Abstract
We present an ultrasound technique to detect the inflammatory changes in developing atheroma. We used contrast enhanced ultrasound imaging (CEUS) with 1) ICAM-1 targeted microbubbles, a molecule of adhesion involved in the inflammatory processes into the lesions of atheroma in New Zealand White rabbits, 2) a pre-treatment with NO-loaded microbubbles and US activation at the site of the endothelium in order to enhance the permeability of the arterial wall and the penetration of the ICAM-1 targeted microbubbles. Following this procedure, the acoustic enhancement is increased by 1.2 fold. NO-ELIP pretreatment with ultrasound activation can potentially facilitate the subsequent penetration of targeted ELIP into the arterial wall, thus allowing improved detection of inflammatory changes in developing atheroma.
Keywords: Echogenic liposomes, intravascular ultrasound imaging, nitric oxide, atherosclerosis, molecular imaging
Introduction
Adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) are expressed on the endothelium of developing atheroma and play an important role in the recruitment of circulatory monocytes into plaques (Dansky et al. 2001; Huo and Ley 2001; Kasper et al. 1996; Misiakos et al. 2001; O’Brien et al. 1993; van der Wal et al. 1992). Adhesion molecules are divided into 3 subgroups: selectins, integrins, and members of the immunoglobulin superfamily of molecules (Dustin et al. 1988). Two subtypes of ICAM coexist: ICAM-1, which is inducible, and ICAM-2 which is constitutively and inducibly expressed on endothelial cells (Staunton et al. 1989).
During atherogenesis, endothelial cells are activated and they express adhesion molecules at high levels, such as ICAM-1 and VCAM-1 (Boyle 2005; Schwartz et al. 2003). These molecules facilitate leukocyte adherence and transmigration into the intima of the arterial wall, and induce inflammation. Molecular imaging of adhesion molecule expression has correlated with the severity of diet-modulated vascular inflammation in late stage apoE−/− mice (Kaufmann et al. 2007). The temporal expression of adhesion molecules was carefully studied in a rabbit model of balloon injury to the aorta (Tanaka et al. 1993). Ten days after the injury, both ICAM-1 and VCAM-1 were expressed initially in the neointimal endothelium and extended to the neointimal smooth muscle cells. By day 30, VCAM-1 expression was reduced but ICAM-1 expression persisted beyond that time point. The increased levels of adhesion molecules is thought to be related to the increased expression of monocyte chemotactic protein-1 and keratinocyte chemoattractant, which in turn encode inflammatory cytokines (Tanaka et al. 1993). Although transient VCAM-1 expression is considered more specific for the initiation of atherosclerosis (Cybulsky et al. 2001), the persistent expression of ICAM-1 may be a more reliable marker for molecular imaging of the inflammatory activity in the arterial wall. Apart from mechanical injury to the aorta, thrombosis by bead embolization or external ligation also resulted in an increase in ICAM-1 expression in the vascular media (Toursarkissian et al. 1997).
Our laboratory has developed techniques to target adhesion molecule expression with echogenic liposomes (ELIP). A number of formulations for molecular imaging and drug and gene delivery have been developed using the intrinsically echogenic liposomal technology (Alkan-Onyuksel et al. 1996; Buchanan et al. 2008; Hamilton et al. 2002b; Huang et al. 2002b). The encapsulation of air into these liposomal formulations results in a small, traversable contrast agent that is suitable for ultrasound image enhancement and controlled release of therapeutic agents, while being stable in the circulation for a prolonged period (Buchanan et al. 2008). Intrinsically ELIP compare favorably to commercially available microbubbles in terms of in vivo stability and targeting capabilities, as well as drug and gene delivery capacities (Demos et al. 1999; Huang et al. 2002a; Huang et al. 2002b). The conjugation of antibodies against a variety of adhesion molecules, tissue factors and thrombotic markers on the liposomal surface of ELIP have proven very effective in identifying developing atheroma with transvascular and intravascular ultrasound (Hamilton et al. 2004).
Most ultrasound contrast agents are considered intravascular contrast agents that lack the capability to penetrate the arterial wall. Unlike other ultrasound contrast agents that are homogeneous in size and distribution and tend to be greater than 3 μm, our ELIP formulations vary in sizes from < 100 nm to several micrometers, with bimodal medians of ~90 and 800 nm due to the ELIP formation from lyophilized lipids during rehydration (Kopechek et al. 2011). Smaller sizes allow these contrast agents to penetrate all layers of the vascular bed, including the adventitia via the vasa vasorum.
In agreement with previous studies using rabbit models of balloon injury(Tanaka et al. 1993), thrombosis(Toursarkissian et al. 1997) and LDL receptor deficiency (Broisat et al. 2007) as well as histological examination of human atherosclerosis(Galkina and Ley 2007; O’Brien et al. 1993), we have observed adhesion molecule expression is not restricted to the endothelial surface but spans the entire thickness of the neointima and the vasa vasorum (Hamilton et al. 2004). As ICAM-1 expression in association with developing atherosclerosis is not only restricted to the endothelial surface of the neointima but also involves the upper medial layer (O’Brien et al. 1993) and the microvessels of the vasa vasorum (Galkina and Ley 2007), it is likely that identification of ICAM-1 expression within the deeper neointimal layers may provide better assessment of chronic arterial wall inflammation, allowing discrimination of transient inflammatory states such as infection. However, effective penetration of microbubbles or ELIP into the neointima and the vasa vasorum requires novel techniques to allow transmigration of the contrast agent through the endothelium, which motivates our development of a new class of ELIP for such an application.
