Abstract
Purpose
To estimate the association between diabetes mellitus (DM) and all-cause mortality during tuberculosis (TB) treatment.
Methods
From 2009 to 2012 a retrospective cohort study among reported TB cases in Georgia was conducted. Patients aged ≥16 years were classified by DM and HIV status at time of TB diagnosis and followed during TB treatment to assess mortality. Hazard ratios (HR) were used to estimate the association between DM and death.
Results
Among 1,325 patients with TB disease, 151 (11.4%) had DM, 147 (11.1%) were HIV-infected, and 7 (0.5%) had both DM and HIV. Patients with TB-DM were more likely to have cavitary lung disease compared to those with TB alone (51.0% vs. 34.7%) and those with TB-HIV were more likely to have military/disseminated disease (12.9% vs. 3.4%) and resistance to rifampin or isoniazid (21.8% vs. 9.0%) compared with those without HIV infection (p<0.05). In multivariable analysis, DM was not associated with death during TB treatment (HR 1.22, 95% CI 0.70–2.12) or any death (aOR 1.05, 95% CI 0.60–1.84).
Conclusions
Among TB patients in Georgia, the prevalence of co-morbid DM and co-infection with HIV was nearly identical. In adjusted models, TB patients with DM did not have increased risk of all-cause mortality.
Keywords: Tuberculosis, Diabetes mellitus, Mortality, HIV, Georgia
INTRODUCTION
The incidence of active tuberculosis (TB) disease in the United States (US) has steadily declined during the past two decades from 26,673 reported TB cases (10.4 per 100,000) in 1992 to 9,951 reported cases (3.2 cases per 100,000) in 2012 [1]. Mortality among patients with TB in the US has also decreased. In 1992 the TB-related mortality rate was 0.7 per 100,000 and by 2011 had decreased to 0.2 per 100,000 [2]. Despite these decreases, subgroups of patients with TB remain at higher risk of death. Increased mortality has consistently been reported among those with multidrug-resistant (MDR) TB,[3–5] HIV co-infection,[6, 7] extrapulmonary TB,[8, 9] substance abuse,[10, 11] and concurrent chronic non-communicable diseases [12, 13]. Diabetes mellitus (DM), a non-communicable disease with a rapidly expanding prevalence in the US,[14] has been estimated to increase the risk of active TB disease approximately 3-fold [15] and may impact TB-related mortality [16]. A 2011 meta-analysis estimated that mortality was more likely among patients with TB and DM (TB-DM) compared to those without DM (unadjusted risk ratio [RR] 1.89, 95% CI 1.53–2.36) [17].
While the prevalence of DM among newly diagnosed patients with TB in the US is not well quantified a few regional studies have reported DM prevalence between 14 to 28% among US adults with TB [18–20]. The impact of DM on TB related mortality is incompletely understood and prior studies have generally not assessed the effect of DM on TB mortality in the US. The primary objectives of this study were: 1) to compare the demographic and clinical presentation characteristics of adult patients with TB and DM, TB and HIV, and TB without HIV or DM; 2) to estimate the association between DM and time until death during TB treatment. A secondary objective was to estimate the association between 1) DM and 2) HIV with any death (before or during TB treatment) among patients with TB.
METHODS
Setting and Participants
All TB cases reported between January 2009 and September 2012 in the state of Georgia, US, were included in this retrospective cohort study. In Georgia, all health care providers and laboratories are required by law to report clinical and laboratory confirmed TB cases to the Georgia Department of Public Health department (GDPH) [21]. Eligible study patients included all patients with pulmonary or extrapulmonary TB (diagnosed by positive culture or a combination of compatible clinical symptoms, radiological findings, and/or response to empiric therapy) aged ≥16 years reported to the Georgia state registry during the study period. TB cases were followed during TB treatment until the date of therapy completion, death, loss to follow-up, or until March 2013, whichever occurred first.
Study Measures and Data Collection
The GDPH verified reported TB cases, monitored patients on directly observed therapy (DOT), and was responsible for systematic collection of all patient information. Standardized TB reporting forms documented TB diagnosis, patient demographic and clinical characteristics, and treatment outcomes. All data was entered into the State Electronic Notifiable Disease Surveillance System (SendSS), a secure web-based software tool.
The primary study outcome was time until death, measured among patients who died from any cause during TB treatment. Patients with a date of death (determined from SendSS) prior to TB treatment completion date were defined as a death during TB treatment. Time to death was calculated as the number of days between TB treatment start and death date. Patients who died before initiating TB treatment were defined as death before TB treatment. Any death included patients that died during TB treatment or before TB treatment initiation.
The primary exposures of interest in this study were DM and HIV status. Medical records were reviewed to determine DM status. All patients were asked if they had ever been diagnosed with DM, patients who self-reported having DM were categorized as DM patients. Patients were not systematically screened for DM but had blood chemistry tests (including glucose) performed at the time of TB diagnosis and treatment initiation. Standard state TB protocols included offering all TB patients HIV screening with an Enzyme-Linked Immunoabsorbent Assay (ELISA) test, positive ELISA were confirmed by Western Blot. Patients with missing HIV results were classified as HIV negative.
