to autocrine and paracrine VEGF signals.
VEGF signalling in tumour cells affects tumour functions independently of angiogenesis.
VEGF signalling in tumour cells is mediated by VEGF receptor tyrosine kinases (RTKs) and neuropilins (NRPs).
NRPs may be at the centre of VEGF signalling because they regulate the function of RTKs and integrins that are crucial for tumour cell function.
Autocrine VEGF signalling may be essential for tumour initiation because it regulates the size of the cancer stem cell pool and the self-renewal of cancer stem cells.
Therapeutic approaches that aim to target NRPs and VEGF RTKs on tumour cells could be useful to promote tumour regression and to diminish the probability of tumour recurrence, especially when used in combination with VEGF-specific antibodies and other modes of therapy.
Acknowledgments
Work in the authors’ laboratory is supported by US National Institutes of Health (NIH) grants CA168464 and CA159856, and by a US Department of Defense prostate cancer grant W81XWH-12-1-0308.
Glossary
- Integrins
A family of more than 20 heterodimeric cell surface extracellular matrix (ECM) receptors. Integrins connect the ECM to the cytoskeleton and can transmit signalling information bidirectionally
- Plexins
A large family of transmembrane proteins that share homology in their extracellular domains with the MET receptor and semaphorins
- Epithelial–mesenchymal transition (EMT)
A conversion from an epithelial to a mesenchymal phenotype, which is a normal component of embryonic development. In carcinomas, this transformation results in altered cell morphology, the expression of mesenchymal proteins and increased invasiveness
- Hypoxia-inducible factor (HIF)
A dimeric transcription factor that is formed of α- and β-subunits and that is involved in the hypoxia-sensitive regulation of numerous genes, including glycolytic enzymes, glucose transporters and angiogenic factors
- Focal adhesions
Dynamic, macromolecular protein complexes that link the extracellular matrix to the actin cytoskeleton through integrins
- PDZ-binding domain (PSD95
DLG and ZO1-binding domain), A structural, protein–protein interaction domain, which is ~80–90 amino acids in length, that often functions as a scaffold for signalling complexes and/or as a cytoskeletal anchor for transmembrane proteins
- Immunoreceptor tyrosine-based activation motif
(ITAM), A motif (YXXL or YXXI) that can be phosphorylated in response to receptor ligation and that functions as a docking site for other proteins involved in signal transduction
- Autophagy
A cellular response in which the cell metabolizes its own contents and organelles to maintain energy production. Although such a process can eventually result in cell death, it can also be used to maintain cell survival in conditions of limiting nutrients
- CD133
A cell-surface glycoprotein, which is also known as Prominin 1, that can be used as a marker for some cancer stem cells
- Polycomb group
Proteins that were first described in Drosophila melanogaster and that are required for normal development. They work in multiprotein complexes that are called Polycomb repressive complexes, which establish regions of chromatin in which gene expression is repressed
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 2.Tischer E, et al. Vascular endothelial growth factor: a new member of the platelet-derived growth factor gene family. Biochem Biophys Res Commun. 1989;165:1198–1206. doi: 10.1016/0006-291x(89)92729-0. [DOI] [PubMed] [Google Scholar]
- 3.Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50:1774–1778. [PubMed] [Google Scholar]
- 4.Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 5.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69 (Suppl 3):11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 8.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 9.Senger DR. Vascular endothelial growth factor: much more than an angiogenesis factor. Mol Biol Cell. 2010;21:377–379. doi: 10.1091/mbc.E09-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen W, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001–2016. doi: 10.1084/jem.20111497. This study rigorously shows the importance of NRP1 on immune cells in regulating tumour growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaqoob U, et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res. 2012;72:4047–4059. doi: 10.1158/0008-5472.CAN-11-3907. This study shows a novel role for NRP1 that is expressed on tumour myofibroblasts in regulating tumour growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 13.Waldner MJ, et al. VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J Exp Med. 2010;207:2855–2868. doi: 10.1084/jem.20100438. This is a thought-provoking study showing that chronic inflammation induces VEGFR2 expression on intestinal epithelial cells and that VEGFR2 signalling is necessary for tumour growth. These findings establish an important link between inflammation and cancer that involves VEGF signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamerlik P, et al. Autocrine VEGF–VEGFR2–neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209:507–520. doi: 10.1084/jem.20111424. This study defines the importance of autocrine VEGF signalling in the function of glioma stem cells, and it shows that this signalling can occur in an intracellular compartment, which is a result that has considerable implications for therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goel HL, et al. VEGF/neuropilin-2 regulation of Bmi-1 and consequent repression of IGF-1R define a novel mechanism of aggressive prostate cancer. Cancer Discov. 