Nitric oxide (NO) is a potent bioactive gas with a wide range of vasoactive properties (Fischer et al. 2004). The desirable characteristics of NO in modulating the development of various vascular diseases have prompted the development of various NO precursors, synthetic NO promoters such as L-arginine, endothelial NO synthase gene, NO donors, and NO gas. One of the most popular modes of NO gas delivery clinically is via inhalation (Miller and Megson 2007; Radomski et al. 1987; Tsao et al. 1994). Although the delivery of NO to the arterial wall has a number of potential benefits, successful NO delivery to targeted tissues is challenging because of the presence of endogenous NO scavengers such as hemoglobin (Tsao et al. 1994). Our laboratory has developed techniques for encapsulating NO (Huang et al. 2009) and other bioactive gases (Britton et al. 2010) into ELIP, resulting in agents that are suitable for both ultrasound imaging and ultrasound-mediated bioactive gas delivery. Our NO-ELIP have been shown to release NO into the arterial wall with effect without NO degradation by scavenging hemoglobin in the bloodstream (Huang et al. 2009). Nitric oxide induces increased vascular permeability in guinea pig conjunctiva (Meijer et al. 1995).
There is accumulating evidence showing that NO signaling and gap junction communication are interdependent and may be mediated by the key components such as eNOS, connexins, and caveolin-1 (Looft-Wilson et al. 2012). Furthermore, we have refined the antibody conjugation techniques in NO-loaded ELIP (NO-ELIP) to preserve the antibody immunoreactivity and ultrasound-mediated bioactive gas delivery (Klegerman et al. 2010). For the first time, it is possible to target the inflamed endothelium for effective ultrasound-triggered local release of NO to facilitate penetration of the ELIP into the arterial wall. In the current study, in order to standardize the delivery of anti-ICAM-1 antibody-conjugated ELIP (anti-ICAM-1 ELIP), NO-ELIP was administered separately prior to the administration of anti-ICAM-1 ELIP.
We hypothesize that a vasoactive agent such as NO may improve the permeability of the vascular bed and allow penetration of anti-ICAM-1 ELIP into all layers of the arterial wall for better quantitation of ICAM-1 expression.
Materials and Methods
ELIP preparation
The preparation of ELIP, anti-ICAM conjugated ELIP and nitric oxide-loaded ELIP were described previously (Hamilton et al. 2002a; Huang et al. 2009; Lanza 2000).
Preparation of standard ELIP
A 27:42:8:8:15 molar ratio of the lipid components L-α-phosphatidylcholine (chicken egg; EPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dipalmitoyl-sn-glycero-3-[phosphor-rac-1-glycerol] (DPPG), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE), and cholesterol (CH) were mixed in a round-bottom flask as chloroform solutions. For preparation of fluorescent ELIP, 0.2 mole% lissamine rhodamine B-DPPE (Avanti Polar Lipids, Alabaster, AL) was included in the formulation, which was subsequently protected from exposure to light. The chloroform was then removed by evaporation under argon, while the flask was rotated in a 50°C water bath. The resulting lipid film was placed under vacuum for 4 hours at ≤100 mTorr pressure for complete removal of the solvent, followed by rehydration of the dry lipid film with 0.32 M mannitol to a concentration of 10 mg lipid/ml. The hydrated lipid was incubated at 55°C for 30 minutes to ensure that all lipids were in the liquid phase during hydration. The mixture was then sonicated in a water bath for 5 minutes. Aliquots of the suspension were frozen at −80°C and lyophilized for 24–48 hours. Each lyophilized dry cake was resuspended with the original volume of nanopure water immediately before use.
Preparation of NO-ELIP
Liposomes of the above composition were prepared according to a previously developed pressurization-freeze method (Huang et al. 2009). Briefly, after drying and hydrating the lipid film, 300-μl aliquots of the suspension were transferred to 2-ml borosilicate glass vials (12×32 mm), which were then sealed with Teflon-coated silicon rubber septal screw caps. Nitric oxide (5.4 ml STP), washed and purified by passage through a saturated sodium hydroxide solution in order to remove nitrogen dioxide produced by contaminating oxygen, or a mixture of NO and argon was introduced into each vial through the septum and pressurized to 9 atm using a syringe fitted with a 27G×1/2″ needle. The pressurized gas/liposome dispersion was incubated for 30 minutes at room temperature, followed by freezing on dry ice for ≥ 30 minutes. Vials were stored at −80° C. Prior to use, the pressure was released by loosening the caps immediately after removal from storage, followed by thawing of the NO-ELIP suspension at room temperature.
Conjugation of IgG Preparations to ELIP
Mouse IgG and mouse monoclonal anti-rabbit ICAM-1 (Rb2/3 hydridoma cell line from Dr. M. Cybulsky, Toronto General Hospital, Toronto, Ontario, Canada) were conjugated to ELIP through a thioether linkage as previously described (Hitchcock et al. 2010; Kim et al. 2010).
