Abstract
Hematopoietic stem cell populations require DNA repair pathways to maintain their long term survival and reconstitution capabilities, but mediators of these processes are still being elucidated. Exonuclease1 (Exo1) participates in homologous recombination (HR) and Exo1 loss results in impaired 5’ HR end resection. We use cultured Exo1mut fibroblasts and bone marrow to demonstrate that loss of Exo1 function results in defective HR in cycling cells. Conversely in Exo1mut mice HR is not required for maintenance of quiescent HSCs at steady state, confirming the steady state HSC reliance on non-homologous end joining (NHEJ). Exo1mut mice sustained serial repopulation, displayed no defect in competitive repopulation or niche occupancy, and exhibited no increased sensitivity to whole body ionizing radiation. However when Exo1mut HSCs were pushed into cell cycle in vivo with 5-Fluorouracil or poly IC, the hematopoietic population became hypersensitive to IR, resulting in HSC defects and animal death. We propose Exo1 mediated HR is dispensable for stem cell function in quiescent HSC, whereas it is essential to HSC response to DNA damage processing after cell cycle entry, and its loss is not compensated by intact NHEJ. In HSCs the maintenance of stem cell function after DNA damage is dependent on the DNA repair capacity, segregated by active vs. quiescent points in cell cycle.
Keywords: Hematopoietic stem cells, DNA repair, cell cycle, homologous recombination
Introduction
Hematopoietic stem cell (HSC) maintenance is essential for sustained longevity and tissue function. The HSC population has lifelong self-renewing capabilities and gives rise to subsets of multipotent progenitor cells, and in turn a progeny of terminally differentiated mature cells consisting of all subtypes of the myeloid and lymphoid lineages. Long term reconstituting HSCs are necessary to replace the differentiated cells after losses caused by normal cell turnover or environmental stress such as infection, radiation, or chemotherapy[1, 2] . Failure to replenish these stores has been linked to a variety of hematopoietic disorders in humans as well as aging phenotypes [3-5].
Under steady state conditions the majority of the HSC population (>95%) is believed to be in a quiescent state with 1-2% turnover a day [6-8]. HSCs remain in the G0 phase of the cell cycle in order to minimize the potential for genomic instability, and only enter the cell cycle when necessary to replenish the hematopoietic system [9]. Therapeutic intervention and environmental stresses can mobilize HSCs into cycle where they remain until homeostasis is restored in the circulating blood cell populations. [6, 10] Since HSCs are required throughout the lifetime of an organism for blood repopulation, mutation and damage avoidance is crucial, and thus the bridge between quiescence and cycling must be finely monitored [11].
The DNA Damage Response (DDR) system is a series of highly conserved pathways that function to repair damaged DNA in cells. The damage can range from single strand breaks (SSB) and double strand breaks (DSB), mispaired bases, or other genomic lesions such as thymine dimers or depurinated bases[12, 13]. Perhaps the most dangerous lesion in cells is the DSB which can be caused by exogenous factors, internal metabolic reaction products, and other sources including mechanical stress on the cell[14]. Two distinct pathways exist to repair DSBs in cells, homologous recombination (HR) and non-homologous end-joining (NHEJ). HR occurs only in cycling cells in the S or G2 phases because it requires a sister chromatid to serve as a homologous template for the damaged strand, resulting in high fidelity repair. NHEJ is a more error prone method to repair DSBs whose basic function is to ligate the two broken ends of the DNA [15] [16]. The interplay between HR and NHEJ is quite complex, with examples of inter-pathways negative regulation to promote proper choice of repair mechanism. [17]
In hematopoietic stem cells much remains to be understood about DDR processes after induction of damage. Loss of DSB repair function in DNA-PK (SCID), LigaseIV−/− mice, Ku80−/− mice, all components of NHEJ, and others has been shown to result in no change in overall stem cell numbers but instead a drastic decrease in stem cell function- characterized by loss of self-renewal capability, competitive repopulation defects, and increases in apoptosis and stem cell exhaustion[18-20]. These data suggest a fundamental role for DDR in HSC maintenance. However, only recently are the DDR pathways used in HSCs being elucidated. Mohrin et al (2010) used immunofluorescence studies in cultured HSCs to demonstrate that quiescent HSCs use NHEJ for DSB response while proliferating HSCs transition to a dual reliance on NHEJ and HR for repair. Thus HSCs appear to rely on NHEJ as the default repair mechanism under quiescent steady state conditions. However, when and how HSC convert to utilization of and perhaps reliance on HR either solely or complemented by NHEJ, has not been evaluated.
Here we have investigated the multifaceted enzyme Exonuclease 1 (Exo1) in the context of hematopoietic function. Exo1 has been implicated in the early 5’ end resection step of HR and loss of Exo1 has been shown to result in DNA DSB repair defects[21] [22, 23]. We used nuclease-dead Exo1mut mice[24] and showed at steady state that these mice displayed no defects in HSC function, including IR sensitivity, indicating that intact NHEJ can maintain quiescent HSCs. However when we pushed the HSC into cycle with 5-FU or poly IC we observed that Exo1mut mice demonstrated IR hypersensitivity which resulted in HSC dysfunction and animal death. Thus when HSCs transition from quiescence to active cell cycle the DNA repair of DSBs transitions from a dependence on NHEJ alone to a requirement for HR. This study suggests that in HSCs the point of greatest reliance on HR for DDR is the point at which HSC enter active cell cycle.
Materials and Methods
Animals
Exo1mut mice -C57BL/6J background- used in these studies were donated by Dr. Winfried Edelmann [24]. Animals were used along with WT littermates throughout. All mouse studies were approved by the institutional animal care and use committee at Case Western Reserve University.
Reporter Plasmid Assays
DR-GFP
The DR-GFP reporter construct was used as previously described by Pierce et al[25]. The plasmid was stably incorporated into WT and Exo1mut fibroblasts followed by transfection of the I-Sce1 endonuclease plasmid. Cytometric analysis was performed on these cells 48 hours after I-Sce1 to measure the levels of GFP fluorescence. Student's t-tests were used to determine statistical significance.
Pem1-Ad2-GFP
The NHEJ reporter construct Pem1-Ad2-GFP [26] was cut with the I-Sce1 endonuclease and subsequently transfected into WT and Exo1mut MEFs. 48 hours post transfection cytometric analysis was performed to determine levels of GFP fluorescence. Student's t-tests were used to determine statistical significance.
