Abstract
Recent studies have identified a subset of memory T cells with stem cell-like properties (TSCM) that include increased longevity and proliferative potential. Here, we examined the dynamics of CD4+ TSCM during pathogenic SIV infection of rhesus macaques (RM) and nonpathogenic SIV infection of sooty mangabeys (SM). While SIV-infected RM show selective numeric preservation of CD4+ TSCM, SIV infection induced a complex perturbation of these cells defined by depletion of CD4+CCR5+ TSCM, increased rates of CD4+ TSCM proliferation, and high levels of direct virus infection. The increased rates of CD4+ TSCM proliferation in SIV-infected RM correlated inversely with the levels of central memory CD4+ T cells (TCM). In contrast, nonpathogenic SIV infection of SM evidenced preservation of both CD4+ TSCM and CD4+ TCM, with normal levels of CD4+ TSCM proliferation, and lack of selective depletion of CD4+CCR5+ TSCM. Importantly, SIV DNA was below the detectable limit in CD4+ TSCM from eight out of ten SIV-infected SM. We propose that increased proliferation and infection of CD4+ TSCM may contribute to the pathogenesis of SIV infection in RM.
Introduction
Pathogenic HIV infection of humans and SIV infection of rhesus macaques (RM) are characterized by progressive depletion of CD4+ T cells and development of a lethal state of immunodeficiency termed AIDS (1). In contrast, SIV infection of African nonhuman primate species that are natural hosts for the virus, such as the sooty mangabeys (SM) and the African green monkeys (AGM), are typically nonpathogenic despite high virus replication (2). While the mechanism responsible for the development of AIDS remains incompletely understood, a series of recent studies have emphasized the role played by chronic immune activation and the direct infection of CD4+ central memory T cells (CD4+ TCM), with these two phenomena being significantly reduced in SIV-infected SM as compared to RM (3). During pathogenic HIV and SIV infections, high levels of direct virus infection of CD4+ TCM are associated with the depletion of CD4+ T cells in blood, lymph nodes, and mucosal tissues, thus suggesting a direct link between virus-mediated CD4+ TCM killing, CD4+ TCM depletion, and onset of a clinically relevant immunodeficiency (4-8). According to this model, CD4+ TCM are essential to maintain the overall CD4+ T cell homeostasis due to their in vivo longevity and high proliferative potential resulting in the ability to maintain their pool as well as the more differentiated pool of CD4+ effector memory (TEM) T cells (9, 10). Interestingly, infection of CD4+ TCM seems to play a significant role in the persistence of reservoirs of latently infected cells in HIV-infected individuals treated with antiretroviral therapy (11), and low levels of latent CD4+ TCM infection are present in HIV-infected individuals that control virus replication either spontaneously (7) or after ART (8).
A series of recent studies has identified a phenotypically and functionally novel subset of memory T cells with stem cell-like properties that were termed “T memory stem cells” or TSCM (12-15). These cells represent the “stem cells” of the memory T cell compartment, as they are uniquely able to self-renew as well as differentiate into all other memory T cell subsets (i.e., TCM, TEM, and transitional memory TTM) (13). Additional properties of TSCM include increased longevity and higher proliferative potential when compared to other T cell memory subsets (13). Phenotypically, TSCM are defined in humans as CD45RA+CD45RO−CD62L+CCR7+CD27+CD28+CD127+CD95+CD122+, and in RM and pigtailed macaques as CD45RA+CCR7+CD27+CD28+CD127+CD95+ (13, 15). In both humans and RM, TSCM express levels of CXCR3, Bcl-2, and LFA-1 intermediate between naive and TCM (13, 15). TSCM are found predominantly in peripheral blood and secondary lymphoid tissues, but not in mucosal tissues (15). In the context of SIV infection, CD8+ TSCM are involved in the long-term maintenance of virus-specific CD8+ T cell-mediated responses (15). At this time, however, the contribution of CD4+ TSCM to HIV or SIV pathogenesis remains unknown.