Animal model
All animal experiments were approved by the Animal Welfare Committee at the University of Texas Health Science Center at Houston. Early developing atherosclerosis was induced in 20 New Zealand White rabbits with a standard procedure of combined balloon denudation and hyperlipidemic diet. Briefly, NZW rabbits of 9 months of age were pre-treated with a hyperlipidemic diet containing 0.2% cholesterol and 4% coconut oil for one week. The animals were anesthetized with ketamine (35 mg/kg), xylazine (5 mg/kg) and 1% to 3% inhaled isoflurane. Under fluoroscopic guidance, a 4F Fogarty catheter (Edwards LifeSciences, Irvine, CA) was inserted into the right femoral artery and advanced to the proximal abdominal aorta. The balloon tip of the 4F Fogarty catheter was inflated with 0.5 ml of diluted iodixanol (Visipaque) solution to aid its visualization under fluoroscopy. The infradiaphragmatic aorta was denuded by pulling the 4F Fogarty catheter towards the aortic bifurcation. A total of three balloon denudations were performed and the right femoral artery was tied off at the end of the procedure. Afterwards, the animal continued on the hyperlipidemic diet for another 3 months prior to the imaging experiments.
Imaging procedure
Intravascular imaging was performed with a 20-MHz, Eagle Eye Gold high-frequency IVUS imaging catheter (Volcano Corp, Rancho Cordova, CA) attached to an automatic pullback device that withdraws at a rate of 0.5 mm/s. We utilized the same IVUS catheter for pre- (i.e., baseline) and post-imaging of anti-ICAM-1 ELIP treatment in each aorta. The time interval between the pre- and post-imaging is 5 minutes without NO-ELIP pretreatment, and 10 minutes with NO-ELIP pretreatment. Therefore, little variance in acoustic output is expected between the IVUS imaging in the same aorta. We presented the data as acoustic enhancement, i.e., normalized acoustic enhancement with respect to baseline in each aorta. The IVUS catheter was inserted via the left femoral artery and advanced retrogradely to the abdominal aorta. It was assumed that there was no curvature in the abdominal aorta and the withdrawing direction of the IVUS catheter was parallel to the blood flow of the abdominal aorta. Instrumental gain and zoom settings were set such that the luminal border and aortic wall structure were clearly detectable at baseline, and remained constant throughout IVUS imaging. For intra-arterial delivery of the ELIP preparations, a 3F catheter was advanced through a sheath inserted into the left carotid artery and into the upper abdominal aorta for direct delivery of ELIP into the previously denuded region.
The animals were divided into 5 experimental groups with following group allocation: Saline + IgG-ELIP (Group 1), saline + anti-ICAM-1 ELIP (Group 2), saline with ultrasound + anti-ICAM-1 ELIP (Group 3), NO-ELIP + anti-ICAM-1 ELIP (Group 4) and NO-ELIP with ultrasound + anti-ICAM-1 ELIP (Group 5). For the pre-treatment, 0.5 ml of NO-ELIP or saline was administered into the arterial sheath. Each milliliter of NO-ELIP contained 1.0 mg of lipid, 2.0 μl of NO and 18 μl of argon. For imaging ELIP administration, 5 mg (1-2 ml) of IgG-ELIP or anti-ICAM-1 ELIP lipid was injected into the arterial sheath. There was a 5 minute interval between pretreatment (saline or NO-ELIP) and anti-ICAM-1 ELIP administration and a 5 minute interval between anti-ICAM-1 ELIP treatment and IVUS imaging. In the pretreatment groups receiving ultrasound (Group 3 and 5), ultrasound was applied for 2 minutes beginning immediately upon injection of the NO-ELIP or saline.
Image analysis
IVUS images were recorded in a digital format with 764×500 pixel spatial resolution (0.02 mm/pixel) and 8-bit gray scale (256 levels) at 0.5 mm intervals and a frame rate of 30 f/s. The boundary borders of the endothelium/atheroma, the external elastic lamina, and the outer edge of the dense adventitia in each IVUS slice were manually segmented using Image-Pro Plus software (Media Cybernetics Inc, Rockville, MD). Acoustic enhancement within the entire abdominal aortic wall borders (i.e., from the luminal border to the outer edge of the adventitia) following each treatment was quantitated by densitometric analysis of gray scale values. Acoustic enhancement within the two layers (atheroma/media and dense adventitia) of the abdominal aortic wall was also quantitated using the same image analysis method to determine the penetration of the ELIP into the vessel wall.
A gray scale value of 0 refers to black color indicating little acoustic reflectivity in ultrasound imaging, whereas the maximum gray scale value of 255 displays in white color indicating highly intensified acoustic reflectivity. Acoustic enhancement (%) within the abdominal aortic wall was determined by comparing mean grayscale values in the same region of interest between the baseline and treatment images for each group.
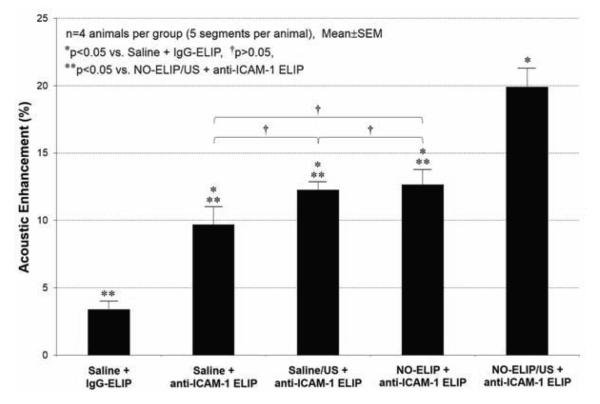
Four rabbits were utilized for each treatment group. Five segments of the aorta in a sequence were imaged for each animal. The percentage acoustic enhancement following the treatment with respect to the baseline was calculated for each segment. Finally, the acoustic enhancement data in Figure 2 were calculated by averaging a total of twenty data sets for each treatment group.
Figure 2.