Immunostaining
Fibroblasts were grown on coverslips and treated with 4Gy of ionizing radiation. At the indicated time points, cells were fixed, incubated at 1:500 in primary phospho-histone H2AX antibody (Millipore- 05-636) and 1:500 for with secondary Alexa Fluor 488. Images were acquired with a fluorescent microscope with a 40X objective and analyzed in AIM Image Browser. Student's t-tests were used to determine statistical significance.
CFU Assay
Whole bone marrow was isolated from WT and Exo1mut mice and irradiated at varying doses of IR. Fifty thousand cells were plated in 3 cm2 plates coated with complete methylcellulose media containing IL3, IL6, SCF, Epo (Stem Cell Technologies M3434) and scored after 14 days. These conditions allow for outgrowth of GCU-GM, CFU-E, BFU-E and CFU-GEMM., Summation of differential CFU counts is reported. Student's t-tests were used to determine statistical significance.
Blood and Marrow Characterization Studies
Peripheral eye blood was extracted from mice, the red blood cells lysed, and cytometric analysis performed examining B220, CD3 and Mac1. To examine the SKL population WT and Exo1mut mice were sacrificed, bone marrow flushed from femurs and tibias, and cytometric analysis performed using sca1, c-kit, and lineage markers CD3,B220,CD11b,Ter119,Cd4 (BD Biosciences) on a BD LSRII cytometer (BD Biosciences). Data was analyzed on Flowjo Version 8.8 software (Treestar), [27]. Student's t-tests were used to determine statistical significance.
Competitive Repopulation Studies
One million whole bone marrow cells from 8-12 week WT or Exo1mut mice (CD45.2) were mixed with age matched WT mice (CD45.1) at a 1:1 ratio and injected via tail vein into lethally irradiated CD45.1 recipients. At eight and sixteen weeks post transplant the mouse chimerism was measured via peripheral blood measuring CD45.1/CD45.2 as well as surface markers B220, Mac1, and CD3 on a BD LSRII cytometer[28]. Student's t-tests were used to determine statistical significance.
Serial Transplant Studies
Recipient BoyJ mice (CD45.1) were lethally irradiated (11Gy) and 16 hours later 2 million WT or Exo1mut (CD45.2) whole bone marrow cells were injected via tail vein. 16 weeks post transplant these mice were sacrificed, bone marrow flushed and 2 million cells were subsequently transplanted into the next batch of lethally irradiated BoyJ mice for 4 rounds of transplant [29].
Niche Occupancy Assay
HSC niche occupancy retention was measured by injecting 5 million whole bone marrow cells from WT 8-12 week old CD45.1 mice into non myeloablated WT or Exo1mut CD45.2 mice. At 8 and 16 weeks post transplant peripheral eye blood was obtained from recipient mice, stained for CD45.1/CD45.2, B220, Mac1, and CD3 and analyzed on a BD LSR II [27]. Student's t-tests were used to determine statistical significance.
5-FU Mobilization Studies
WT and Exo1mut mice were IP injected with 150 mg/kg 5-FU (Sigma). To measure BrdU incorporation 1mg/mouse BrdU (Sigma-Aldrich) was IP injected into the mice 4 days after 5-FU treatment. 16 hours after 5-FU treatment the mice were sacrificed, marrow was flushed from the femurs and tibias, and stained for SKL and BRDU. The percent of BRDU+ SKL cells was measured using a BD LSRII [30].
Poly(I:C) Studies
Mice were treated twice with 10ug/g poly(i:c) (Invivogen) separated by 48 hours. After another 48 hours the mice were treated with 6.5Gy IR and the assays described previously were performed on these treated mice.
Results
Exo1mut Fibroblasts Display DNA DSB Repair Defects
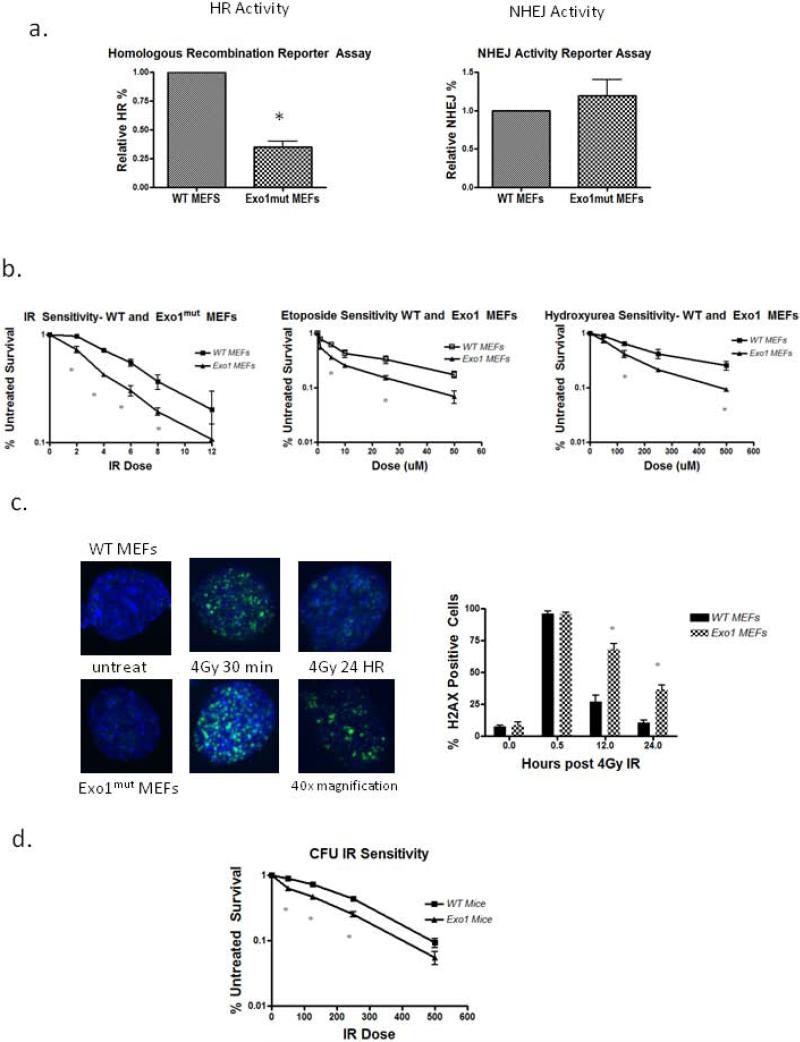
We used Exo1mut mice [24] to characterize the DSB response. These mice contain nuclease dead Exo1 but possess a slightly truncated form of the full length protein. Exo1mut mice have been characterized as displaying MMR defects, increased cancer susceptibility, and sterility [24]. Murine embryonic fibroblasts (MEFs) were derived from gestation day 13.5 females and DSB repair proficiency was measured by reporter plasmids. Homologous recombination proficiency was determined using the DR GFP plasmid [25] in which a double strand break is introduced through expression of the I-SCE1 endonuclease with functional gene conversion resulting in GFP expression. Exo1mut MEFs displayed a marked decrease in HR-reporter activity when compared to WT MEFs indicating an impaired HR pathway (Figure 1a, p<0.05), a link previously established by Exo1 depletion in MCF7 cells [21]. Conversely, using the nonhomologous end joining reporter plasmid pEGFP-Pem1-Ad2 [26] in an assay in which this plasmid is linearized by HINDIII digestion such that upon recircularization via end joining GFP is detected, we found that Exo1mut MEFs had normal NHEJ activity (Figure 1a, p>0.05). This confirmed that the DSB repair defect in Exo1mut MEFs is HR pathway specific, which additional studies have demonstrated is due to impaired 5’→3’ resection and initiation of HR [23] [31] [32].