In this study, we examined for the first time CD4+ TSCM in both healthy and SIV-infected RM and SM. We found that the absolute number of CD4+ TSCM is preserved during both pathogenic and nonpathogenic SIV infections, but SIV-infected RM showed a selective depletion of CD4+CCR5+ TSCM. We also found that, in SIV-infected RM, but not in SIV-infected SM, CD4+ TSCM display significantly higher levels of proliferation that correlate inversely with both percentage and absolute number of CD4+ TCM. Importantly, substantial levels of direct virus infection of CD4+ TSCM were seen only in SIV-infected RM, with the majority of SIV-infected SM lacking SIV DNA within CD4+ TSCM. Based on these data we propose that increased proliferation and infection rates of CD4+ TSCM may play a role in the pathogenesis of SIV infection in RM. Based on their longevity and high levels of direct virus infection in pathogenic SIV-infection, we postulate CD4+TSCM may be an important site for the HIV/SIV reservoir as well as for maintaining memory T cell homeostasis.
Materials and Methods
Animals
Twenty-seven SIV-uninfected RM and 13 SIV-uninfected SM, plus 39 chronically SIV-infected RM and 19 chronically SIV-infected sooty SM were included in this study. All SIV-infected RM had been previously infected intravenously (i.v.) with SIVmac239 or SIVmac251. To obtain frequency of infection data from SM, blood was collected from six experimentally infected and four naturally infected SM. Four experimentally infected SM were infected i.v. with 0.5 ml of plasma (titrated to 107 SIV RNA copies/ml) from a naturally SIVsmm-infected SM and two experimentally infected SM were inoculated i.v. with 25 ng p27 equivalent of SIVsmE041 (primary isolate derived from a naturally SIVsmm-infected SM, 31). Of the SIV-infected SM, eight were heterozygous for the wild type CCR5 allele and the previously described delta 2 or delta 24 alleles that are not associated with reduced susceptibility to infection in the heterozygous state (29). In RM, acute infection was defined as day 7 to 14 post infection, early stage chronic infection was defined as day 42 to 84 post infection, and late chronic infection was defined as day 128 to 365 post infection. We obtained complete blood counts (CBC) for 16 SIV-uninfected, 18 acutely infected, 11 early chronic, and 10 late chronic RM, thus absolute numbers could only be calculated from these animals. All animals were anesthetized prior to the performance of any procedure, and proper steps were taken to ensure the welfare and to minimize the suffering of all animals in these studies. The animals were housed at the Yerkes National Primate Research Center of Emory University and maintained in accordance with US National Institutes of Health guidelines under IACUC approved protocols. Anesthesia was used for all blood collections.
Tissue processing
Peripheral blood mononuclear cells (PBMCs) were freshly isolated from whole blood by density centrifugation or sodium citrate CPT tubes. Frozen PBMCs were thawed in 37 degree water bath and used immediately.
Immunophenotyping
Immunophenotyping was performed according to standard procedures and monoclonal antibodies cross-reactive in both SM and RM were used. The following antibodies were used for immunophenotyping of CD4+TSCM in SM and RM PBMCs: Live/Dead Fixable Aqua from Invitrogen, CD14-V500 (M5E2), CD16-V500 (3G8), CD3-APC-Cy7 (SP34-2), CD45RA-APC (5H9), CCR7-PE-Cy7 (3D12), Ki67-Alexa 700 (B56), CCR5-PE (3A9), CD95-PE-Cy5 (DX2), CXCR3-PerCP-Cy5.5 (1C6/CXCR3), CD11a-FITC (HI111), CD122-Biotin (Mik-β3), streptavidin-PE, all from Becton Dickinson. CD4-BV650 (OKT4), CD8-BV711 (RPA-T8), CD27-BV570 or -BV605 (O323), streptavidin-605 all from Biolegend, CD28-ECD (CD28.2) from Beckman Coulter. Flow cytometric acquisition was carried out on an LSRII flow cytometer driven by the FACS DiVa software package (Becton Dickinson). Analysis of the acquired data was carried out using FlowJo (Tree Star) and PRISM (Graph Pad) software.