2012;2:906–921. doi: 10.1158/2159-8290.CD-12-0085. This study shows the ability of NRP2-mediated VEGF signalling to regulate BMI1, which is a crucial stem cell factor. Importantly, this study also shows that therapeutic targeting of NRP2 results in compensatory IGF1R signalling and that combined NRP2 and IGF1R therapy can be effective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y, et al. VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res. 2012;72:3912–3918. doi: 10.1158/0008-5472.CAN-11-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–5605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 18.Bates RC, et al. Flt-1-dependent survival characterizes the epithelial–mesenchymal transition of colonic organoids. Curr Biol. 2003;13:1721–1727. doi: 10.1016/j.cub.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Soker S, et al. Vascular endothelial growth factor-mediated autocrine stimulation of prostate tumor cells coincides with progression to a malignant phenotype. Am J Pathol. 2001;159:651–659. doi: 10.1016/S0002-9440(10)61736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prud’homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012;3:921–939. doi: 10.18632/oncotarget.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker MW, Guo HF, Li X, Linkugel AD, Vander Kooi CW. Function of members of the neuropilin family as essential pleiotropic cell surface receptors. Biochemistry. 2012;51:9437–9446. doi: 10.1021/bi3012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolodkin AL, et al. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 24.Winberg ML, et al. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 26.Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11:31–39. doi: 10.1007/s10456-008-9097-1. [DOI] [PubMed] [Google Scholar]
- 27.Parker MW, Xu P, Li X, Vander Kooi CW. Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J Biol Chem. 2012;287:11082–11089. doi: 10.1074/jbc.M111.331140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossignol M, Gagnon ML, Klagsbrun M. Genomic organization of human neuropilin-1 and neuropilin-2 genes: identification and distribution of splice variants and soluble isoforms. Genomics. 2000;70:211–222. doi: 10.1006/geno.2000.6381. [DOI] [PubMed] [Google Scholar]
- 29.Frankel P, et al. Chondroitin sulphate-modified neuropilin 1 is expressed in human tumour cells and modulates 3D invasion in the U87MG human glioblastoma cell line through a p130Cas-mediated pathway. EMBO Rep. 2008;9:983–989. doi: 10.1038/embor.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shintani Y, et al. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. EMBO J. 2006;25:3045–3055. doi: 10.1038/sj.emboj.7601188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zachary IC, Frankel P, Evans IM, Pellet-Many C. The role of neuropilins in cell signalling. Biochem Soc Trans. 2009;37:1171–1178. doi: 10.1042/BST0371171. [DOI] [PubMed] [Google Scholar]
- 32.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. This is a seminal study showing that NRP1 can function as a VEGF receptor and that it is expressed on tumour cells. [DOI] [PubMed] [Google Scholar]
- 33.Neufeld G, Sabag AD, Rabinovicz N, Kessler O. Semaphorins in angiogenesis and tumor progression. Cold Spring Harb Perspect Med. 2012;2:a006718. doi: 10.1101/cshperspect.a006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kigel B, Rabinowicz N, Varshavsky A, Kessler O, Neufeld G. Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling. Blood. 2011;118:4285–4296. doi: 10.1182/blood-2011-03-341388. [DOI] [PubMed] [Google Scholar]
- 35.Neufeld G, Kessler O, Herzog Y. The interaction of neuropilin-1 and neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol. 2002;515:81–90. doi: 10.1007/978-1-4615-0119-0_7. [DOI] [PubMed] [Google Scholar]
- 36.Bachelder RE, et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- 37.Perrot-Applanat M, Di Benedetto M. Autocrine functions of VEGF in breast tumor cells: adhesion, survival, migration and invasion. Cell Adh Migr. 2012;6:547–553. doi: 10.4161/cam.23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel HL, et al. GLI1 regulates a novel neuropilin-2/α6β1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol Med. 2013;5:488–508. doi: 10.1002/emmm.201202078. This study provides the best example so far of how NRP2-mediated VEGF signalling can promote tumour initiation through a mechanism that involves the integrin-mediated regulation of GLI1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J, et al. Blockade of VEGF-A suppresses tumor growth via inhibition of autocrine signaling through FAK and AKT. Cancer Lett. 2012;318:221–225. doi: 10.1016/j.canlet.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Beck B, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. This is a seminal study that establishes the importance of NRP1-mediated autocrine VEGF signalling in regulating the size of the cancer stem cell pool and the stemness of these cells. [DOI] [PubMed] [Google Scholar]