Figure 1. Exo1mut fibroblasts and hematopoietic progenitors display DSB repair defects.
(A) Reporter plasmid quantitation of DR-GFP (HR) and Pem1-Ad2-GFP (NHEJ) in MEFs. Flow cytometry data measured % GFP positive cells. (B) Drug survival studies in MEFs quantified using MTT assay. Error bars represent SEM. (C) Confocal images of γ-H2AX immunostaining. 50 cells per treatment were analyzed using 40x magnification for foci formation and the % positive was plotted. (D) Colony survival analysis of whole bone marrow. Numbers of colonies at each IR dose were counted 14 days post plating in methylcellulose. Error bars represent SEM. P values were calculated by 2-tailed student t test. * p<0.05 vs. WT.
To determine the functional repair defect in Exo1mut MEFs, we examined the response to ionizing radiation. Using MTT survival assays, we show that the Exo1mut fibroblasts displayed a ~2-fold increase in radiation sensitivity compared to WT MEFs. This sensitivity was also observed using additional DSB inducing agents etoposide and hydroxyurea (Figure 1b). Furthermore, we quantified γ-H2AX immunofluorescence staining as a measure of DSBs on WT and Exo1mut MEFs at various time points following irradiation and found that the Exo1mut cells contained persistent γ-H2AX foci after 24 hours (Figure 1c, p<0.05). Together these data suggest that the loss of Exo1 function in cells undergoing active proliferation results in a survival defect after DSB formation.
To compare the fibroblast response to the hematopoietic population, we used a colony forming unit (CFU) assay of unfractionated mouse bone marrow after radiation. Whole bone marrow was collected from WT and Exo1mut mice, treated with an IR dose range (0.5-5Gy) following which the cells were cultured in methylcellulose in the presence of cytokines, and colony forming unit (CFU) survival enumerated on day 14. This assay induces cell division of the HSC due to cytokine exposure, so the read out is of cytokine responsive proliferating progenitor cells [33]. We examined the ability of WT and Exo1mut marrow to produce multiple CFU types: CFU-GM, CFU-GEMM, and BFU-E. Exo1mut marrow had normal numbers of CFU. Since the distribution of CFU among types was not altered, and the impact affected all sublineages, we report the collective data. However, marrow CFU from these mice were more IR sensitive than WT marrow at all doses tested, indicating that when HSC progenitor cells are treated with IR and immediately subject to cytokine proliferative signals, the loss of Exo1 activity results in DNA repair defects and a survival disadvantage (p<.05, Figure 1d).
Exo1mut Mice Display Normal Marrow Characteristics
To understand the general effect that inactivation of Exo1 had on animal physiology and HSC maintenance, we measured body weight and blood counts of age matched WT and Exo1mut mice and found no change in weight (WT- 23.11 +/− 1.006g vs. Exomut - 22.22 +/− 1.152g) nor numbers of white blood cells (WT-8.2 +/− 0.35 K cells/ul vs. Exomut-7.5 +/− 0.59 K cells/ul), red blood cells (WT- 7.8 +/− 0.49 M cells/ul vs Exomut- 7.8 +/− 0.44 M cells/ul), or platelets (WT- 699 +/− 38.45 K cells/ul vs. Exomut- 697 +/− 34.86 K cells/ul). Additionally we observed no differences in total numbers of lineage-, Sca1+, Kit+ (SKL) or the more primitive SKL CD150+,CD48-(SLAM) HSCs in age matched 8-12 week old mice (p>0.05). We also found that levels of T-cells, B-cells and myeloid cells were unchanged in the Exo1mut mice, suggesting that the loss of Exo1 activity has no effect on steady state HSC progenitor multilineage differentiation (Supplemental 1, p>0.05). This apparently normal steady state hematopoietic phenotype appears much milder than FA, Rad50, or BRCA2 mouse models [34-36], which are associated with loss of HR.
Exo1mut Mice Display no Competitive Repopulation, Serial Transplant or Niche Occupancy Defects
To determine whether HSCs from Exo1mut mice had inherent stem cell defects we performed HSC engraftment and transplant studies. Competitive repopulation studies were performed with Exo1mut mice (these mice express the CD45.2 variant) to determine whether the loss of Exo1 function would lead to a defect in engraftment and reconstitution when directly competed against WT marrow from mice that express the CD45.1 variant of CD45. One million CD45.1 WT whole bone marrow cells and one million CD45.2 Exo1mut marrow cells from 8-12 week age matched mice were transplanted into lethally irradiated CD45.1 WT recipients. Chimerism was determined by flow cytometry of CD45.1 or CD45.2 marking in blood after 8 and 16 weeks[28]. Exo1 deficient marrow cells displayed no competitive repopulation defect when competed against marrow from WT mice after transplantation (Figure 2a, p>0.05). Since this tests the competency of resting and quiescent HSC, this suggested that there is no defect in the Exo1mut mice.
Figure 2. Exo1mut mice exhibit no defects in competitive repopulation, serial transplant, or niche occupancy.