Additional patient covariates of interest measured by interview in the cohort included demographic information, socio-behavioral characteristics, comorbidities, and clinical features. MDR TB was defined as resistance to both rifampin and isoniazid. Occupation, country of birth, history of homelessness, incarceration history, alcohol use in the past year, and drug use (injection or non-injection) were self-reported during patient interviews conducted by health care providers. End stage renal disease (ESRD) was determined from medical records and patient charts. Sputum acid fast bacilli (AFB) smear status, AFB culture for Mycobacterium tuberculosishistory of TB, tuberculin skin test (TST) result, chest radiograph findings (e.g., presence of lung cavity or miliary TB), and TB drug susceptibility information was abstracted SendSS.
Data Analyses
Data analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC). The association between patient characteristics with TB-DM, TB-HIV, and TB only was analyzed using bivariate analyses. We used χ2 tests to calculate p-values for categorical variables, ANOVA procedures to compare differences in normally distributed continuous variables (means), and the Kruskal-Wallis test for comparison of non-normally distributed variables (medians). A two-sided p-value less than 0.05 was considered statistically significant throughout the analyses. Cox proportional hazards regression models were used to estimate the hazard rate ratios (HR) and 95% confidence intervals (CI) for time to mortality during TB treatment. Patients were censored at the time of treatment completion or last documented clinical visit date if death did not occur on or before either date of completion or last visit date. Proportional hazard assumptions were assessed graphically (log negative log curves), with goodness-of-fit tests (Schoenfeld residuals) and using time-dependent models [22]. Logistic regression models were used to estimate the odds ratio (OR) and 95% CI for the outcomes 1) death before TB treatment initiation and 2) any death (before or during TB treatment). Separate regression models and covariate selection strategies were created for estimates of the two primary exposures: 1) DM status and 2) HIV status. Selected covariates considered to be known confounders were included in Cox and logistic regression models based on bivariate associations or biologic plausibility with the primary exposures and outcomes, previous literature, or directed acyclic graph theory [23]. Statistical interaction was assessed between the primary exposures of interest and all covariates included in the final Cox model.
Ethical approval
The study was approved by the Institutional Review Boards (IRB) of Emory University and Georgia Department of Health.
RESULTS
A total of 1,428 patients with TB were reported to the state of Georgia during the study period. After excluding patients <16 years (N=103), 1,325 were included in baseline analyses. A total of 1,238 (93.4%) were included in longitudinal analyses, after excluding patients who died before TB treatment initiation (N=34) or who had no TB treatment follow-up information (N=53).
Among the 1,325 patients with TB included in the study, 151 (11.4%) had DM, 147 patients had HIV (11.1%), and seven (0.5%) patients had both DM and HIV. Most patients were male (66.5%) and US born (54.6%); the most common race/ethnicity was Non-Hispanic Black (48.2%) and the median age was 45 years (Table 1). Compared to patients with TB only (no DM or HIV), TB-DM patients had higher prevalence of ESRD (6.6% vs. 1.6%) but were less likely to be diagnosed in a correctional facility (2.0% vs. 9.4%) (p<0.01). Patients with TB and HIV were more likely to report heavy alcohol use (25.2% vs. 14.5%), drug use (28.0% vs. 7.7%), and recent homelessness (29.2% vs. 7.1%) than TB only patients (p<0.01) (Table 1).
Table 1.
Diabetes mellitus, HIV, and baseline characteristics of adult TB patients in the state of Georgia 2009–2012
| Patient characteristic (at TB treatment start) |
No HIV/DM N=1020 (77.4) N (%) |
HIV N=147 (11.2) N (%) |
DMA N=151 (11.5) N (%) |
TotalB N=1318 N (%) |
P- value |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 44.3 (17.8) | 41.2 (10.0) | 57.4 (14.4) | 45.5 (17.3) | <0.01 |
| Median (IQR)C | 43.0 (27.0) | 43.0 (16.0) | 56.0 (19.0) | 45.0 (26.0) | |
| 16–24B | 151 (14.8) | 9 (6.1) | 0 | 160 (12.1) | <0.01 |
| 25–34 | 217 (21.3) | 33 (22.5) | 12 (8.0) | 262 (19.9) | |
| 35–44 | 164 (16.1) | 46 (31.3) | 11 (7.3) | 221 (16.8) | |
| 45–54 | 195 (19.1) | 51 (34.7) | 48 (31.8) | 294 (22.3) | |