- 41.Lichtenberger BM, et al. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. This is a study that uses a transgenic mouse model to rigorously show the importance of autocrine VEGF signalling during the genesis of squamous carcinoma. [DOI] [PubMed] [Google Scholar]
- 42.Matsuura M, et al. Autocrine loop between vascular endothelial growth factor (VEGF)-C and VEGF receptor-3 positively regulates tumor-associated lymphangiogenesis in oral squamoid cancer cells. Am J Pathol. 2009;175:1709–1721. doi: 10.2353/ajpath.2009.081139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoeffner DJ, et al. VEGF contributes to mammary tumor growth in transgenic mice through paracrine and autocrine mechanisms. Lab Invest. 2005;85:608–623. doi: 10.1038/labinvest.3700258. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt T, et al. Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1+ leukemia. Cancer Cell. 2011;19:740–753. doi: 10.1016/j.ccr.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Senger DR, Van De Water L. VEGF expression by epithelial and stromal cell compartments: resolving a controversy. Am J Pathol. 2000;157:1–3. doi: 10.1016/S0002-9440(10)64508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mak P, et al. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Porath I, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nature Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wanami LS, Chen HY, Peiro S, Garcia de Herreros A, Bachelder RE. Vascular endothelial growth factor-A stimulates Snail expression in breast tumor cells: implications for tumor progression. Exp Cell Res. 2008;314:2448–2453. doi: 10.1016/j.yexcr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med. 2013;17:30–54. doi: 10.1111/jcmm.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grugel S, Finkenzeller G, Weindel K, Barleon B, Marme D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995;270:25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- 51.Mak P, Chang C, Pursell B, Mercurio AM. Estrogen receptor-β sustains epithelial differentiation by regulating prolyl hydroxylase 2 transcription. Proc Natl Acad Sci USA. 2013;110:4708–4713. doi: 10.1073/pnas.1221654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hillman RT, et al. Neuropilins are positive regulators of Hedgehog signal transduction. Genes Dev. 2011;25:2333–2346. doi: 10.1101/gad.173054.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao Y, et al. Neuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axes. Cancer Res. 2008;68:8667–8672. doi: 10.1158/0008-5472.CAN-08-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin FJ, et al. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest. 2010;120:1694–1707. doi: 10.1172/JCI40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin J, et al. COUP-TFII inhibits TGF-β-induced growth barrier to promote prostate tumorigenesis. Nature. 2013;493:236–240. doi: 10.1038/nature11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You LR, et al. Suppression of notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 57.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113:5680–5688. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- 59.Lee TH, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakayama M, Berger P. Coordination of VEGF receptor trafficking and signaling by coreceptors. Exp Cell Res. 2013;319:1340–1347. doi: 10.1016/j.yexcr.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Lu KV, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. This is a thought-provoking study showing that VEGF–VEGR2 signalling inhibits the HGF-mediated invasion of glioblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsushita A, Gotze T, Korc M. Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 2007;67:10309–10316. doi: 10.1158/0008-5472.CAN-07-3256. [DOI] [PubMed] [Google Scholar]
- 63.Hu B, et al. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene. 2007;26:5577–5586. doi: 10.1038/sj.onc.1210348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rizzolio S, et al. Neuropilin-1-dependent regulation of EGF-receptor signaling. Cancer Res. 2012;72:5801–5811. doi: 10.1158/0008-5472.CAN-12-0995. [DOI] [PubMed] [Google Scholar]
- 65.Grandclement C, et al. Neuropilin-2 expression promotes TGF-β1-mediated epithelial to mesenchymal transition in colorectal cancer cells. PLoS ONE. 2011;6:e20444. doi: 10.1371/journal.pone.0020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glinka Y, Stoilova S, Mohammed N, Prud’homme GJ. Neuropilin-1 exerts co-receptor function for TGF-β-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-β. Carcinogenesis. 2011;32:613–621. doi: 10.1093/carcin/bgq281. [DOI] [PubMed] [Google Scholar]
- 67.Migdal M, et al. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem. 1998;273:22272–22278. doi: 10.1074/jbc.273.35.22272. [DOI] [PubMed] [Google Scholar]