(A) Representative FACS profiles. Competitive repopulation assay on WT and Exo1mut HSCs. 1*106 whole bone marrow cells from 8-12 week old Exo1mut mice (CD45.2) were mixed 1:1 with age-matched WT competitors (CD45.1) and transplanted via tail vein injection into lethally irradiated CD45.1 recipients. (B) Serial transplant results from WT and Exo1mut mice. Marrow was flushed from age matched CD45.2 mice and 2*106 cells were transplanted into lethally irradiated CD45.1 recipients sequentially. (C) Niche occupancy proficiency results 16 weeks post transplant via flow cytometry. P values were calculated by 2-tailed student t test. * p<0.05 vs. WT.
Long term HSC reconstitution and engraftment capabilities were measured via serial transplantation. Two million mouse bone marrow cells were injected into lethally irradiated WT recipients. Marrow from these mice was subsequently injected into a new set of lethally irradiated recipients sequentially at 16 week intervals until the mice can no longer survive – typically 4-6 passages for WT mice [37], a biologic marker of the HSC exhaustion. HSCs with intrinsic defects, specifically those with NHEJ or mismatch repair defects, lose the capability to reconstitute recipients after fewer rounds of serial transplantation [19, 29]. Exo1mut mice were able to repopulate recipient mice equivalent to WT mice after four rounds of transplantation (Figure 2b, p>0.05).
We also measured HSC hematopoietic niche occupancy of Exo1mut mice by performing transplantation assays into unconditioned recipient mice. NHEJ deficiency has been reported to lead to long term engraftment of WT donor cells into unconditioned NHEJ deficient mice [27], demonstrating an inherent defect in HSC hematopoietic niche occupancy. As reported, this assay identifies HSC that are not secure in the quiescent hematopoietic stem cell niche, allowing engraftment and long term hematopoiesis from the invading cell population to a far greater extent than observed in normal recipients. We found that Exo1mut mice, like WT mice, displayed no engraftment (< 0.5%) of WT donor marrow in the absence of conditioning (Figure 2c, p>0.05).
Exo1mut mice display no radiation sensitivity or competitive repopulation defect after IR treatment
Loss of Exo1 expression results in increased IR sensitivity in differentiated dividing cells [21] The HSC population at steady state is predominantly quiescent and thus reliant on NHEJ. Therefore, we predicted that Exo1 mediated loss of HR would not affect HSC IR sensitivity in otherwise unperturbed Exo1mut mice. This is distinctly different than the studies observed in Figure 1 that evaluated dividing hematopoietic CFU. Exo1mut and WT mice were treated with either a lethal (8Gy) or sublethal (4-6Gy) dose of IR and animal survival monitored over 6 months. We observed no survival differences between the two groups of mice in the dose range of 4-8Gy (Figure 3a). To specifically look at Exo1mut HSC IR sensitivity, we performed competitive repopulation transplantation of cells into lethally irradiated WT mice as described above. After initial reconstitution, the recipients were given an additional 6 Gy of ionizing radiation at 8 weeks post transplantation to challenge the donor HSCs in order to determine whether loss of Exo1 function impacted DSB repair of HSC after transplantation. At 1, 5, and 8 weeks following the radiation dose, cytometric analysis was performed on peripheral blood. We found that one week post IR treatment, the Exo1mut cells demonstrated decreased chimerism compared to WT (p<0.05) but this recovered by five and eight weeks post transplant to equal chimerism (Figure 3b). This suggests that the proliferating short term repopulating Exo1mut HSCs were IR sensitive whereas the quiescent long term repopulation cells were not dependent on Exo1 after irradiation.
Figure 3. Exo1mut HSCs at steady state are not more IR sensitive than WT HSCs.
(A) Age matched (8-12 week) WT and Exo1mut mice were treated with whole body IR and animal survival was monitored at 6 months post treatment. (B) WT and Exo1mut whole bone marrow was mixed 1:1 and injected into lethally irradiated CD45.1 recipients. 8 weeks post transplant the recipient mice were treated with 6Gy IR. Cytometric analysis was performed on peripheral blood 1,5, and 8 weeks following the IR treatment to determine percent chimerism.
Exo1mut mice Display Increased IR Sensitivity after HSC cell cycle entry
The CFU analysis and the competitive repopulation studies (Figures 1d and 2a) suggest that Exo1 is dispensable for radiation survival at steady state but needed for mediating HR after irradiation induced DSB. Because HSCs normally reside in a quiescent state, it is not surprising that they rely on NHEJ, and that Exo1 and HR function cannot be elucidated. However, we hypothesized that HSCs in active cell cycle would be dependent on Exo1 and intact HR after IR. We wanted to determine whether NHEJ would complement for loss of Exo1 and HR or whether Exo1 and HR were essential after DSB for maintenance of HSC function. If HR is required for DSB repair in cycling HSCs, Exo1 deficiency should result in increased IR sensitivity. To test the role of Exo1 in DSB repair for cycling cells, we induced the HSC population from quiescent to cycling in vivo using the chemotherapeutic agent 5-fluorouracil, and 5 days later, at a point of active cell cycle, treated these mice with whole body radiation. 5-FU treatment forces the quiescent HSC population to enter cell cycle 4-6 days after treatment in order to replenish the depleted hematopoietic pool, leading to reconstitution through self-replication. [38-40] Under these conditions cells without functional HR could become susceptible to exogenous IR stress and might rely on NHEJ which, if sufficient, would compensate for loss of HR.