The effects of nitric oxide delivery on the acoustic enhancement with various ELIP formulations in the inflammatory atheroma (n=4 animals per group). Incremental enhancement of the arterial wall by anti-ICAM-1 ELIP was observed by pre-treatment with NO-ELIP and ultrasound activation (Group 5).
Histology
Twelve centimeters of aorta were excised from each animal at the time of sacrifice. The artery was sectioned at 5 mm intervals, into approximately 20-24 rings, 2 mm-thick. The rings were embedded in Tissue-Tek® O.C.T. compound and immediately frozen. Five micron-thick histological sections were cut and stained with H&E. Immunohistochemistry was performed with anti-ICAM-1 antibody (1:100 dilution).
Statistical analysis
SigmaStat (Systat Software Inc., San Jose, CA) was utilized for statistical analyses. The Kruskal-Wallis analysis of variance of ranks and median test was used to assess if there was a global difference in acoustic enhancement between the groups, followed by all pairwise comparisons. Data were shown as mean and standard error of means. Immunoreactivity data of treatment groups were compared by one-way ANOVA and the Holm-Sidak method. Proportions of two-color staining liposome preparations in flow cytometry were compared by chi-squared (χ2) analysis.
Results
Physicochemical characteristics of antibody-conjugated ELIP and NO release profile of NO-ELIP
The conjugation efficiencies were 19-20 μg IgG/ mg lipid for the IgG-ELIP and 10.7-12.2 μg antibody/ mg lipid for the anti-rabbit ICAM-1 ELIP. The number average spherical equivalent diameter of conjugated ELIP > 0.4 μm in size was 596 and 631 nm and comprised 1.08 × 1010 and 7.4 × 109 liposomes/mg lipid, respectively. Zeta potential for ELIP was −41.5 mV but was not performed for the antibody-conjugated ELIP. The particle size distribution of antibody-conjugated ELIP remained stable for several months when the lyophilized preparation was stored at 4 °C.
Intravascular imaging
The luminal border and the outer edge of the adventitia were well visualized by IVUS imaging and indicated by the two concentric dotted circular tracings (Figure 1). The luminal border and the outer edge of the adventitia were well visualized by IVUS imaging and indicated by the two concentric dotted circular tracings. Visually, little change was seen in animals treated with saline + IgG-ELIP, saline + anti-ICAM-ELIP, or saline with ultrasound activation + anti-ICAM-1 ELIP (Group 1 to 3). Visually increased echogenicity was seen in animals treated with NO-ELIP without ultrasound activation + anti-ICAM-1 ELIP (Group 4) and NO-ELIP with ultrasound activation + anti-ICAM-1 ELIP (Group 5), with the latter being most prominent. In several vessels pretreated with NO-ELIP (Group 4 and 5), enlarged luminal diameters were observed indicating the vasodilative effect by NO-ELIP (Fig.1, lower 2 panels).
Figure 1.
Representative intravascular ultrasound images of matched segments demonstrating acoustic enhancement in the arterial wall at baseline and after the delivery of various ELIP formulations. The luminal border and the outer edge of the adventitia are indicated by the two concentric dotted circular tracings (left panel). For comparison, the non-annotated images are also shown (right panel). Visually, little change was seen in animals treated with saline + IgGELIP (Group 1), saline + anti-ICAM-ELIP (Group 2), or saline with ultrasound activation + anti-ICAM-1 ELIP (Group 3). Visually increased echogenicity was seen in animals pre-treated with NO-ELIP ± ultrasound activation prior to anti-ICAM-1 ELIP (Group 4 and 5).
Image analysis revealed detailed changes within the aortic wall after various treatments (Fig. 2). There was no highlighting of inflammatory atheroma with IgG-ELIP (Group 1) (3.4±1.2%). Anti-ICAM-1 ELIP (Group 2) was effective in targeting the inflamed arterial wall, resulting in acoustic enhancement in the abdominal aortic wall by 9.7±1.3% with respect to baseline. The use of ultrasound during the pretreatment of saline followed by anti-ICAM-1 ELIP injection (Group 3) showed no additional acoustic enhancement (12.3±0.6%). NO-ELIP pretreatment without ultrasound activation prior to anti-ICAM-1 ELIP administration (Group 4) also did not show any difference in acoustic enhancement (12.7±1.1%). Ultrasound-triggered pretreatment with NO-ELIP (Group 5), however, demonstrated further acoustic enhancement from anti-ICAM-1 ELIP targeting (19.9±1.4%).
Acoustic enhancement within the atheroma/media layer and the dense adventitia layer following various ELIP treatments was shown (Table 1). There was no acoustic enhancement in both layers with IgG-ELIP treatment (Group 1). The dense adventitia of the abdominal aorta demonstrated greater acoustic enhancement (9.0±1.0%) following anti-ICAM-1 ELIP treatment (Group 2) compared to the atheroma/media layer (3.8±0.9%). Acoustic enhancement measured across the entire aortic wall did now show any difference among the saline pretreatment + anti-ICAM-1 ELIP (Group 2), saline pretreatment with ultrasound + anti-ICAM-1 ELIP (Group 3), and NO-ELIP pretreatment with ultrasound + anti-ICAM-1 ELIP (Group 5) (Fig. 1). However, acoustic enhancement in the atheroma/media layer demonstrated greater highlighting with saline pretreatment with ultrasound + anti-ICAM-1 ELIP (Group 3) (13.4±1.1%) and NO-ELIP pretreatment with ultrasound + anti-ICAM-1 ELIP (Group 5) (13.3±1.8%) compared to saline pretreatment + anti-ICAM-1 ELIP (Group 2) (3.8±0.9%). With ultrasound application while NOELIP pretreatment (Group 5), acoustic enhancement within the atheroma/media layer (23.6±2.3%) further increased but there was no difference in acoustic enhancement in the dense adventitia layer (5.3±0.9%). This indicates that NO-ELIP pretreatment and ultrasound application facilitates the penetration of subsequent delivery of targeted ELIP into the arterial wall.