| 55–64 | 144 (14.1) | 7 (4.8) | 35 (23.2) | 186 (14.1) | |
| >65 | 149 (14.6) | 1 (0.7) | 45 (29.8) | 195 (14.8) | |
| Sex | |||||
| Female | 353 (34.6) | 40 (27.2) | 48 (31.8) | 441 (33.5) | 0.19 |
| Male | 667 (65.4) | 107 (72.8) | 103 (68.2) | 877 (66.5) | |
| Race/Ethnicity | |||||
| NH BlackC | 447 (43.9) | 114 (77.5) | 73 (48.3) | 634 (48.2) | <0.01 |
| NH Asian | 206 (20.2) | 6 (4.1) | 22 (14.6) | 234 (17.8) | |
| NH White | 171 (16.8) | 7 (4.8) | 26 (17.2) | 204 (15.5) | |
| Hispanic | 194 (19.1) | 20 (13.6) | 30 (19.9) | 244 (18.5) | |
| OccupationC | |||||
| Employed | 426 (41.8) | 44 (29.9) | 48 (31.8) | 518 (39.3) | <0.01 |
| Unemployed | 347 (34.0) | 94 (64.0) | 53 (35.1) | 494 (37.5) | |
| Retired | 108 (10.6) | 1 (0.7) | 33 (21.9) | 142 (10.8) | |
| Other/unknownD | 139 (13.6) | 8 (5.4) | 17 (11.3) | 164 (12.4) | |
| Foreign bornC | |||||
| No | 516 (50.8) | 109 (74.2) | 92 (60.9) | 717 (54.6) | <0.01 |
| Yes | 500 (49.2) | 38 (25.9) | 59 (39.1) | 597 (45.4) | |
| Recent homelessness | |||||
| No | 942 (92.9) | 102 (70.8) | 139 (92.7) | 1183 (90.4) | <0.01 |
| Yes | 72 (7.1) | 42 (29.2) | 11 (7.3) | 125 (9.6) | |
| In correctional facility when TB diagnosedB | |||||
| No | 920 (90.6) | 133 (91.7) | 147 (98.0) | 1200 (91.6) | 0.01 |
| Yes | 95 (9.4) | 12 (8.3) | 3 (2.0) | 110 (8.4) | |
| Heavy alcohol use | |||||
| No | 858 (85.5) | 107 (74.8) | 129 (86.0) | 1094 (84.4) | <0.01 |
| Yes | 146 (14.5) | 36 (25.2) | 21 (14.0) | 203 (15.7) | |
| Drug use | |||||
| No | 928 (92.3) | 103 (72.0) | 142 (94.0) | 1173 (90.3) | <0.01 |
| Yes | 77 (7.7) | 40 (28.0) | 9 (6.0) | 126 (9.7) | |
| End stage renal diseaseC | |||||
| No | 1004 (98.4) | 139 (94.6) | 141 (93.4) | 1284 (97.4) | <0.01 |
| Yes | 16 (1.6) | 8 (5.4) | 10 (6.6) | 34 (2.6) | |
| TB Characteristics | |||||
| AFB smear status | |||||
| Negative | 542 (58.6) | 76 (56.3) | 64 (47.8) | 682 (57.1) | 0.06 |
| Positive | 383 (41.4) | 59 (43.7) | 70 (52.2) | 512 (42.9) | |
| Unavailable | 95 | 12 | 17 | 124 | |
| Baseline culture | |||||
| Negative | 177 (17.4) | 26 (17.7) | 18 (11.9) | 221 (16.8) | 0.53 |
| Pulm TB positive | 576 (56.5) | 89 (60.5) | 89 (58.9) | 754 (57.2) | |
| EPTB positive | 192 (18.8) | 24 (16.3) | 34 (22.5) | 250 (19.0) | |
| Unavailable | 75 (7.4) | 8 (5.4) | 10 (6.6) | 93 (7.1) | |
| TB site of disease | |||||
| Pulm only | 749 (73.4) | 91 (61.9) | 112 (74.2) | 952 (72.2) | <0.01 |
| Pulm and EPTB | 66 (6.5) | 30 (20.4) | 14 (9.3) | 110 (8.4) | |
| EPTB only | 205 (20.1) | 26 (17.7) | 25 (16.6) | 256 (19.4) | |
| Previous TB treatment | |||||
| No | 959 (94.5) | 135 (91.8) | 142 (94.0) | 1236 (94.1) | 0.44 |
| Yes | 56 (5.5) | 12 (8.2) | 9 (6.0) | 77 (5.9) | |
| TST status | |||||
| Negative | 175 (23.5) | 43 (55.8) | 42 (42.4) | 260 (28.3) | <0.01 |
| Positive | 569 (76.5) | 34 (44.2) | 57 (57.6) | 660 (71.7) | |
| Not done/unknown | 276 | 70 | 52 | 398 | |
| Any lung cavity | |||||
| No | 643 (65.3) | 113 (80.1) | 72 (49.0) | 828 (65.0) | <0.01 |
| Yes | 342 (34.7) | 28 (19.9) | 75 (51.0) | 445 (35.0) | |
| Milliary TB | |||||
| No | 933 (96.6) | 122 (87.1) | 139 (96.5) | 1194 (95.5) | <0.01 |
| Yes | 33 (3.4) | 18 (12.9) | 5 (3.5) | 56 (4.5) | |
| DST profile | |||||
| None to RIF/INH | 683 (90.6) | 85 (77.3) | 109 (90.1) | 877 (89.0) | <0.01 |
| RIF or INH | 68 (9.0) | 24 (21.8) | 10 (8.3) | 102 (10.4) | |
| MDR | 3 (0.4) | 1 (0.9) | 2(1.7) | 6 (0.6) | |
| Unavailable | 266 | 37 | 30 | 333 | |
| TB Outcomes | |||||
| Treatment duration (days)E | |||||
| Mean (SD) | 221 (101) | 282 (127) | 250 (137) | 231 (111) | <0.01 |
| Median (IQR) | 207 (90) | 292 (164) | 223 (112) | 212 (99) | |
| Death before treatment initiation | |||||
| No | 997 (97.8) | 139 (94.6) | 148 (98.0) | 1284 (97.4) | 0.07 |
| Yes | 23 (2.3) | 8 (5.4) | 3 (2.0) | 34 (2.6) | |
| Death during TB treatment | |||||
| No | 945 (94.8) | 125 (89.9) | 132 (89.2) | 1202 (93.6) | <0.01 |
| Yes | 52 (5.2) | 14 (10.1) | 16 (10.8) | 82 (6.4) | |
| Time to death during treatment (days)F | |||||
| Mean (SD) | 81 (82) | 77 (82) | 82 (90) | 81 (83) | |
| Median (IQR) | 57 (82) | 46 (139) | 64 (112) | 57(87) | 0.32 |
| Death before or during TB treatment | |||||
| No | 945 (92.7) | 125 (85.0) | 132 (87.4) | 1202 (91.2) | <0.01 |
| Yes | 75 (7.4) | 22 (15.0) | 19 (12.6) | 116 (8.8) | |
Abbreviations: DM-diabetes mellitus; SD-standard deviation; IQR-interquartile range; NH-Non-Hispanic; Pulm-pulmonary; EPTB-Extrapulmonary TB; RIF-Rifampin; INH-Isoniazid; AFB-acid fast bacilli; MDR-multi-drug resistant
Diabetes mellitus status was self-reported or from abstracted from medical records.