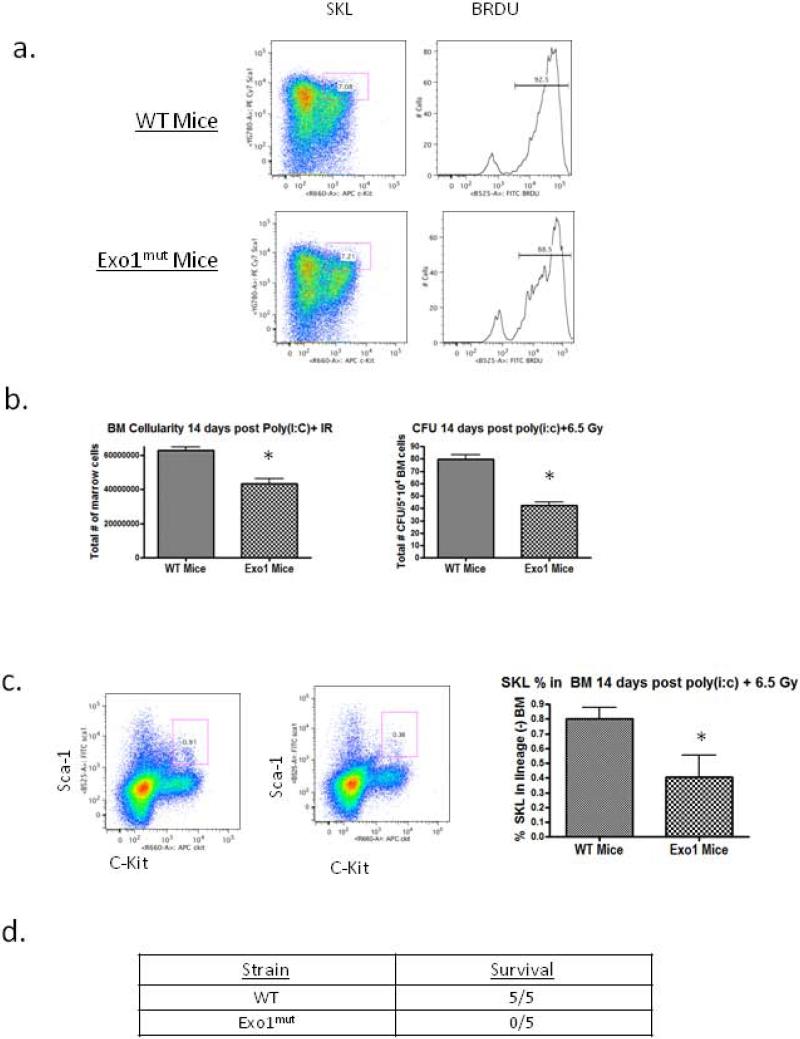
To confirm that 5-FU induced a damage mediated proliferative response in HSC in vivo, we treated mice with 5-FU alone and measured proliferation via incorporation of the synthetic thymidine analog BrdU in the SKL (lin-/Sca1+/c-Kit+) progenitor population. Five days post 150mg/kg 5-FU treatment, bone marrow was harvested from WT and Exo1mut mice and the percent of BrdU+ SKL cells was measured. We found that in both mouse strains the percentage of cycling BrdU+ SKL cells increases approximately 2.5 fold (Supplemental 2). We also showed that a single dose of 150 mg/kg 5-FU was not more toxic to Exo1mut mice than to WT (Supplemental 2).
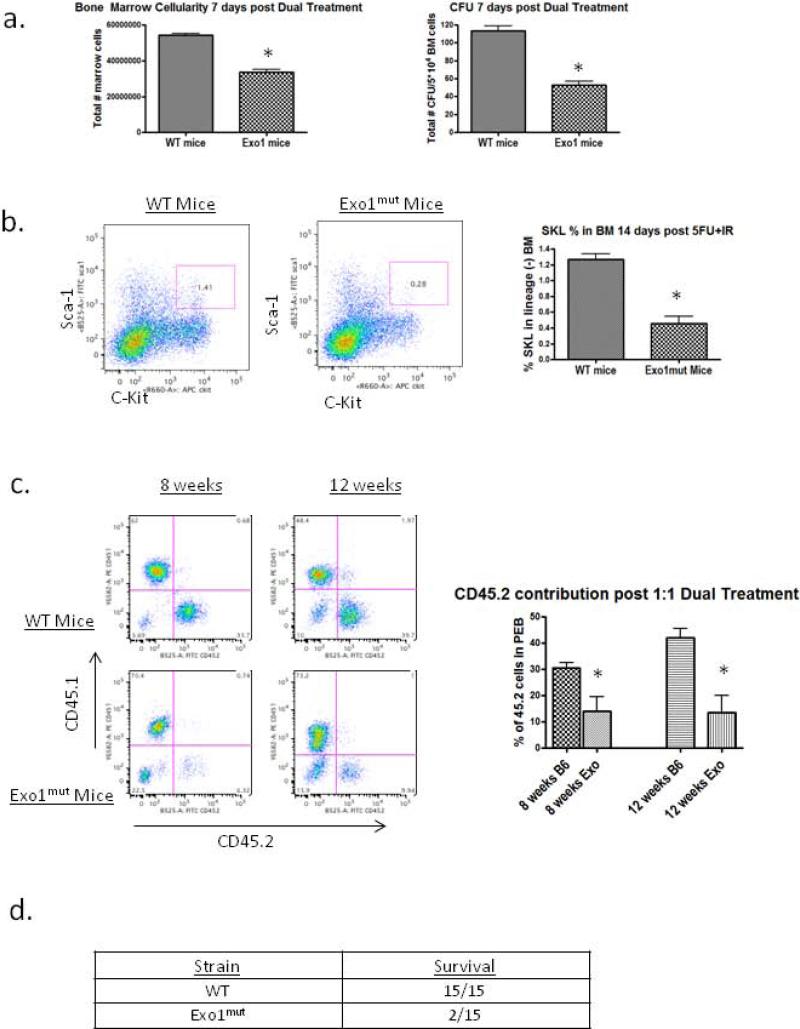
We asked whether cycling HSCs become dependent on Exo1 for survival following IR. WT and Exo1mut mice were injected with 150 mg/kg 5-FU IP, and 5 days later were treated with 4 Gy IR. We measured bone marrow cellularity and colony forming unit potential of both groups seven days following the second treatment. Bone marrow cell counts in Exo1mut mice contained approximately 40% fewer total bone marrow cells than their WT counterparts (Figure 5a). Whole bone marrow cell preparations from these mice were plated in methylcellulose containing cytokines and the number of colonies formed was counted at 14 days. A drop in each of the major classes of CFU were observed, so only composite CFU data are reported. Marrow cells from Exo1mut mice formed ~50% fewer CFU colonies than those from WT mice (Figure 4a). This demonstrated that HSCs from Exo1mut mice exhibited more severe toxicity compared to WT marrow HSC.
Figure 5. Poly IC mediated cell cycle entry also results in HSC IR hypersensitivity in Exo1mut mice only.
(A) WT and Exo1mut mice were treated twice with 10 ug/g poly(i:c) separated by 48 hours. 48 hours following the second treatment the percentBRDU+SKL cells were recorded via flow cytometry of whole bone marrow. (B) WT and Exo1mut mice were treated with poly(i:c) followed by 6.5 Gy IR. Fourteen days post treatment 3 mice from each group were sacrificed and bone marrow cellularity and CFU counts were measured. Error bars represent SEM. (C) WT and Exo1mut mice were treated with poly(i:c)+6.5Gy and fourteen days post treatment 3 mice were sacrificed and flow cytometry was performed on whole bone marrow to determine SKL numbers. Error bars represent SEM. (D) Numbers of WT and Exo1mut mice that survived the poly(i:c) + IR combination treatment two months post treatment. P values were calculated by 2-tailed student t test. * p<0.05 vs. WT.