Table 1.
Acoustic enhancement (%) in the arterial layers following various ELIP treatments (n=4 animals per group, 5 segments per animal).
| Saline + IgG-ELIP |
Saline + anti-ICAM-1-ELIP |
Saline/US + anti-ICAM-1-ELIP |
NO-ELIP + anti-ICAM-1-ELIP |
NO-ELIP/US + anti-ICAM-1-ELIP |
|
|---|---|---|---|---|---|
| Atheroma/media | 2.3±0.5 | 3.8±0.9 | *13.4±1.1† | *13.3±1.8† | *23.6±2.3† |
| Dense adventitia | 3.2±0.9 | *9.0±1.0 | 5.7±0.6† | 6.5±0.9† | 5.3±0.9† |
p>0.05 vs. each other,
p<.01 vs. Saline + IgG-ELIP.
ICAM-1 expression and ELIP localization in the aortic wall
The combination of balloon denudation and cholesterol feeding resulted in prolific neointimal hyperplasia (Fig. 3a). ICAM-1 is highly expressed in the neointimal tissues and all layers of the aortic wall as shown in Fig. 3b, corresponding to the image highlighting strategies using anti-ICAM-1 ELIP. In animals injected with rhodamine-labeled anti-ICAM-1 ELIP without pretreatment with NO-ELIP or ultrasound, sparse distribution of rhodamine-labeled anti-ICAM-1 ELIP was seen in all layers of the plaque (Fig. 4a). In contrast, in animals pre-treated with NOELIP and ultrasound, abundant amount of rhodamine-labeled anti-ICAM-1 ELIP was visualized within all layers of the plaque in the aortic wall (Fig. 4a).
Figure 3.

(A) H&E image of a prototypical neointimal lesion. (B) Anti-ICAM-1 immunohistochemical staining shows ICAM-1 is highly expressed in the endothelium, neointimal tissues and all layers of the aortic wall.
Figure 4.

Representative immunofluoresence microscopic images of the aortic wall to detect the distribution of rhodamine-labeled anti-ICAM-1 ELIP. The leftmost panel of each row represents a low magnification (10X) of the aortic wall and the white squares denote the regions where high magnification (60X) images were obtained at increasing depth from the lumen/ endothelial, neointimal and neointimal/ medial regions, respectively. (A) Anti-ICAM-1 ELIP only without pre-treatment with NO-ELIP or ultrasound (Group 2): Minimal amount of rhodamine-labeled anti-ICAM-1 ELIP was detected. (B) Pre-treatment with NO-ELIP and ultrasound, followed by anti-ICAM-1 ELIP (Group 5): Rhodamine-labeled anti-ICAM-1 ELIP were visualized throughout all layers of the plaque (white arrows).
Discussion
We have demonstrated (1) the transmural nature of adhesion molecules in the developing atheroma, (2) the underestimation of ICAM-1 expression when using ICAM-1 targeting ELIP alone, and (3) the effectiveness of NO-ELIP pretreatment and ultrasound activation in vasodilatation and endothelial permeability for effective targeting of ICAM-1 expression in all layers of the developing atheroma.
The physicochemical characteristics, NO release profile and in vivo biological activities of NO-ELIP were previously reported (Huang et al. 2009). In brief, about 2 μl of NO gas and 18 μl of argon were entrapped in each milligram of liposomes. Unlike the antibody-conjugated ELIP, the preparation of the “ready-to-administer” NO-ELIP was not dependent on the reconstitution of lyophilized lipids and NO loading in NO-ELIP was expected to be uniform. The retention of biological activities of NO within NO-ELIP, the efficacy of NO delivery to the arterial wall and inhibition of neointimal hyperplasia were demonstrated In a rabbit model of carotid denudation and neointimal hyperplasia (Huang et al. 2009). The stability of echogenic liposomes in the blood pool was demonstrated in a physiologic flow phantom (Radhakrishnan et al. 2012).