Patients with both HIV and DM (n=7) are excluded from the table.
Statistically significant, two-sided p-value <0.05
Other indicates disabled, not eligible for employment, student, or homemaker E.
Among patients with treatment completion date, excluding deaths during treatment (N=1155)
Among patients who died during TB treatment (N=83)
Clinical TB characteristics at baseline differed among TB-DM patients, TB-HIV patients, and TB only patients (Table 1). TB-DM patients were more likely to have cavitary lung disease at time of TB diagnosis (51%) compared to patients with TB-HIV (19.9%) and TB only (34.7%) (p<0.01). Patients with TB-DM were more likely to be sputum AFB smear positive (52.5%) compared to patients with TB-HIV co-infection (43.7%) and those with TB only (41.4%) (p=0.06). Overall extrapulmonary TB was common (28.8%) but more frequent among those patients with TB-HIV co-infection (38.1%) than among patients with TB-DM (25.9%) or TB only (26.6%) (p<0.01). Miliary or disseminated TB was more common among those with TB-HIV co-infection compared to patients with TB only (12.9% vs. 3.4%) (p<0.01). The overall prevalence of multidrug resistance was low (0.6%) among the cohort. Patients with TB-HIV were more likely to have single drug resistance (i.e., resistance to either isoniazid or rifampin) (21.8%) compared to patients with TB-DM (8.3%) or TB only (9.0%) (p<0.01).
Overall, 116 (8.8%) TB patients died, including 34 before TB treatment initiation (Table 1). During TB treatment, 83 (6.4%) patients died including 5.2% (52/1020) of TB only patients, 10.8% (16/151) of TB-DM, 10.1% (14/147) of TB-HIV, and 14.3% (1/7) of patients with DM, HIV, and TB (p<0.01). Among TB patients who completed treatment and did not die, the median treatment time was 212 days (interquartile range [IQR] 99 days). In unadjusted analyses, increased age, male sex, white race/ethnicity, being unemployed or retired, born in the US, ESRD, DM, HIV, and baseline positive culture status were associated with increased hazard of death during TB treatment (Table 2).
Table 2.
Unadjusted hazard rate ratios for baseline patient characteristics associated with death during TB treatment among patients in the state of Georgia, 2009–2012
| Patient characteristic (at TB treatment start) |
Died N=83/1238 (6.7) Died/N (%) |
cHR (95% CI) |
|---|---|---|
| Diabetes mellitus | ||
| No | 66/1089 (6.1) | 1.00 |
| Yes | 17/149 (11.4) | 1.88 (1.10, 3.20) |
| Age (years) | ||
| 16–34 | 6/402 (1.5) | 1.00 |
| 35–44 | 9/213 (4.2) | 2.78 (0.99, 7.8) |
| 45–54 | 19/276 (6.9) | 4.58 (1.83,11.48) |
| ≥55 | 49/347 (14.1) | 10.24 (4.39, 23.92) |
| Sex | ||
| Female | 14/414 (3.4) | 1.00 |
| Male | 69/824 (8.4) | 2.55 (1.44, 4.53) |
| Race/ethnicity | ||
| White | 20/194 (10.3) | 1.00 |
| NH Black | 54/592 (9.1) | 0.85 (0.51, 1.42) |
| NH Asian | 4/220 (1.8) | 0.17 (0.06, 0.49) |