Figure 4. Exo1mut mice become IR sensitive after 5-FU induced HSC cell cycle entry.
(A) Age matched WT and Exo1mut mice were treated with 150 mg/kg 5-FU followed by 4Gy IR 5 days post 5-FU. Seven days post dual treatment 3 mice from each group were sacrificed and bone marrow cellularity and CFU counts were measured. Error bars represent SEM. (B) WT and Exo1mut mice were treated with the combination of 5-FU and IR and 14 days post treatment 3 mice were sacrificed and flow cytometry was performed on whole bone marrow to determine SKL numbers. Error bars represent SEM. (C) Dual treated WT and Exo1mut whole marrow were mixed 1:1 and injected into lethally irradiated recipients. Animal chimerism was measured using CD45.1/2 flow cytometry 8 weeks post transplant. (D) Numbers of WT and Exo1mut mice that survived the 5-FU + IR combination treatment two months post treatment. P values were calculated by 2-tailed student t test. * p<0.05 vs. WT.
We measured the concentration and total numbers of SKL cells in mice 14 days after the dual treatment and showed that while WT mice were able to restore normal levels of SKL cells in their bone marrow, Exo1mut mice demonstrate a significant decrease in SKL levels 14 days post treatment (p<0.05). Thus, both SKL and CFU recovery after sequential 5-FU and irradiation in Exo1mut mice indicate a significant loss of HSC at short term time points (Figure 4b).
To determine if the HSCs in WT or Exo1mut mice differed in long term repopulation function after sequential treatment, we collected whole bone marrow from WT and Exo1mut mice 14 days after the 5-FU+ 4 Gy IR, and performed competitive repopulation assays at a 1:1 ratio of dual treated marrow using CD45.1/CD45.2 to distinguish the populations. We observed that Exo1mut marrow was significantly impaired in repopulating capability 8 weeks post transplant compared to WT marrow (Figure 4c). This confirmed the observations showing that the combination of 5-FU+IR was more toxic to Exo1mut marrow than to WT marrow HSC. In additional experiments, we observed that 13 of 15 Exo1mut mice died within 6 weeks of the combination 5-FU+IR treatment while none of the WT mice died (Figure 4d). Thus both short term and long term HSC function during and after irradiation induced DSBs is impaired with loss of Exo1 activity. Further, intact NHEJ is not able to compensate for loss of HR in cycling HSCs.
Since 5-FU may induce DNA damage that is impaired by Exo1 loss of function, we confirmed these results using an additional agent that could induce HSC into cell cycle prior to irradiation. WT and Exo1mut mice were treated with the double stranded RNA mimetic poly IC which has been shown to promote the proliferation of dormant HSCs in vivo [41]. We confirmed this proliferation induction in WT and Exo1mut mice by treating twice with poly IC and measuring BRDU incorporation 2 days after treatment (Figure 5a). We treated mice with poly IC followed by 6.5 Gy IR and observed that Exo1mut mice displayed significantly reduced bone marrow cellularity, CFU formation, and SKL compared to WT marrow (Figure 5b,c, p<0.05). Additionally, all Exo1mut mice treated in this manner died within 6 weeks of treatment while all WT mice survived the poly IC + IR (Figure 5d). These data confirm that HSC proliferation results in an increased reliance on Exo1-mediated HR for repair of DSBs. Further, it indicates that proliferating HSC cannot compensate for loss of HR by using NHEJ alone for DSB repair.
Discussion
These results indicate that lack of fully functional HR, mediated by Exo1mut, in HSCs leads to a proliferation-dependent sensitivity to irradiation induced DSB even though such sensitivity is not observed in steady state. The IR hypersensitivity is uncovered by treating mice with 5-FU to induce death of proliferating hematopoietic progenitor cells or by poly IC, both of which induce HSC into cell cycle. In mice containing the Exo1 mutation, these cycling HSCs lack fully competent HR, cannot repair DSBs by that mechanism, are not compensated in function by NHEJ, and lose repopulating function and marrow regenerating capacity. However, this functional HSC defect is not observed under steady state conditions. As a consequence, mice treated under conditions of HSC proliferation die of marrow hypoplasia and the residual marrow is not capable of competitive repopulation. Although others have shown that HSC utilize both HR and NHEJ in response to DSBs after IR, our data indicate that intact NHEJ is insufficient to compensate for loss of HR in DSB repair among proliferating HSCs.
There are a number of novel findings in this study. First, we noted normal hematopoietic function of the Exo1mut mouse under steady state conditions and under conditions of physiologic proliferation during transplantation and competitive repopulation. In the absence of exogenous DNA damage, HSC function was normal. We showed that under quiescent steady state conditions Exo1mut mice displayed no characteristics of stem cell failure as measured by competitive repopulation, serial transplant, and niche occupancy. Second, Exo1mut HSCs response to IR induced DSB was normal in vivo. Most likely this indicates that DSB repair in quiescent HSC is mediated by intact NHEJ.
Third, our work further clarifies the link between DNA repair dependence and cell cycle status of HSCs. When Exo1mut hematopoietic progenitors are exposed to irradiation in vitro and then assayed in the presence of cytokines for CFU, a defect is seen. Further, when HSC of these Exo1mut mice are forced into cell cycle by 5-FU (Poly IC, or likely other sequentially administered chemotherapy), the DSB repair defects results in significant loss of HSC function. What appears to be a normal response to a single irradiation dose in HSC without HR is severely compromised when HSC are proliferating. In addition to the HSC population, the hematopoietic failure after cell cycle activation may be caused by damage to multiple cycling populations including myeloid and lymphoid progenitors. Since hypoplastic hematopoiesis leading to animal death is the essence of inadequate marrow reserve, the susceptibility observed in Exomut mice reflects a combined impact of loss of HR on HSC and progenitor cell death.
These results model in vivo the switch between sole NHEJ reliance in HSCs to a preferred reliance on HR during proliferation. Utilizing the HR impaired Exo1mut mice allowed us to contrast our data with those of the well established NHEJ mouse models (SCID, KU80, KU70) which demonstrate steady state HSC DNA repair defects, hematopoietic stem cell deficiency and irradiation hypersensivitity. The results clarify in greater detail the relationship between cell cycle status and DNA repair dependency, and demonstrate that HR becomes essential for DSB processing after HSC cell cycle entry. This in vivo model of pushing dormant HSCs into cycle is a novel way of clarifying how DNA repair shifts along with cell cycle status in HSCs and could potentially be used to study how other DNA repair pathways are altered by cell cycle as well. Additionally this data may have an impact in the field of leukemic and other cancer stem cells, another population believed to live between states of quiescence and active cell cycle and it may be interesting to determine how cell cycle entry in this population affects DNA repair reliance.