- 68.West DC, et al. Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J Biol Chem. 2005;280:13457–13464. doi: 10.1074/jbc.M410924200. [DOI] [PubMed] [Google Scholar]
- 69.Glinka Y, Prud’homme GJ. Neuropilin-1 is a receptor for transforming growth factor β-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302–310. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banerjee S, et al. Breast cancer cells secreted platelet-derived growth factor-induced motility of vascular smooth muscle cells is mediated through neuropilin-1. Mol Carcinog. 2006;45:871–880. doi: 10.1002/mc.20248. [DOI] [PubMed] [Google Scholar]
- 71.Byzova TV, et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–860. This study provided the first indication that VEGF signalling can regulate integrin activation in tumour cells. [PubMed] [Google Scholar]
- 72.Soldi R, et al. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukasawa M, Matsushita A, Korc M. Neuropilin-1 interacts with integrin β1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol Ther. 2007;6:1173–1180. doi: 10.4161/cbt.6.8.4363. [DOI] [PubMed] [Google Scholar]
- 74.Goel HL, Pursell B, Standley C, Fogarty K, Mercurio AM. Neuropilin-2 regulates α6β1 integrin in the formation of focal adhesions and signaling. J Cell Sci. 2012;125:497–506. doi: 10.1242/jcs.094433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell. 2011;22:2766–2776. doi: 10.1091/mbc.E09-12-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen TT, et al. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zachary IC. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem Soc Trans. 2011;39:1583–1591. doi: 10.1042/BST20110697. [DOI] [PubMed] [Google Scholar]
- 78.De Vries L, Lou X, Zhao G, Zheng B, Farquhar MG. GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP. Proc Natl Acad Sci USA. 1998;95:12340–12345. doi: 10.1073/pnas.95.21.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katoh M. Functional proteomics, human genetics and cancer biology of GIPC family members. Exp Mol Med. 2013;45:e26. doi: 10.1038/emm.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Snuderl M, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152:1065–1076. doi: 10.1016/j.cell.2013.01.036. This is an important study that establishes the potential for treating medulloblastoma by antibody-mediated targeting of PLGF and NRP1 on tumour cells and by negating the contribution of VEGFR1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valdembri D, et al. Neuropilin-1/GIPC1 signaling regulates α5β1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barr MP, Bouchier-Hayes DJ, Harmey JJ. Vascular endothelial growth factor is an autocrine survival factor for breast tumour cells under hypoxia. Int J Oncol. 2008;32:41–48. [PubMed] [Google Scholar]
- 83.Parikh AA, et al. Neuropilin-1 in human colon cancer: expression, regulation, and role in induction of angiogenesis. Am J Pathol. 2004;164:2139–2151. doi: 10.1016/S0002-9440(10)63772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parikh AA, et al. Expression and regulation of the novel vascular endothelial growth factor receptor neuropilin-1 by epidermal growth factor in human pancreatic carcinoma. Cancer. 2003;98:720–729. doi: 10.1002/cncr.11560. [DOI] [PubMed] [Google Scholar]
- 85.Samuel S, et al. Neuropilin-2 mediated β-catenin signaling and survival in human gastro-intestinal cancer cell lines. PLoS ONE. 2011;6:e23208. doi: 10.1371/journal.pone.0023208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spannuth WA, et al. Functional significance of VEGFR-2 on ovarian cancer cells. Int J Cancer. 2009;124:1045–1053. doi: 10.1002/ijc.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 88.Wey JS, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–438. doi: 10.1002/cncr.21145. [DOI] [PubMed] [Google Scholar]
- 89.Wey JS, et al. Overexpression of neuropilin-1 promotes constitutive MAPK signalling and chemoresistance in pancreatic cancer cells. Br J Cancer. 2005;93:233–241. doi: 10.1038/sj.bjc.6602663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dallas NA, et al. Neuropilin-2-mediated tumor growth and angiogenesis in pancreatic adenocarcinoma. Clin Cancer Res. 2008;14:8052–8060. doi: 10.1158/1078-0432.CCR-08-1520. [DOI] [PubMed] [Google Scholar]
- 91.Itakura J, Ishiwata T, Shen B, Kornmann M, Korc M. Concomitant over-expression of vascular endothelial growth factor and its receptors in pancreatic cancer. Int J Cancer. 2000;85:27–34. doi: 10.1002/(sici)1097-0215(20000101)85:1<27::aid-ijc5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 92.Fan F, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–2653. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 93.Stanton MJ, et al. Autophagy control by the VEGF-C/NRP-2 axis in cancer and its implication for treatment resistance. Cancer Res. 2013;73:160–171. doi: 10.1158/0008-5472.CAN-11-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Philip B, Ito K, Moreno-Sanchez R, Ralph SJ. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis. 2013;34:1699–1707. doi: 10.1093/carcin/bgt209. [DOI] [PubMed] [Google Scholar]