Molecular imaging of adhesion molecule expression in atherosclerosis has been reported using radioactive (Broisat et al. 2007; Kelly et al. 2005; Nahrendorf et al. 2009) and paramagnetic (Jaffer et al. 2006; McAteer et al. 2008; Nahrendorf et al. 2006) contrast agents as well as other forms of ultrasound contrast agents (Kaufmann et al. 2007). Due to the size of ultrasound contrast agents, a number of approaches have been attempted to improve their interactions with the endothelial surface in the setting of high systemic blood flow. These include partial deflation of microbubbles (Rychak et al. 2006), multivalent targeting with the display of two homing ligands (Ferrante et al. 2009; Kaufmann et al. 2010), acoustic radiation forces (Lum et al. 2006; Rychak et al. 2007) and encapsulation of magnetic components in the microbubbles to facilitate adhesion using a magnet (Soetanto and Watarari 2000; Wu et al. 2011). Our group has demonstrated the use of the ELIP contrast agents in detecting adhesion molecule expression in atherosclerosis in vivo (Demos et al. 1999; Hamilton et al. 2004). The endothelium represents an effective barrier against the entry of large macromolecules, including ELIP and microbubbles, which are considered pure intravasacular contrast agents. To gain access to subendothelial components, in this case adhesion molecules in the neointimal media and smooth muscle cells, we have demonstrated the effectiveness of NO delivery by NO-ELIP in increasing the permeability of the endothelium and allowing the penetration of anti-ICAM-1 ELIP into the subendothelial layers. A number of mechanisms have been proposed for enhanced NO-mediated endothelial permeability and are related to its interactions with eNOS, connexins, and caveolin-1 (Looft-Wilson et al. 2012). Our group has also demonstrated that NO delivery by bifunctional ELIP containing both NO-ELIP and anti-ICAM-1 antibody enhances the permeability of TNFα-stimulated human coronary artery endothelial cell monolayers and facilitate the passage of CD34+ human peripheral mononuclear cells (Klegerman et al. 2010). Recent evidence also suggests that there are NO-independent relaxation factors that could mediate the transfer through gap junctions (Chaytor et al. 1998). These NO-independent relaxation agonists include acetylcholine, adenosine triphosphate and cyclopiazonic acid that directly interact with connexin protein subunits in connexon that form the gap junctions between endothelial cells (Taylor et al. 1998). These NO-independent agonists could be considered in the future development of our ELIP platform and may provide additional opportunities for enhanced contrast agent, drug and stem cell delivery into the arterial wall. Although we have been successful in preparing bifunctional ELIP that deliver NO and targets ICAM-1 and retains the immunoreactivity of the anti-ICAM-1 antibody, the presence of biotin-streptavidin bridges in this formulation may preclude clinical translation. Thus, sequential administration of NO-ELIP and anti-ICAM-1 ELIP was tested in the current rabbit model.
Study limitations
Some factors will need to be considered when interpreting the data. Both the pretreatment NO-ELIP and targeting anti-ICAM-1 ELIP were administered intra-arterially into the aorta. Previous studies have demonstrated the re-emergence of ELIP in the systemic circulation after intravenous administration (Hamilton et al. 2002a) and highlighted the excellent versatility of our agent for a range of applications, both for intravenous and intra-arterial administration at the time of IVUS imaging. The employment of an intra-arterial route for ELIP administration in the current study was to minimize the dose of the injected tracer in a large animal model and maximize the local concentration of the injected tracer. In certain clinical scenarios such as coronary and carotid catheterization, intra-arterial route of administration may be clinically relevant for diagnostic and therapeutic applications.
We did not evaluate the tissue concentration of NO in the atherosclerotic lesions. However, based on our previous in vivo observation in a rabbit model of carotid atherosclerosis, the payload within NO-ELIP was likely to be retained and its delivery into the arterial wall was associated with inhibition of neointimal hyperplasia (Huang et al. 2009).
Another limitation in this study relates to the use of B-mode fundamental imaging to evaluate the signal enhancement in the aortic wall, which poses some challenges in identifying specific signals from the retained ELIP in the aortic wall. The mean gray scale measured by image analysis was a summation of ultrasound backscattering from the arterial wall that was present at baseline and additional enhancement from targeted ELIP in the arterial wall. B-mode fundamental imaging by IVUS was unable to measure the net signal enhancement from the targeted ELIP specifically. We have recently reported the use of radiofrequency signal from the raw data as a methodology to improve the specific measurement of signal from retained ELIP in the arterial wall (Kim et al. 2012). We have recently applied this technique in studies in atherosclerotic miniswine (unpublished results) and demonstrated much improvement in contrast agent detection in the arterial wall. A number of studies have reported the use of harmonic (Goertz et al. 2006), and subharmonic (Goertz et al. 2007) and ultraharmonic (Maresca et al. 2013) IVUS imaging with custom-made IVUS catheters for contrast-specific imaging, but those devices are not commercially available. In this study, we improved the validity of the analysis by careful matching of the aortic segments based on major branch points and landmark and analysis at multiple levels within the same aortic segment. The utility of the technique and methodology in this study is most applicable to ultrasound imaging as presently used to evaluate vascular wall components and thus has the most clinical relevance.
Conclusions
Ultrasound-facilitated NO delivery can effectively enhance atheroma permeability and improve anti-ICAM1-ELIP targeting to inflammatory components in the arterial wall. Since the expression of adhesion molecules is not restricted to the endothelium but in all the layers in the inflammatory atheroma, the use of this NO pretreatment strategy has potential to improve the accuracy of targeted molecular imaging and staging of developing atheroma.