At steady state, long term HSCs are not completely quiescent but instead have small proportions of cells entering cell cycle over a 24 hour period giving rise to low population cycling rates [6] [42] [43]. Our study examines a point in time radiation exposure and observes a shift in DNA repair reliance from solely NHEJ for the quiescent population to HR dependency when there is a rapid shift of the HSC population from quiescence into cell cycle. This study design simply shifts the HSC population from largely quiescent to one with a sizable proportion in cell cycle, uncovering this rather significant sensitivity to DSB in the absence of functional HR. This is also likely to occur for the population in cell cycle under steady state conditions, albeit at an inconsequential frequency. Alternatively, the DSB stress response of IR, known to be coupled with induction of stimulatory cytokines, may shorten the cell cycle time for individual stem and progenitor cells, increasing their dependency on HR.
Schaetzlein et. al have recently described the derivation of an Exo1null mouse which has a very similar DSB response phenotype to that of the Exo1mut mouse used in these studies. In that report, the authors propose that the nuclease function of Exo1 is important for its role in end resection of DSBs but appears dispensable for its roles in DNA mismatch repair and class switch recombination [44]. Thus because our studies focus on the DSB response of Exo1mut HSCs, we would anticipate that the Exo1null mouse would have a similar IR response phenotype to the Exo1mut mouse.
Other pathways have also linked loss of HR with HSC dysfunction. Studies of mutations in a BRCA2/FANCD1 mutant mouse model have demonstrated defects in both HSC repopulation and proliferation capabilities. As is currently understood the core nuclear FA complex responds to DNA damage by monoubiquitinating FANC1 and FANCD2, this subsequent complex interacts with other downstream proteins including BRCA2 and RAD51C[17]. The effect of these interactions is still being elucidated but it is believed that the FA activation results in enhancement of HR. While BRCA2 is a well established HR protein, it also has been implicated in the Fanconi anemia (FA) pathway and the HSC phenotype is similar to those previously described in FA mouse models including Fancc−/− and Fancd2−/− mice. [45] [34] The Fancc−/− and the Fancd2−/− mice exhibit a loss of HSC quiescence (different than the more mild phenotype of Exo1mut mice), with both models displaying significantly higher percentages of HSCs in cell cycle. The BRCA1/Fancd1 mice have been shown to allow engraftment of donor HSCs in the absence of any preconditioning, again suggesting a loss of quiescence [36]. We have shown that loss of quiescence results in a transition to greater HR dependence in the cycling HSCs, similar to that observed in FA mice and humans with FA disorders. As we have shown in the Exo1mut mouse, increased cycling HSCs with compromised HR results in HSC defects especially in response to exogenous stress. The BRCA2/FANCD1 mice demonstrated a severe IR sensitivity, dying within 5-9 days of 7Gy IR, potentially due to the combined effect of quiescence loss and impaired HR in the cycling HSCs. Thus, the fanconi anemia hematopoietic phenotype can be partially explained by an increase in cycling HSCs that are HR dependent but also HR impaired, and thus unable to repair damage from exogenous stress due to their cell cycle status and DNA DSB reliance.
Exo1 polymorphisms have been associated with multiple types of human cancers including gastric, breast, and lung[46-48]. While conclusive evidence linking Exo1 polymorphisms to disease pathogenesis has yet to be uncovered, our work also suggests that patients containing these polymorphisms may be susceptible to HSC toxicity from chemotherapy and irradiation. For instance sequential doses of IR may result in HSC activation and subsequent hypersensitivity due to loss of Exo1 function. Studies involving these patients and their sensitivity to chemotherapy may yield new evidence of HSC activation and chemosensitivity in humans with Exo1 or other HR DNA repair gene polymorphisms.
These observations raise new questions. Does a defect in NHEJ also have differential effects among quiescent vs. proliferating cells? Can counter measures to retain HSC in quiescence reduce or eliminate the DSB repair defect or at least its impact on robust HSC function? Does this cycling dependent defect explain other hypersensitivity genetic defects of DNA repair? Further, are there human defects in these pathways that give rise to DSB hypersensitivity, marrow failure and leukemic transformation? These studies provide a clear teleological rationale for maintaining stem cell populations in quiescence and point to the feedback and complementation mechanisms to protect from forced induction of proliferation, especially after DNA damage.
Supplementary Material
Supplemental Figure 1:
(A) Animal weight of age matched (8-12 week) mice. Error bars represent SEM. (B) Peripheral eye blood counts were recorded from five age matched mice. (C) Representative FACs profiles of whole bone marrow pregated for lineage negative cells. The Sca1+,C-Kit+ (SKL) cells and the SKL CD48-,CD150+ (SLAM) cells are represented. (D) Flow cytometry of peripheral eye blood stained for CD3,B220 and Mac1 cells from B6 WT and Exo1mut mice.
Supplemental Figure 2:
(A) WT and Exo1mut mice were treated with 150 mg/kg 5-FU and the percent BRDU+ SKL cells were recorded 5 days after treatment via flow cytometry of whole bone marrow. (B) SKL levels of WT and Exo1mut mice 14 days following either 150 mg/kg 5-FU or 4Gy IR only. Number of animals alive 3 months post either treatment is displayed.
Acknowledgements
Grant Support
This work was supported by the Cytometry & Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center and the Radiation Resources Core Facility of the Case Comprehensive Cancer Center (2P30 CA043703-23 (Gerson, PI)). It was also supported by National Institutes of health grant 5R42 CA128269-02 (Gerson, PI) and molecular therapeutics grant 5T32GM008803-10.
Footnotes
Authorship Contributions
A.D. designed and performed the experiments, analyzed the data and wrote the manuscript with help from S.L.G. Y.Q. helped design some experiments and provided necessary reagents for others. S.L.G. and Y.Q. provided helpful discussions. S.L.G. designed the study and supervised the overall project.
Disclosure of Conlicts of Interest:
The authors declare that there are no conflicts of interest.