- 96.Lathia JD, et al. Integrin α 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cariati M, et al. α-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int J Cancer. 2008;122:298–304. doi: 10.1002/ijc.23103. [DOI] [PubMed] [Google Scholar]
- 98.Liu S, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 7:682–693. doi: 10.1016/j.stem.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maru Y, Yamaguchi S, Shibuya M. Flt-1, a receptor for vascular endothelial growth factor, has transforming and morphogenic potentials. Oncogene. 1998;16:2585–2595. doi: 10.1038/sj.onc.1201786. [DOI] [PubMed] [Google Scholar]
- 101.Scheel C, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh RP, Franke K, Wielockx B. Hypoxia-mediated regulation of stem cell fate. High Alt Med Biol. 2012;13:162–168. doi: 10.1089/ham.2012.1043. [DOI] [PubMed] [Google Scholar]
- 104.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J Clin Oncol. 2012;30:4026–4034. doi: 10.1200/JCO.2012.41.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31:1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 107.Geretti E, et al. A mutated soluble neuropilin-2 B domain antagonizes vascular endothelial growth factor bioactivity and inhibits tumor progression. Mol Cancer Res. 2010;8:1063–1073. doi: 10.1158/1541-7786.MCR-10-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pan Q, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 109.Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized Phase III trial. J Clin Oncol. 2012;30:2119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 110.Jubb AM, et al. Impact of exploratory biomarkers on the treatment effect of bevacizumab in metastatic breast cancer. Clin Cancer Res. 2011;17:372–381. doi: 10.1158/1078-0432.CCR-10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caunt M, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–342. doi: 10.1016/j.ccr.2008.01.029. References 108 and 111 establish the efficacy of using function-blocking antibodies to inhibit NRP1 and NRP2 with minimal side effects. [DOI] [PubMed] [Google Scholar]
- 112.Liang WC, et al. Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J Mol Biol. 2007;366:815–829. doi: 10.1016/j.jmb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 113.Gray MJ, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008;100:109–120. doi: 10.1093/jnci/djm279. [DOI] [PubMed] [Google Scholar]
- 114.Nasarre C, et al. Peptide-based interference of the transmembrane domain of neuropilin-1 inhibits glioma growth in vivo. Oncogene. 2010;29:2381–2392. doi: 10.1038/onc.2010.9. [DOI] [PubMed] [Google Scholar]
- 115.Xin Y, et al. Anti-neuropilin-1 (MNRP1685A): unexpected pharmacokinetic differences across species, from preclinical models to humans. Pharm Res. 2012;29:2512–2521. doi: 10.1007/s11095-012-0781-x. [DOI] [PubMed] [Google Scholar]
- 116.Weekes CD, et al. A Phase 1b study for MNRP1685A(anti-NRP1) administered intravenously with bevacizumab with or without paclitaxel to patients with advanced solid tumors. J Clin Oncol Abstr. 2011;29:3050. References 115 and 116 raise concerns about using NRP-specific antibody therapy, and these studies show the need to develop more targeted approaches. [Google Scholar]
- 117.Roth L, et al. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene. 2012;31:3754–3763. doi: 10.1038/onc.2011.537. This study describes a novel approach to deliver drugs to tumour cells using NRPs. [DOI] [PubMed] [Google Scholar]
- 118.Heffelfinger SC, et al. Inhibition of VEGFR2 prevents DMBA-induced mammary tumor formation. Lab Invest. 2004;84:989–998. doi: 10.1038/labinvest.3700128. [DOI] [PubMed] [Google Scholar]
- 119.Adham SA, Sher I, Coomber BL. Molecular blockade of VEGFR2 in human epithelial ovarian carcinoma cells. Lab Invest. 2010;90:709–723. doi: 10.1038/labinvest.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wedam SB, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 121.Fukasawa M, Korc M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res. 2004;10:3327–3332. doi: 10.1158/1078-0432.CCR-03-0820. [DOI] [PubMed] [Google Scholar]
- 122.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. This study shows that conditional deletion of VEGFA in pancreatic carcinoma cells increased tumour invasiveness, which is a result that provides a note of caution because VEGFA loss should disrupt autocrine VEGF signalling and decrease tumour invasiveness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galdiero MR, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 124.Gaur P, et al. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin Cancer Res. 2009;15:6763–6770. doi: 10.1158/1078-0432.CCR-09-1810. [DOI] [PubMed] [Google Scholar]