| Hispanic | 5/226 (2.2) | 0.21 (0.08, 0.56) |
| Occupation | ||
| Employed | 11/492 (2.2) | 1.00 |
| Unemployed | 34/479 (7.1) | 3.17 (1.61, 6.26) |
| Retired | 24/124 (19.4) | 9.87 (4.83, 20.16) |
| Other/unknown | 14/143 (9.8) | 4.84 (2.20,10.66) |
| Foreign born | ||
| No | 69/669 (10.3) | 1.00 |
| Yes | 14/568 (2.5) | 0.24 (0.13, 0.42) |
| Recent homeless | ||
| No | 75/1111 (6.8) | 1.00 |
| Yes | 7/124 (5.7) | 0.80 (0.37, 1.74) |
| Currently in correctional facility | ||
| No | 81/1132 (7.2) | 1.00 |
| Yes | 1/103 (1.0) | 0.14 (0.02, 1.03) |
| Heavy alcohol use | ||
| No/unknown | 65/1047 (6.2) | 1.00 |
| Yes | 18/191 (9.4) | 1.51 (0.90, 2.55) |
| Drug use | ||
| No | 73/1113 (6.6) | 1.00 |
| Yes | 6/117 (5.1) | 0.73 (0.32, 1.68) |
| End stage renal disease | ||
| No | 75/1206 (6.2) | 1.00 |
| Yes | 8/32 (25.0) | 4.21 (2.03, 8.73) |
| HIV serologic status | ||
| Negative/unknown | 68/1098 (6.2) | 1.00 |
| Positive | 15/140 (10.7) | 1.59 (0.90, 2.79) |
| AFB sputum smear status | ||
| Negative | 33/655 (5.0) | 1.00 |
| Positive | 35/498 (7.0) | 1.40 (0.87, 2.25) |
| Unknown | 15/85 (17.7) | 3.74 (2.03, 6.89) |
| Baseline culture | ||
| Negative/unknown | 12/298 (4.0) | 1.00 |
| Positive | 71/940 (7.6) | 1.86 (1.01, 3.43) |
| TB site of disease | ||
| Pulmonary only | 56/913 (6.1) | 1.00 |
| Pulmonary and extra-pulmonary | 11/105 (10.5) | 1.67 (0.87, 3.18) |
| Extra-pulmonary only | 16/220 (7.3) | 1.09 (0.63, 1.91) |
| Previous TB treatment | ||
| No | 78/1167 (6.7) | 1.00 |
| Yes | 4/70 (5.7) | 0.86 (0.31, 2.34) |
| Cavitary lung disease on chest radiograph at time of TB diagnosis | ||
| No | 54/780 (6.9) | 1.00 |
| Yes | 28/427 (6.6) | 0.95 (0.60, 1.51) |
| Miliary/Disseminated TB | ||
| No | 78/1187 (6.6) | 1.00 |
| Yes | 5/51 (9.8) | 1.51 (0.61, 3.72) |
| Drug Susceptibility | ||
| None to RIF or INH | 59/827 (7.1) | 1.00 |
| Single Drug (RIF or INH) | 7/96 (7.3) | 0.97 (0.44, 2.13) |
| MDR-TB | 1/6 (16.7) | 1.86 (0.26, 13.57) |
| Unavailable | 16/309 (5.2) | 0.73 (0.42, 1.27) |
Abbreviations: cHR-crude hazard ratio; CI-confidence interval; STD-standard deviation; IQR-interquartile range; NH-Non-Hispanic; RIF-Rifampin; INH-Isoniazid; AFB-acid fast bacilli; MDR-multi-drug resistant (resistance to at least both INH and RIF)
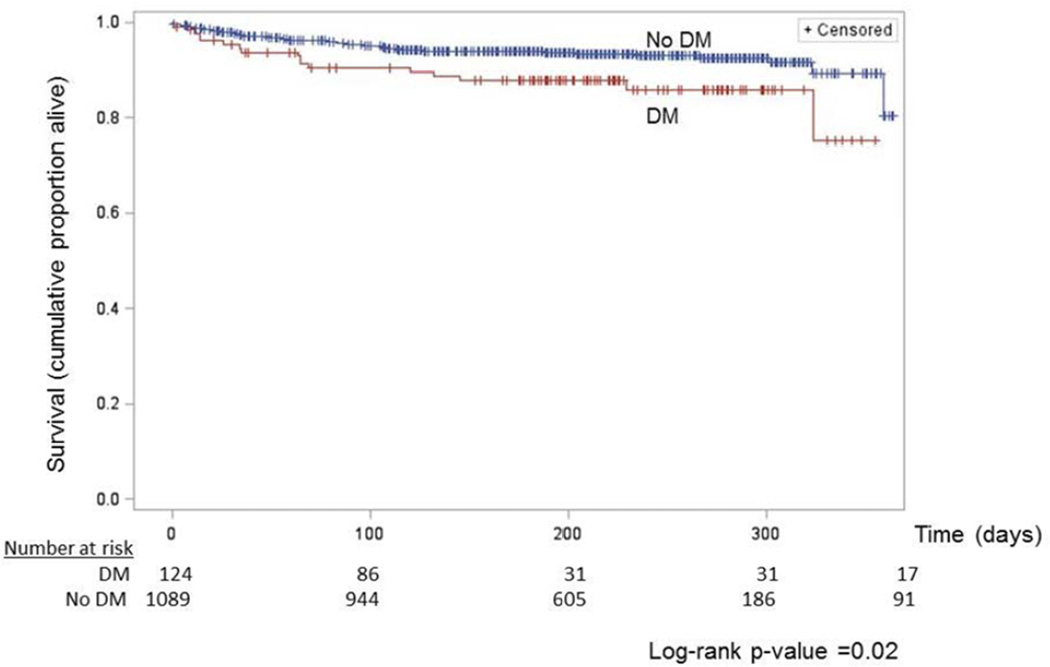
Compared to TB only patients, the unadjusted hazard of time to death during treatment was greater among TB-DM (crude hazard rate ratio [cHR] 1.88, 95% CI 1.10–3.20) (Table 3 and Figure 1). After adjusting for covariates associated with both mortality during TB treatment and DM status, the adjusted hazard of death among TB-DM patients was 1.22 (95% CI 0.70–2.12) times the hazard of death among TB patients without DM (Table 3). The estimated adjusted odds of any death among TB-DM patients was nearly the same as TB only patients (aOR 1.05, 95% CI 0.60–1.84).
Table 3.