Cell Sorting
Sorting of CD4+ TN, TSCM, TCM, and TEM cells from SIV-infected rhesus macaques and sooty mangabeys was performed on a FACS Aria II flow cytometer (Becton Dickinson). Cells were initially gated on the basis of light scatter, followed by positive staining for CD3 and CD4 and negative staining for Live/Dead Aqua. CD4+ TN (CD28+CD95−CCR7+), TCM (CD45RA−CD28+CD95+CCR7+) and TEM (CD45RA−CD95+CCR7−) cell subsets were gated on characteristic patterns of CD28, CD95, CCR7, and CD45RA. CD4+ TSCM cells were sorted as CD45RA+CCR7+CD95+CD122+. This population was determined to be uniformly positive for CD27, CD28 (Figure 1A) and CD127 (data not shown).
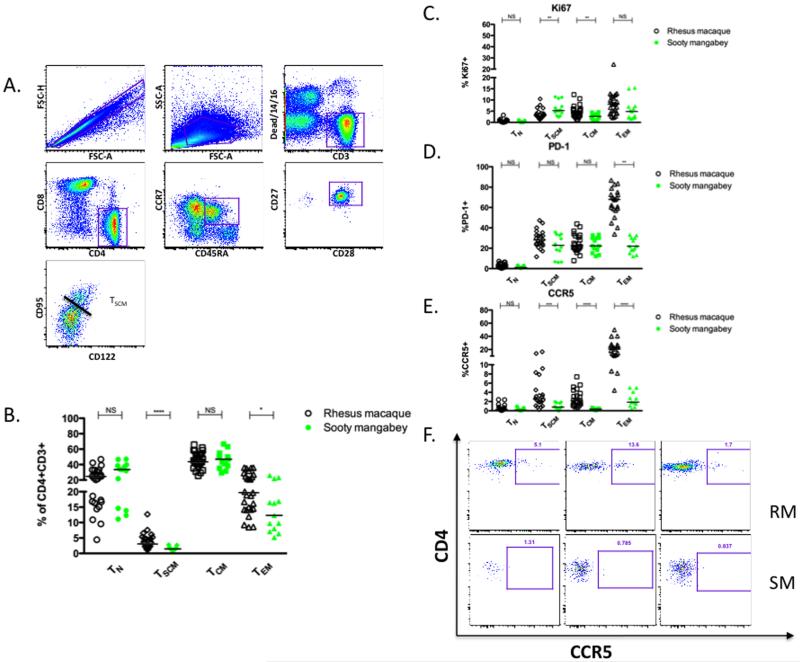
Figure 1. Identification of CD4+ TSCM in healthy RM and SM.
(A) Flow cytometric analysis of PBMC from a representative SIV-uninfected RM. CD4+ TSCM were defined as shown by expression of CD45RA+CCR7+CD27+CD28+CD95+CD122+. (B) Frequencies of circulating CD4+ T cell subsets (TN, TSCM, TCM, TEM) in 27 SIV-uninfected RM and 13 SIV-uninfected SM along with the fraction of each subset expressing Ki67 (C), PD-1 (D), and CCR5 (E). TN, TCM, TEM were defined using CD95, CD28 and CCR7: TN (CD28+CD95−CCR7+), TCM (CD45RA−CD28+CD95+CCR7+), TEM (CD45RA−CD95+CCR7−), not excluding TSCM from TCM compartment for phenotypic analysis. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, NS = not significant (Mann-Whitney). Bars are drawn at the median. (F) Representative CCR5 staining on CD4+ TSCM cells from 3 SIV-uninfected RM and SIV-uninfected SM.
Plasma viral load
Plasma viral RNA quantification was determined as previously described (16).
Quantitative PCR for SIV gag DNA
Quantification of SIVmac gag or SIVsmm utr DNA was performed as described in (17, 18). For cell number quantification, quantitative PCR was performed simultaneously for monkey albumin gene copy number. Albumin primers and probe along with qPCR conditions were previously described in (5). The sensitivity of the assay is 5 SIV DNA copies per 105 cells. Samples with undetectable SIV DNA were assigned a level of half of the lower limit of detection for graphical purposes and statistical analysis.