- 125.Bender RJ, Mac Gabhann F. Expression of VEGF and semaphorin genes define subgroups of triple negative breast cancer. PLoS ONE. 2013;8:e61788. doi: 10.1371/journal.pone.0061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Driessens MH, et al. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol. 2001;11:339–344. doi: 10.1016/s0960-9822(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 127.Wong OG, et al. Plexin-B1 mutations in prostate cancer. Proc Natl Acad Sci USA. 2007;104:19040–19045. doi: 10.1073/pnas.0702544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Worzfeld T, et al. ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Invest. 2012;122:1296–1305. doi: 10.1172/JCI60568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xia G, et al. Expression and significance of vascular endothelial growth factor receptor 2 in bladder cancer. J Urol. 2006;175:1245–1252. doi: 10.1016/S0022-5347(05)00736-6. [DOI] [PubMed] [Google Scholar]
- 130.Sato K, et al. Expression of vascular endothelial growth factor gene and its receptor (flt-1) gene in urinary bladder cancer. Tohoku J Exp Med. 1998;185:173–184. doi: 10.1620/tjem.185.173. [DOI] [PubMed] [Google Scholar]
- 131.Hlobilkova A, et al. Analysis of VEGF, Flt-1, Flk-1, nestin and MMP-9 in relation to astrocytoma pathogenesis and progression. Neoplasma. 2009;56:284–290. doi: 10.4149/neo_2009_04_284. [DOI] [PubMed] [Google Scholar]
- 132.Knizetova P, et al. Autocrine regulation of glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle. 2008;7:2553–2561. doi: 10.4161/cc.7.16.6442. [DOI] [PubMed] [Google Scholar]
- 133.de Jong JS, van Diest PJ, van der Valk P, Baak JP. Expression of growth factors, growth inhibiting factors, and their receptors in invasive breast cancer. I: An inventory in search of autocrine and paracrine loops. J Pathol. 1998;184:44–52. doi: 10.1002/(SICI)1096-9896(199801)184:1<44::AID-PATH984>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 134.Mylona E, et al. The prognostic value of vascular endothelial growth factors (VEGFs)-A and -B and their receptor, VEGFR-1, in invasive breast carcinoma. Gynecol Oncol. 2007;104:557–563. doi: 10.1016/j.ygyno.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 135.Ghosh S, et al. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol. 2008;39:1835–1843. doi: 10.1016/j.humpath.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wei SC, et al. Placenta growth factor expression is correlated with survival of patients with colorectal cancer. Gut. 2005;54:666–672. doi: 10.1136/gut.2004.050831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ozdemir F, et al. The effects of VEGF and VEGFR-2 on survival in patients with gastric cancer. J Exp Clin Cancer Res. 2006;25:83–88. [PubMed] [Google Scholar]
- 138.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11:1434–1440. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 139.Riedel F, et al. Serum levels of vascular endothelial growth factor in patients with head and neck cancer. Eur Arch Otorhinolaryngol. 2000;257:332–336. doi: 10.1007/s004059900208. [DOI] [PubMed] [Google Scholar]
- 140.Carrillo de Santa Pau E, et al. Prognostic significance of the expression of vascular endothelial growth factors A, B, C, and D and their receptors R1, R2, and R3 in patients with nonsmall cell lung cancer. Cancer. 2009;115:1701–1712. doi: 10.1002/cncr.24193. [DOI] [PubMed] [Google Scholar]
- 141.Decaussin M, et al. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): correlation with angiogenesis and survival. J Pathol. 1999;188:369–377. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 142.Seto T, et al. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung Cancer. 2006;53:91–96. doi: 10.1016/j.lungcan.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 143.Lantuejoul S, et al. Expression of VEGF, semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. J Pathol. 2003;200:336–347. doi: 10.1002/path.1367. [DOI] [PubMed] [Google Scholar]
- 144.Strizzi L, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193:468–475. doi: 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 145.Padro T, et al. Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR-2) in the bone marrow of patients with acute myeloid leukemia. Leukemia. 2002;16:1302–1310. doi: 10.1038/sj.leu.2402534. [DOI] [PubMed] [Google Scholar]
- 146.Chen H, Ye D, Xie X, Chen B, Lu W. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol Oncol. 2004;94:630–635. doi: 10.1016/j.ygyno.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 147.Hall GH, et al. Neuropilin-1 and VEGF correlate with somatostatin expression and microvessel density in ovarian tumours. Int J Oncol. 2005;27:1283–1288. [PubMed] [Google Scholar]
- 148.von Marschall Z, et al. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology. 2000;119:1358–1372. doi: 10.1053/gast.2000.19578. [DOI] [PubMed] [Google Scholar]
- 149.Kollermann J, Helpap B. Expression of vascular endothelial growth factor (VEGF) and VEGF receptor Flk-1 in benign, premalignant, and malignant prostate tissue. Am J Clin Pathol. 2001;116:115–121. doi: 10.1309/1LBM-6X32-JH6W-ENUD. [DOI] [PubMed] [Google Scholar]
- 150.Latil A, et al. VEGF overexpression in clinically localized prostate tumors and neuropilin-1 overexpression in metastatic forms. Int J Cancer. 2000;89:167–171. doi: 10.1002/(sici)1097-0215(20000320)89:2<167::aid-ijc11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 151.Yacoub M, et al. Differential expression of the semaphorin 3A pathway in prostatic cancer. Histopathology. 2009;55:392–398. doi: 10.1111/j.1365-2559.2009.03406.x. [DOI] [PubMed] [Google Scholar]