Crude and adjusted estimates of the association between DM and HIV with death before TB treatment, during TB treatment, and any death among TB patients in the state of Georgia, 2009–2012
| Death before TB treatment | Death during TB treatment | Any death | ||||
|---|---|---|---|---|---|---|
| Models | cOR(95% CI) | aOR (95% CI) | cHR (95% CI) | aHR (95% CI) | cOR (95% CI) | aOR (95% CI) |
| 1. Diabetes mellitusA | ||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.71 (0.21, 2.35) | 0.42 (0.13, 1.42)C | 1.88 (1.10, 3.20) | 1.22 (0.70, 2.12)D | 1.60 (0.96, 2.67) | 1.05 (0.60, 1.84) |
| 2. HIV statusB | ||||||
| Negative/unknown | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Positive | 2.41 (1.07, 5.43) | 4.42 (1.80, 10.86)C | 1.59 (0.90, 2.79) | 2.18 (1.15, 4.15) | 2.01 (1.23, 3.29) | 3.32 (1.74, 6.32) |
Models 1: Three models were used to estimate the association between DM and death (defined separately in each of the three adjusted columns). In addition to DM, models were adjusted for age, sex, race/ethnicity, occupation, foreign born, heavy alcohol use, HIV status, and baseline TB culture.
Models 2: Three models were used to estimate the association between HIV and death (defined separately in each of the three adjusted columns). In addition to HIV, models were adjusted for age, sex, race/ethnicity, occupation, foreign born, DM, ESRD, heavy alcohol use, drug use, and baseline TB culture.
Adjusted for sex and age only.
In the adjusted model age, male sex, unemployed status, and HIV positive remained statistically (p<0.05) associated with death during TB treatment.
Abbreviations: cOR-crude odds ratio; CI-confidence interval; aOR-adjusted odds ratio; cHR-crude hazard ratio; aHR-adjusted hazard ratio
Figure 1.
Unadjusted cumulative all-cause mortality among tuberculosis patients with and without diabetes mellitus one year from initiation of TB treatment
The unadjusted hazard of death during TB treatment among patients with TB-HIV co-infection was 1.59 (95% CI 0.90, 2.79) times the hazard among TB patients who were HIV negative or unknown. After adjusting for covariates, the estimated adjusted hazard of death among TB-HIV patients increased to 2.18 (95% CI 1.15–4.15) times the hazard of death among TB patients without HIV (Table 3). Co-infection with HIV was also associated with increased odds of death before TB treatment initiation (adjusted OR [aOR] 4.42, 95% CI 1.80–10.86) and any death (aOR 3.32, 95% CI 1.74–6.32).
DISCUSSION
In this cohort of patients with TB from the US state of Georgia, DM was common among TB patients (11.5%) and similar in prevalence to co-infection with HIV (11.2%). In unadjusted analysis, DM was found to be associated with a nearly two-fold hazard of all-cause mortality during TB treatment. After adjusting for age and other confounders, TB-DM patients, compared to TB patients without DM, did not have significantly higher hazard of death during TB treatment (aHR 1.22, 95% CI 0.70–2.12). HIV co-infection, a well-described risk factor for poor outcomes among TB patients, was associated with an increased hazard of death during TB treatment (aHR 2.18, 95% CI 1.15–4.15) and any death (aOR 3.32, 95% CI 1.74–6.32).
Although HIV co-infection among TB patients is well-described, we found DM equally as common among this cohort of patients with TB. This finding is important to highlight as DM prevalence is increasing globally[24], which will likely also lead to an increase in TB-DM, a comorbidity that presents several new clinical challenges. The presence of TB may exacerbate hyperglycemia[16, 25], there are potential drug interactions between rifampicin and oral hypoglycemic agents[26], and there may be an increased risk of peripheral neuropathy in TB-DM patients with the use of isoniazid[27].
Several hypotheses have been postulated to explain mechanisms that could lead to increased risk of TB in patients with DM, and increased risk of mortality in TB-DM patients. Both mouse and human models have demonstrated that DM alters adaptive and cell-mediated immune responses [28–30]. Impaired alveolar macrophage activation due to glycation of binding sites may inhibit subsequent granuloma formation in TB-DM patients [31]. In addition, altered T-helper (Th) 1, Th 2, and Th 17 cytokine responses have been demonstrated among patients with TB-DM [29, 32]. Chronic hyperglycemia may disrupt the regulation of key cytokines, such as interferon-gamma,[30, 33] which in turn may increase the M. tuberculosis bacterial burden and subsequent risk of death in TB-DM patients.
The majority of previous studies that have examined mortality in TB patients with DM have reported an increased risk of death among TB-DM patients. For example, a 2011 systematic review showed that 95.5% (21 of 23) of studies found an increased unadjusted risk of death among TB-DM patients when compared to TB patients without DM [17]. However, the systematic-review had important limitations. First, follow-up time and mortality measurement was inconsistent across studies, some followed patients to the end of TB treatment while others followed patients beyond TB treatment completion. Second, of the 21 studies that reported increased unadjusted mortality risk, only 9 were powered to detect a statistically significant difference in the comparison, and only 4 studies adjusted for age and other confounders. Our unadjusted estimate (cHR 1.88, 95% CI 1.10–3.20) for death during treatment was remarkably similar to the unadjusted pooled risk ratio (RR) in the systematic review (RR 1.89, 95% CI 1.52–2.36). Our unadjusted estimate was confounded (mostly by age) and after controlling for covariates the adjusted estimate moved closer to the null (aHR 1.22, 95% CI 0.70–2.12). Given our findings and the association of DM with age and age with death, all future studies evaluating the association of DM with death among TB patients should at a minimum control for age.