Acknowledgements
This study was supported by NIH grants HL059586 and HL074002.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Alkan-Onyuksel H, Demos SM, Lanza GM, Vonesh MJ, Klegerman ME, Kane BJ, Kuszak J, McPherson DD. Development of inherently echogenic liposomes as an ultrasonic contrast agent. J Pharm Sci. 1996;85:486–90. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005;3:63–8. doi: 10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]
- Britton GL, Kim H, Kee PH, Aronowski J, Holland CK, McPherson DD, Huang SL. In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation. 2010;122:1578–87. doi: 10.1161/CIRCULATIONAHA.109.879338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broisat A, Riou LM, Ardisson V, Boturyn D, Dumy P, Fagret D, Ghezzi C. Molecular imaging of vascular cell adhesion molecule-1 expression in experimental atherosclerotic plaques with radiolabelled B2702-p. Eur J Nucl Med Mol Imaging. 2007;34:830–40. doi: 10.1007/s00259-006-0310-4. [DOI] [PubMed] [Google Scholar]
- Buchanan KD, Huang S, Kim H, Macdonald RC, McPherson DD. Echogenic liposome compositions for increased retention of ultrasound reflectivity at physiologic temperature. J Pharm Sci. 2008;97:2242–9. doi: 10.1002/jps.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol. 1998;508(Pt 2):561–73. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–62. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, Cybulsky MI, Smith JD. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–7. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj A, Greene R, Klegerman M, McPherson DD. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol. 1999;33:867–75. doi: 10.1016/s0735-1097(98)00607-x. [DOI] [PubMed] [Google Scholar]
- Dustin M, Staunton D, Springer T. Supergene families meet in the immune system. Immunol Today. 1988;9:213–5. doi: 10.1016/0167-5699(88)91216-9. [DOI] [PubMed] [Google Scholar]
- Ferrante EA, Pickard JE, Rychak J, Klibanov A, Ley K. Dual targeting improves microbubble contrast agent adhesion to VCAM-1 and P-selectin under flow. J Control Release. 2009;140:100–7. doi: 10.1016/j.jconrel.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JW, Hawkins S, Clowes AW. Pharmacologic inhibition of nitric oxide synthases and cyclooxygenases enhances intimal hyperplasia in balloon-injured rat carotid arteries. J Vasc Surg. 2004;40:115–22. doi: 10.1016/j.jvs.2004.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- Goertz DE, Frijlink ME, Tempel D, Bhagwandas V, Gisolf A, Krams R, de Jong N, van der Steen AF. Subharmonic contrast intravascular ultrasound for vasa vasorum imaging. Ultrasound Med Biol. 2007;33:1859–72. doi: 10.1016/j.ultrasmedbio.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Goertz DE, Frijlink ME, Tempel D, van Damme LC, Krams R, Schaar JA, Ten Cate FJ, Serruys PW, de Jong N, van der Steen AF. Contrast harmonic intravascular ultrasound: a feasibility study for vasa vasorum imaging. Invest Radiol. 2006;41:631–8. doi: 10.1097/01.rli.0000229773.11715.da. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Huang SL, Warnick D, Stein A, Rabbat M, Madhav T, Kane B, Nagaraj A, Klegerman M, MacDonald R, McPherson D. Left ventricular thrombus enhancement after intravenous injection of echogenic immunoliposomes: studies in a new experimental model. Circulation. 2002a;105:2772–8. doi: 10.1161/01.cir.0000017500.61563.80. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Rabbat M, Jain P, Belkind N, Huang SL, Nagaraj A, Klegerman M, Macdonald R, McPherson DD. A physiologic flow chamber model to define intravascular ultrasound enhancement of fibrin using echogenic liposomes. Invest Radiol. 2002b;37:215–21. doi: 10.1097/00004424-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Hamilton AJ, Huang SL, Warnick D, Rabbat M, Kane B, Nagaraj A, Klegerman M, McPherson DD. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004;43:453–60. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- Hitchcock KE, Caudell DN, Sutton JT, Klegerman ME, Vela D, Pyne-Geithman GJ, Abruzzo T, Cyr PE, Geng YJ, McPherson DD, Holland CK. Ultrasound-enhanced delivery of targeted echogenic liposomes in a novel ex vivo mouse aorta model. J Control Release. 2010;144:288–95. doi: 10.1016/j.jconrel.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Hamilton AJ, Tiukinhoy SD, Nagaraj A, Kane BJ, Klegerman M, McPherson DD, MacDonald RC. Liposomes as ultrasound imaging contrast agents and as ultrasound-sensitive drug delivery agents. Cell Mol Biol Lett. 2002a;7:233–5. [PubMed] [Google Scholar]
- Huang SL, Hamilton AJ, Pozharski E, Nagaraj A, Klegerman ME, McPherson DD, MacDonald RC. Physical correlates of the ultrasonic reflectivity of lipid dispersions suitable as diagnostic contrast agents. Ultrasound Med Biol. 2002b;28:339–48. doi: 10.1016/s0301-5629(01)00512-9. [DOI] [PubMed] [Google Scholar]
- Huang SL, Kee PH, Kim H, Moody MR, Chrzanowski SM, Macdonald RC, McPherson DD. Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J Am Coll Cardiol. 2009;54:652–9. doi: 10.1016/j.jacc.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Ley K. Adhesion molecules and atherogenesis. Acta Physiol Scand. 2001;173:35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006;5:85–92. [PubMed] [Google Scholar]
- Kasper HU, Schmidt A, Roessner A. Expression of the adhesion molecules ICAM, VCAM, and ELAM in the arteriosclerotic plaque. Gen Diagn Pathol. 1996;141:289–94. [PubMed] [Google Scholar]