- 152.Gunningham SP, et al. VEGF-B expression in human primary breast cancers is associated with lymph node metastasis but not angiogenesis. J Pathol. 2001;193:325–332. doi: 10.1002/path.814. [DOI] [PubMed] [Google Scholar]
- 153.Su JL, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 154.Van Trappen PO, et al. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different stages of cervical carcinogenesis. J Pathol. 2003;201:544–554. doi: 10.1002/path.1467. [DOI] [PubMed] [Google Scholar]
- 155.Hashimoto I, et al. Vascular endothelial growth factor-C expression and its relationship to pelvic lymph node status in invasive cervical cancer. Br J Cancer. 2001;85:93–97. doi: 10.1054/bjoc.2001.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Witte D, Thomas A, Ali N, Carlson N, Younes M. Expression of the vascular endothelial growth factor receptor-3 (VEGFR-3) and its ligand VEGF-C in human colorectal adenocarcinoma. Anticancer Res. 2002;22:1463–1466. [PubMed] [Google Scholar]
- 157.Akagi K, et al. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. 2000;83:887–891. doi: 10.1054/bjoc.2000.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yonemura Y, et al. Lymphangiogenesis and the vascular endothelial growth factor receptor (VEGFR)-3 in gastric cancer. Eur J Cancer. 2001;37:918–923. doi: 10.1016/s0959-8049(01)00015-6. [DOI] [PubMed] [Google Scholar]
- 159.Neuchrist C, et al. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck. 2003;25:464–474. doi: 10.1002/hed.10235. [DOI] [PubMed] [Google Scholar]
- 160.Kojima H, et al. Clinical significance of vascular endothelial growth factor-C and vascular endothelial growth factor receptor 3 in patients with T1 lung adenocarcinoma. Cancer. 2005;104:1668–1677. doi: 10.1002/cncr.21366. [DOI] [PubMed] [Google Scholar]
- 161.Juttner S, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24:228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- 162.Parr C, Watkins G, Boulton M, Cai J, Jiang WG. Placenta growth factor is over-expressed and has prognostic value in human breast cancer. Eur J Cancer. 2005;41:2819–2827. doi: 10.1016/j.ejca.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 163.Chen CN, et al. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004;213:73–82. doi: 10.1016/j.canlet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 164.Ho MC, et al. Placenta growth factor not vascular endothelial growth factor A or C can predict the early recurrence after radical resection of hepatocellular carcinoma. Cancer Lett. 2007;250:237–249. doi: 10.1016/j.canlet.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 165.Wu Y, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 166.Lalla RV, Boisoneau DS, Spiro JD, Kreutzer DL. Expression of vascular endothelial growth factor receptors on tumor cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:882–888. doi: 10.1001/archotol.129.8.882. [DOI] [PubMed] [Google Scholar]
- 167.Straume O, Akslen LA. Increased expression of VEGF-receptors (FLT-1, KDR, NRP-1) and thrombospondin-1 is associated with glomeruloid microvascular proliferation, an aggressive angiogenic phenotype, in malignant melanoma. Angiogenesis. 2003;6:295–301. doi: 10.1023/B:AGEN.0000029408.08638.aa. [DOI] [PubMed] [Google Scholar]
- 168.Gockel I, et al. Co-expression of receptor tyrosine kinases in esophageal adenocarcinoma and squamous cell cancer. Oncol Rep. 2008;20:845–850. [PubMed] [Google Scholar]
- 169.Jackson MW, et al. A potential autocrine role for vascular endothelial growth factor in prostate cancer. Cancer Res. 2002;62:854–859. [PubMed] [Google Scholar]
- 170.Nobusawa S, Stawski R, Kim YH, Nakazato Y, Ohgaki H. Amplification of the PDGFRA, KIT and KDR genes in glioblastoma: a population-based study. Neuropathology. 2011;31:583–588. doi: 10.1111/j.1440-1789.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- 171.Puputti M, et al. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res. 2006;4:927–934. doi: 10.1158/1541-7786.MCR-06-0085. [DOI] [PubMed] [Google Scholar]
- 172.Nakopoulou L, et al. Expression of the vascular endothelial growth factor receptor-2/Flk-1 in breast carcinomas: correlation with proliferation. Hum Pathol. 2002;33:863–870. doi: 10.1053/hupa.2002.126879. [DOI] [PubMed] [Google Scholar]
- 173.Longatto-Filho A, et al. Molecular characterization of EGFR, PDGFRA and VEGFR2 in cervical adenosquamous carcinoma. BMC Cancer. 2009;9:212. doi: 10.1186/1471-2407-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Giatromanolaki A, et al. Activated VEGFR2/KDR pathway in tumour cells and tumour associated vessels of colorectal cancer. Eur J Clin Invest. 2007;37:878–886. doi: 10.1111/j.1365-2362.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 175.Giatromanolaki A, et al. Phosphorylated KDR expression in endometrial cancer cells relates to HIF1α/VEGF pathway and unfavourable prognosis. Mod Pathol. 2006;19:701–707. doi: 10.1038/modpathol.3800579. [DOI] [PubMed] [Google Scholar]
- 176.Neuchrist C, et al. Vascular endothelial growth factor receptor 2 (VEGFR2) expression in squamous cell carcinomas of the head and neck. Laryngoscope. 2001;111:1834–1841. doi: 10.1097/00005537-200110000-00031. [DOI] [PubMed] [Google Scholar]