In the US only three studies, all from Maryland and with low precision, have estimated the effect of DM on mortality during TB treatment [18, 19, 34]. A study by Dooley et al.[19] was the only work to estimate the association between DM and mortality during TB treatment with a model adjusting for age and HIV status (aOR 6.70, 95% CI 1.11–38.20). The two additional studies estimated the odds of death during TB treatment comparing patients with and without DM after adjusting only for age. Fielder et al.[34] estimated the odds of death among TB-DM was 3.80 (95% CI 1.42–10.16) times that of TB only patients, while Oursler et al.[18] estimated the same measure of effect at 6.70 (95% CI 1.57–28.52). In contrast to previous studies, we adjusted for potential socio-economic (race/ethnicity, occupation) and behavioral characteristics (heavy alcohol use) that could confound the association between DM and mortality. Additional geographically diverse studies that 1) control for confounders and 2) assess DM control and management will provide better measures of association of DM and death among TB patients. Poor blood glucose control and inadequate access to DM care (among patients with TB) in resource limited settings may partially explain differences in the observed risk of mortality across studies. For example, a Tanzanian study of patients with TB conducted from 2006--2008 reported a much stronger association between DM and mortality compared to our findings. [35]
There are important limitations to note in our study. First, we relied on self-report and medical chart abstraction to determine whether TB patients had DM, and therefore the primary exposure of interest was subject to misclassification due to TB patients who did not know they had DM or who had never been screened. Similarly, we were unable to distinguish between type 1 and type 2 DM. However, patients who were classified with DM were unlikely to be non-DM patients, consequently the specificity of DM measurement in our cohort was likely high. Moreover, we do not have reason to believe that the misclassification of DM status was differential with respect to mortality during TB treatment and therefore our estimated effect of the dichotomous exposure (DM) on the outcome (death) is plausibly biased toward the null [36]. Second, our study did not have measures of glucose control or DM duration and therefore we could not estimate the effect of hyperglycemia or chronic DM (vs. acute hyperglycemia). If the effect of DM on mortality during TB treatment is modified by blood glucose level, our estimated null effect could be due to a mixing of the DM patients with higher blood glucose (and higher mortality risk) and those with controlled blood glucose (and lower mortality risk). Third, we did not have any measurements of body mass index (BMI) or anthropometry of TB patients. Similar to measures of glucose control, the effect of DM on mortality during TB treatment may be modified by BMI and our study was unable to assess this potential relationship. Finally, although all-cause mortality in this study was well documented during TB treatment, we were unable to determine if the cause of death was specific to TB disease. Similarly, we did not assess mortality after patients completed TB treatment and were therefore unable to estimate the association between DM and mortality in patients who may have died from TB disease after treatment ended.
Our study had excellent follow-up information from a large, well-characterized cohort of TB patients from US, a major strength of this study. Unlike previously published studies estimating the association between DM and mortality during TB treatment, we were able to estimate the association between DM and time to death adjusting for age, HIV status, and other potentially important confounders. The scope of our study was also innovative. We used survival analysis to estimate the association between DM and all-cause mortality, one of the few studies to do so to date and only the second among TB patients in the US. To our knowledge, no previous studies have compared the clinical presentation of TB-DM patients to clinical characteristics of TB-HIV patients.
Conclusion
With an increasing prevalence of DM across the world, it is critical to improve knowledge regarding the effects of the disease on public health, including co-morbidity with infectious diseases. We showed that DM is common among TB patients in Georgia, with DM co-morbidity prevalence estimates similar to that of HIV co-infection. This study challenges previous unadjusted findings of increased mortality risk among TB patients with DM. Earlier studies concluded that DM increases the risk of death in TB patients, but we did not find a clinically meaningful association between DM and the hazard of all-cause mortality after adjusting for age, HIV, and other potential confounding factors. Nonetheless, more studies are needed to determine if poorly controlled blood glucose in patients with TB-DM increases the risk of mortality during TB treatment.