References
- 1.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–68. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lombard DB, et al. DNA repair, genome stability, and aging. Cell. 2005;120(4):497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Mimeault M, Batra SK. Concise review: recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24(11):2319–45. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Cheshier SH, et al. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96(6):3120–5. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J. Quiescence regulators for hematopoietic stem cell. Exp Hematol. doi: 10.1016/j.exphem.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Takizawa H, et al. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 208(2):273–84. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 10.Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 10(3):201–9. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- 11.Pietras EM, Warr MR, Passegue E. Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 195(5):709–20. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286(5446):1897–905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 13.Wood RD, et al. Human DNA repair genes. Science. 2001;291(5507):1284–9. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 14.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23(5):687–96. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 15.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27(3):247–54. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 16.Sancar A, et al. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 17.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431(7011):997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 19.Nijnik A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447(7145):686–90. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 20.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–9. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 21.Bolderson E, et al. Involvement of Exo1b in DNA damage-induced apoptosis. Nucleic Acids Res. 2009;37(10):3452–63. doi: 10.1093/nar/gkp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolderson E, et al. Phosphorylation of Exo1 modulates homologous recombination repair of DNA double-strand breaks. Nucleic Acids Res. 38(6):1821–31. doi: 10.1093/nar/gkp1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimonkar AV, et al. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 25(4):350–62. doi: 10.1101/gad.2003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei K, et al. Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 2003;17(5):603–14. doi: 10.1101/gad.1060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce AJ, et al. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13(20):2633–8. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seluanov A, et al. DNA end joining becomes less efficient and more error-prone during cellular senescence. Proc Natl Acad Sci U S A. 2004;101(20):7624–9. doi: 10.1073/pnas.0400726101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qing Y, Lin Y, Gerson SL. An intrinsic BM hematopoietic niche occupancy defect of HSC in scid mice facilitates exogenous HSC engraftment. Blood. doi: 10.1182/blood-2011-05-350611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry JM, Li L. Functional assays for hematopoietic stem cell self-renewal. Methods Mol Biol. 636. :45–54. doi: 10.1007/978-1-60761-691-7_3. [DOI] [PubMed] [Google Scholar]
- 29.Reese JS, Liu L, Gerson SL. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood. 2003;102(5):1626–33. doi: 10.1182/blood-2002-10-3035. [DOI] [PubMed] [Google Scholar]
- 30.Hoggatt J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–55. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao S, Toczylowski T, Yan H. Mechanistic analysis of Xenopus EXO1's function in 5′-strand resection at DNA double-strand breaks. Nucleic Acids Res. 39(14):5967–77. doi: 10.1093/nar/gkr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomimatsu N, et al. Exo1 plays a major role in DNA end resection in humans and influences double-strand break repair and damage signaling decisions. DNA Repair (Amst) 11(4):441–8. doi: 10.1016/j.dnarep.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Os RP, Dethmers-Ausema B, de Haan G. In vitro assays for cobblestone area-forming cells, LTC-IC, and CFU-C. Methods Mol Biol. 2008;430:143–57. doi: 10.1007/978-1-59745-182-6_10. [DOI] [PubMed] [Google Scholar]
- 34.Zhang QS, et al. Fancd2−/− mice have hematopoietic defects that can be partially corrected by resveratrol. Blood. 116(24):5140–8. doi: 10.1182/blood-2010-04-278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales M, et al. The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor. Genes Dev. 2005;19(24):3043–54. doi: 10.1101/gad.1373705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro S, et al. Hematopoietic dysfunction in a mouse model for Fanconi anemia group D1. Mol Ther. 2006;14(4):525–35. doi: 10.1016/j.ymthe.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Srour EF, Yoder MC. Flow cytometric analysis of hematopoietic development. Methods Mol Med. 2005;105:65–80. doi: 10.1385/1-59259-826-9:065. [DOI] [PubMed] [Google Scholar]
- 38.Harrison DE, Lerner CP. Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5-fluorouracil. Blood. 1991;78(5):1237–40. [PubMed] [Google Scholar]
- 39.Kopp HG, et al. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–56. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 40.Rizzo S, et al. Quiescent (5-fluorouracil-resistant) aplastic anemia hematopoietic cells in vitro. Exp Hematol. 2004;32(7):665–72. doi: 10.1016/j.exphem.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Essers MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 42.Bradford GB, et al. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol. 1997;25(5):445–53. [PubMed] [Google Scholar]
- 43.Nygren JM, Bryder D. A novel assay to trace proliferation history in vivo reveals that enhanced divisional kinetics accompany loss of hematopoietic stem cell self-renewal. PLoS One. 2008;3(11):e3710. doi: 10.1371/journal.pone.0003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaetzlein S, et al. Mammalian Exo1 encodes both structural and catalytic functions that play distinct roles in essential biological processes. Proc Natl Acad Sci U S A. 110(27):E2470–9. doi: 10.1073/pnas.1308512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, et al. Fanconi anemia type C-deficient hematopoietic stem/progenitor cells exhibit aberrant cell cycle control. Blood. 2003;102(6):2081–4. doi: 10.1182/blood-2003-02-0536. [DOI] [PubMed] [Google Scholar]
- 46.Bau DT, et al. Single-nucleotide polymorphism of the Exo1 gene: association with gastric cancer susceptibility and interaction with smoking in Taiwan. Chin J Physiol. 2009;52(6):411–8. doi: 10.4077/cjp.2009.amh076. [DOI] [PubMed] [Google Scholar]
- 47.Jin G, et al. Potentially functional polymorphisms of EXO1 and risk of lung cancer in a Chinese population: A case-control analysis. Lung Cancer. 2008;60(3):340–6. doi: 10.1016/j.lungcan.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Wang HC, et al. Association of genetic polymorphisms of EXO1 gene with risk of breast cancer in Taiwan. Anticancer Res. 2009;29(10):3897–901. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1:
(A) Animal weight of age matched (8-12 week) mice. Error bars represent SEM. (B) Peripheral eye blood counts were recorded from five age matched mice. (C) Representative FACs profiles of whole bone marrow pregated for lineage negative cells. The Sca1+,C-Kit+ (SKL) cells and the SKL CD48-,CD150+ (SLAM) cells are represented. (D) Flow cytometry of peripheral eye blood stained for CD3,B220 and Mac1 cells from B6 WT and Exo1mut mice.
Supplemental Figure 2:
(A) WT and Exo1mut mice were treated with 150 mg/kg 5-FU and the percent BRDU+ SKL cells were recorded 5 days after treatment via flow cytometry of whole bone marrow. (B) SKL levels of WT and Exo1mut mice 14 days following either 150 mg/kg 5-FU or 4Gy IR only. Number of animals alive 3 months post either treatment is displayed.