- 177.Huang J, et al. Prognostic significance and potential therapeutic target of VEGFR2 in hepatocellular carcinoma. J Clin Pathol. 2011;64:343–348. doi: 10.1136/jcp.2010.085142. [DOI] [PubMed] [Google Scholar]
- 178.Yang F, et al. Increased VEGFR-2 gene copy is associated with chemoresistance and shorter survival in patients with non-small-cell lung carcinoma who receive adjuvant chemotherapy. Cancer Res. 2011;71:5512–5521. doi: 10.1158/0008-5472.CAN-10-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Giatromanolaki A, et al. Hypoxia and activated VEGF/receptor pathway in multiple myeloma. Anticancer Res. 2010;30:2831–2836. [PubMed] [Google Scholar]
- 180.Badalian G, Derecskei K, Szendroi A, Szendroi M, Timar J. EGFR and VEGFR2 protein expressions in bone metastases of clear cell renal cancer. Anticancer Res. 2007;27:889–894. [PubMed] [Google Scholar]
- 181.Sato H, Takeda Y. VEGFR2 expression and relationship between tumor neovascularization and histologic characteristics in oral squamous cell carcinoma. J Oral Sci. 2009;51:551–557. doi: 10.2334/josnusd.51.551. [DOI] [PubMed] [Google Scholar]
- 182.Rodriguez-Antona C, et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr Relat Cancer. 2010;17:7–16. doi: 10.1677/ERC-08-0304. [DOI] [PubMed] [Google Scholar]
- 183.Broholm H, Laursen H. Vascular endothelial growth factor (VEGF) receptor neuropilin-1’s distribution in astrocytic tumors. APMIS. 2004;112:257–263. doi: 10.1111/j.1600-0463.2004.apm11204-0505.x. [DOI] [PubMed] [Google Scholar]
- 184.Ding H, et al. Expression and regulation of neuropilin-1 in human astrocytomas. Int J Cancer. 2000;88:584–592. doi: 10.1002/1097-0215(20001115)88:4<584::aid-ijc11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 185.Jubb AM, et al. Neuropilin-1 expression in cancer and development. J Pathol. 2012;226:50–60. doi: 10.1002/path.2989. [DOI] [PubMed] [Google Scholar]
- 186.Staton CA, et al. Expression of class 3 semaphorins and their receptors in human breast neoplasia. Histopathology. 2011;59:274–282. doi: 10.1111/j.1365-2559.2011.03922.x. [DOI] [PubMed] [Google Scholar]
- 187.Ochiumi T, et al. Neuropilin-1 is involved in regulation of apoptosis and migration of human colon cancer. Int J Oncol. 2006;29:105–116. [PubMed] [Google Scholar]
- 188.Hansel DE, et al. Expression of neuropilin-1 in high-grade dysplasia, invasive cancer, and metastases of the human gastrointestinal tract. Am J Surg Pathol. 2004;28:347–356. doi: 10.1097/00000478-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 189.Kawakami T, et al. Neuropilin 1 and neuropilin 2 co-expression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer. 2002;95:2196–2201. doi: 10.1002/cncr.10936. [DOI] [PubMed] [Google Scholar]
- 190.Osada R, et al. Expression of semaphorins, vascular endothelial growth factor, and their common receptor neuropilins and alleic loss of semaphorin locus in epithelial ovarian neoplasms: increased ratio of vascular endothelial growth factor to semaphorin is a poor prognostic factor in ovarian carcinomas. Hum Pathol. 2006;37:1414–1425. doi: 10.1016/j.humpath.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 191.Baba T, et al. Neuropilin-1 promotes unlimited growth of ovarian cancer by evading contact inhibition. Gynecol Oncol. 2007;105:703–711. doi: 10.1016/j.ygyno.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 192.Muller MW, et al. Association of axon guidance factor semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer. 2007;121:2421–2433. doi: 10.1002/ijc.22949. [DOI] [PubMed] [Google Scholar]
- 193.Li M, et al. Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer. 2004;101:2341–2350. doi: 10.1002/cncr.20634. [DOI] [PubMed] [Google Scholar]
- 194.Fukahi K, Fukasawa M, Neufeld G, Itakura J, Korc M. Aberrant expression of neuropilin-1 and -2 in human pancreatic cancer cells. Clin Cancer Res. 2004;10:581–590. doi: 10.1158/1078-0432.ccr-0930-03. [DOI] [PubMed] [Google Scholar]
- 195.Vanveldhuizen PJ, et al. Differential expression of neuropilin-1 in malignant and benign prostatic stromal tissue. Oncol Rep. 2003;10:1067–1071. [PubMed] [Google Scholar]
- 196.Sanchez-Carbayo M, et al. Gene discovery in bladder cancer progression using cDNA microarrays. Am J Pathol. 2003;163:505–516. doi: 10.1016/S0002-9440(10)63679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jubb AM, et al. Neuropilin-2 expression in cancer. Histopathology. 2012;61:340–349. doi: 10.1111/j.1365-2559.2012.04224.x. [DOI] [PubMed] [Google Scholar]
- 198.Yasuoka H, et al. Neuropilin-2 expression in breast cancer: correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer. 2009;9:220. doi: 10.1186/1471-2407-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rushing EC, et al. Neuropilin-2: a novel biomarker for malignant melanoma? Hum Pathol. 2012;43:381–389. doi: 10.1016/j.humpath.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cao Y, et al. Neuropilin-2 promotes extravasation and metastasis by interacting with endothelial α5 integrin. Cancer Res. 2013;73:4579–4590. doi: 10.1158/0008-5472.CAN-13-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]