LIST OF ABBREVIATIONS AND ACRONYMS
- AFB
Acid fast bacilli
- aHR
Adjusted hazard ratio
- cHR
Crude hazard ratio
- CI
Confidence interval
- DM
Diabetes mellitus
- DOT
Directly observed therapy
- ELISA
Enzyme-linked immunoabsorbent assay
- EPTB
Extrapulmonary tuberculosis
- ESRD
End stage renal disease
- GDPH
Georgia Department of Public Health
- HIV
Human immunodeficiency virus
- HR
Hazard ratio
- INH
Isoniazid
- IQR
Interquartile range
- MDR
Multiple drug-resistant
- RIF
Rifampin
- RR
Risk ratio
- SD
Standard deviation
- SendSS
State Electronic Notifiable Disease Surveillance System
- TB
Tuberculosis
- Th
T-helper
- TST
Tuberculin skin test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Trends in tuberculosis--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013 Mar 22;62(11):201–205. [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Reported Tuberculosis in the United States, 2011. Atlanta, GA: USA: Department of Health and Human Services, CDC; 2012. [Google Scholar]
- 3.Garcia-Garcia Mde L, Ponce-De-Leon A, Garcia-Sancho MC, Ferreyra-Reyes L, Palacios-Martinez M, Fuentes J, et al. Tuberculosis-related deaths within a well-functioning DOTS control program. Emerg Infect Dis. 2002 Nov;8(11):1327–1333. doi: 10.3201/eid0811.020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kliiman K, Altraja A. Predictors and mortality associated with treatment default in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010 Apr;14(4):454–463. [PubMed] [Google Scholar]
- 5.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000 May 17;283(19):2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 6.Marks SM, Magee E, Robison V. Patients diagnosed with tuberculosis at death or who died during therapy: association with the human immunodeficiency virus. Int J Tuberc Lung Dis. 2011 Apr;15(4):465–470. doi: 10.5588/ijtld.10.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au-Yeung C, Kanters S, Ding E, Glaziou P, Anema A, Cooper CL, et al. Tuberculosis mortality in HIV-infected individuals: a cross-national systematic assessment. Clin Epidemiol. 2011;3:21–29. doi: 10.2147/CLEP.S15574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porkert MT, Sotir M, Parrott-Moore P, Blumberg HM. Tuberculous meningitis at a large inner-city medical center. Am J Med Sci. 1997 Jun;313(6):325–331. doi: 10.1097/00000441-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kourbatova EV, Leonard MK, Jr, Romero J, Kraft C, del Rio C, Blumberg HM. Risk factors for mortality among patients with extrapulmonary tuberculosis at an academic inner-city hospital in the U.S. Eur J Epidemiol. 2006;21(9):715–721. doi: 10.1007/s10654-006-9060-7. [DOI] [PubMed] [Google Scholar]
- 10.Sterling TR, Zhao Z, Khan A, Chaisson RE, Schluger N, Mangura B, et al. Mortality in a large tuberculosis treatment trial: modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis. 2006 May;10(5):542–549. [PubMed] [Google Scholar]
- 11.Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, Leonard MK., Jr Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int J Tuberc Lung Dis. 2006 Nov;10(11):1224–1230. [PubMed] [Google Scholar]
- 12.Walpola HC, Siskind V, Patel AM, Konstantinos A, Derhy P. Tuberculosis-related deaths in Queensland, Australia, 1989–1998: characteristics and risk factors. Int J Tuberc Lung Dis. 2003 Aug;7(8):742–750. [PubMed] [Google Scholar]
- 13.Raviglione M, Marais B, Floyd K, Lonnroth K, Getahun H, Migliori GB, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet. 2012 May 19;379(9829):1902–1913. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- 14.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010 Mar;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008 Jul 15;5(7):e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009 Dec;9(12):737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011 Jul 1;9(1):81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oursler KK, Moore RD, Bishai WR, Harrington SM, Pope DS, Chaisson RE. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiologic factors. Clin Infect Dis. 2002 Mar 15;34(6):752–759. doi: 10.1086/338784. [DOI] [PubMed] [Google Scholar]
- 19.Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009 Apr;80(4):634–639. [PMC free article] [PubMed] [Google Scholar]
- 20.Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith B, et al. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007 Apr;135(3):483–491. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GDPH. 2011 Georgia Tuberculosis Report. Atlanta: Georgia Department of Public Health; 2012. [Google Scholar]
- 22.Kleinbaum D, Klein M. Survival Analysis: A Self-Learning Text. 3rd ed. New York: Springer; 2012. [Google Scholar]
- 23.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999 Jan;10(1):37–48. [PubMed] [Google Scholar]
- 24.IDF. Diabetes Atlas, Update 2012. Brussels: International Diabetes Federation; 2012. [Google Scholar]
- 25.Oluboyo PO, Erasmus RT. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle. 1990 Jun;71(2):135–138. doi: 10.1016/0041-3879(90)90010-6. [DOI] [PubMed] [Google Scholar]
- 26.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivisto KT. Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin Pharmacol Ther. 2001 Jun;69(6):400–406. doi: 10.1067/mcp.2001.115822. [DOI] [PubMed] [Google Scholar]
- 27.Kapur A, Harries AD. The double burden of diabetes and tuberculosis - Public health implications. Diabetes Res Clin Pract. 2013 Jan 7; doi: 10.1016/j.diabres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008 Jun 12;3(6):399–407. doi: 10.1016/j.chom.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, Mora F, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008 Sep 1;47(5):634–641. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stalenhoef JE, Alisjahbana B, Nelwan EJ, van der Ven-Jongekrijg J, Ottenhoff TH, van der Meer JW, et al. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis. 2008 Feb;27(2):97–103. doi: 10.1007/s10096-007-0395-0. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee D, Bhattacharyya R, Kaul D, Sharma P. Diabetes and tuberculosis: analysis of a paradox. Adv Clin Chem. 2011;53:139–153. [PubMed] [Google Scholar]
- 32.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013 Sep;208(5):739–748. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992 May-Jun;18(3):187–201. [PubMed] [Google Scholar]
- 34.Fielder JF, Chaulk CP, Dalvi M, Gachuhi R, Comstock GW, Sterling TR. A high tuberculosis case-fatality rate in a setting of effective tuberculosis control: implications for acceptable treatment success rates. Int J Tuberc Lung Dis. 2002 Dec;6(12):1114–1117. [PubMed] [Google Scholar]
- 35.Faurholt-Jepsen D, Range N, PrayGod G, Jeremiah K, Faurholt-Jepsen M, Aabye MG, et al. Diabetes is a strong predictor of mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients from Mwanza, Tanzania. Trop Med Int Health. 2013 Jul;18(7):822–829. doi: 10.1111/tmi.12120. [DOI] [PubMed] [Google Scholar]
- 36.Rothman K, Greenland S, Lash T. Modern Epidemiology. 3 ed. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]