- Kaufmann BA, Carr CL, Belcik JT, Xie A, Yue Q, Chadderdon S, Caplan ES, Khangura J, Bullens S, Bunting S, Lindner JR. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010;30:54–9. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–84. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96:327–36. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- Kim H, Moody MR, Laing ST, Kee PH, Huang SL, Klegerman ME, McPherson DD. In vivo volumetric intravascular ultrasound visualization of early/inflammatory arterial atheroma using targeted echogenic immunoliposomes. Invest Radiol. 2010;45:685–91. doi: 10.1097/RLI.0b013e3181ee5bdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rim Y, Kee P, Moody MR, Klegerman ME, Huang SL, McPherson DD, Laing ST. Molecular Imaging of Atheroma Components in vivo Using Targeted Echogenic Immunoliposomes. Circulation. 2012;126:A17488. [Google Scholar]
- Klegerman ME, Wassler M, Huang SL, Zou Y, Kim H, Shelat HS, Holland CK, Geng YJ, McPherson DD. Liposomal modular complexes for simultaneous targeted delivery of bioactive gases and therapeutics. J Control Release. 2010;142:326–31. doi: 10.1016/j.jconrel.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopechek JA, Haworth KJ, Raymond JL, Douglas Mast T, Perrin SR, Klegerman ME, Huang S, Porter TM, McPherson DD, Holland CK. Acoustic characterization of echogenic liposomes: frequency-dependent attenuation and backscatter. J Acoust Soc Am. 2011;130:3472–81. doi: 10.1121/1.3626124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza GM. Acoustically reflective liposomes and methods to make and use the same. University of Illinois and Northwestern University; 2000. [Google Scholar]
- Looft-Wilson RC, Billaud M, Johnstone SR, Straub AC, Isakson BE. Interaction between nitric oxide signaling and gap junctions: Effects on vascular function. Biochim Biophys Acta. 2012;1818:1895–902. doi: 10.1016/j.bbamem.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum AF, Borden MA, Dayton PA, Kruse DE, Simon SI, Ferrara KW. Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. J Control Release. 2006;111:128–34. doi: 10.1016/j.jconrel.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca D, Renaud G, van Soest G, Li X, Zhou Q, Shung KK, de Jong N, van der Steen AF. Contrast-Enhanced Intravascular Ultrasound Pulse Sequences for Bandwidth-Limited Transducers. Ultrasound Med Biol. 2013 doi: 10.1016/j.ultrasmedbio.2012.10.020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, von zur Muhlen C, Greaves DR, Neubauer S, Channon KM, Choudhury RP. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28:77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer F, Ruijter JM, Van Delft JL, Van Haeringen NJ. Nitric oxide induces vascular permeability changes in the guinea pig conjunctiva. Eur J Pharmacol. 1995;284:61–7. doi: 10.1016/0014-2999(95)00376-v. [DOI] [PubMed] [Google Scholar]
- Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br J Pharmacol. 2007;151:305–21. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiakos EP, Kouraklis G, Agapitos E, Perrea D, Karatzas G, Boudoulas H, Karayannakos PE. Expression of PDGF-A, TGFb and VCAM-1 during the developmental stages of experimental atherosclerosis. Eur Surg Res. 2001;33:264–9. doi: 10.1159/000049716. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–11. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, Aikawa E, Kelly K, Libby P, Weissleder R. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2:1213–22. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–51. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan K, Haworth KJ, Huang SL, Klegerman ME, McPherson DD, Holland CK. Stability of echogenic liposomes as a blood pool ultrasound contrast agent in a physiologic flow phantom. Ultrasound Med Biol. 2012;38:1970–81. doi: 10.1016/j.ultrasmedbio.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–8. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Rychak JJ, Klibanov AL, Ley KF, Hossack JA. Enhanced targeting of ultrasound contrast agents using acoustic radiation force. Ultrasound Med Biol. 2007;33:1132–9. doi: 10.1016/j.ultrasmedbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Rychak JJ, Lindner JR, Ley K, Klibanov AL. Deformable gas-filled microbubbles targeted to P-selectin. J Control Release. 2006;114:288–99. doi: 10.1016/j.jconrel.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Bayes-Genis A, Lesser JR, Sangiorgi M, Henry TD, Conover CA. Detecting vulnerable plaque using peripheral blood: inflammatory and cellular markers. J Interv Cardiol. 2003;16:231–42. doi: 10.1034/j.1600-0854.2003.8025.x. [DOI] [PubMed] [Google Scholar]
- Soetanto K, Watarari H. Development of magnetic microbubbles for drug delivery system (DDS) Jpn J Appl Phys. 2000;39:3230–32. [Google Scholar]
- Staunton DE, Dustin ML, Springer TA. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989;339:61–4. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Sukhova GK, Swanson SJ, Clinton SK, Ganz P, Cybulsky MI, Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788–803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- Taylor HJ, Chaytor AT, Evans WH, Griffith TM. Inhibition of the gap junctional component of endothelium-dependent relaxations in rabbit iliac artery by 18-alpha glycyrrhetinic acid. Br J Pharmacol. 1998;125:1–3. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toursarkissian B, Schwartz D, Eisenberg PR, Rubin BG. Arterial thrombosis induces early upregulation of intercellular adhesion molecule in the media. J Vasc Surg. 1997;26:663–9. doi: 10.1016/s0741-5214(97)70067-3. [DOI] [PubMed] [Google Scholar]
- Tsao PS, McEvoy LM, Drexler H, Butcher EC, Cooke JP. Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by L-arginine. Circulation. 1994;89:2176–82. doi: 10.1161/01.cir.89.5.2176. [DOI] [PubMed] [Google Scholar]
- van der Wal AC, Das PK, Tigges AJ, Becker AE. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am J Pathol. 1992;141:1427–33. [PMC free article] [PubMed] [Google Scholar]
- Wu J, Leong-Poi H, Bin J, Yang L, Liao Y, Liu Y, Cai J, Xie J. Efficacy of contrast-enhanced US and magnetic microbubbles targeted to vascular cell adhesion molecule-1 for molecular imaging of atherosclerosis. Radiology. 2011;260:463–71. doi: 10.1148/radiol.11102251. [DOI] [PubMed] [Google Scholar]