Statistical Analyses
Comparisons between frequencies in RM and SM (Figure 1B-E and Supplementary Figure 1) and SIV-uninfected and SIV-infected SM (Figure 5) were carried out using a non-parametric Mann Whitney test. Comparisons between frequencies of CD4+ T cell subsets over time during pathogenic infection of RM (Figures 2-4) were carried out using a Kruskal Wallis test. Comparisons between frequency of infection in RM and SM (Figure 6) were carried out using non-parametric Mann-Whitney test. Correlations were determined using the non-Gaussian Spearman correlation. Significance was attributed at p < 0.05. All analyses were conducted using GraphPad Prism 5.0.
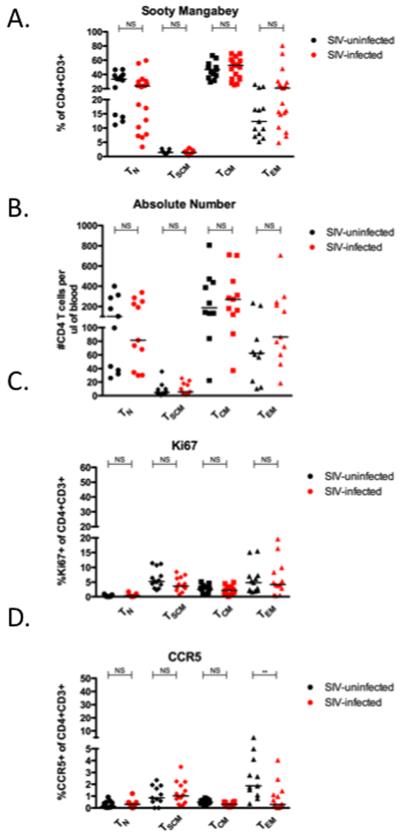
Figure 5. CD4+ TSCM are unperturbed during non-pathogenic SIV infection of SM.
Comparison of frequency (A) and absolute number (B) of CD4+ TN, TSCM, TCM, TEM in SIV-uninfected and chronically SIV-infected SM. (C) Frequency of CCR5+ T cells in each CD4+ T cell subset in SIV-uninfected and chronically SIV-infected SM. (D) Frequency of proliferating CD4+ T cell subsets, as measured by Ki67 expression in both uninfected and chronically SIV-infected SM. ** p < 0.01, NS = not significant (Mann-Whitney). Bars are drawn at the median.
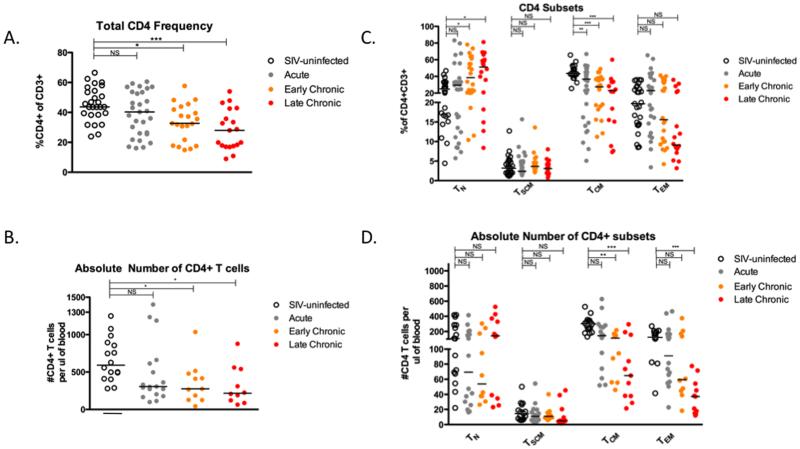
Figure 2. Selective preservation of CD4+ TSCM cells during pathogenic SIV infection of RM.
Frequency (A) and absolute number (B) of total CD3+CD4+ lymphocytes in PBMC of RM during pathogenic SIV infection. Frequency (C) and absolute number (D) of TN, TSCM, TCM, TEM subsets in PBMC of RM during pathogenic SIV infection. Data in (A) and (C) represent the following RM: 27 SIV-uninfected, 29 acutely infected (day 7-14) 22 early chronic infection (day 65-84), and 19 late chronic infection (day 128-365). Data in (B) and (D) represent the following RM: 16 SIV-uninfected, 18 acutely infected (day 7-14), 11 early chronic (day 65), and 10 late chronic (day 128-365). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, NS = not significant (Kruskal-Wallis test, compared to SIV-uninfected). Bars are drawn at the median.
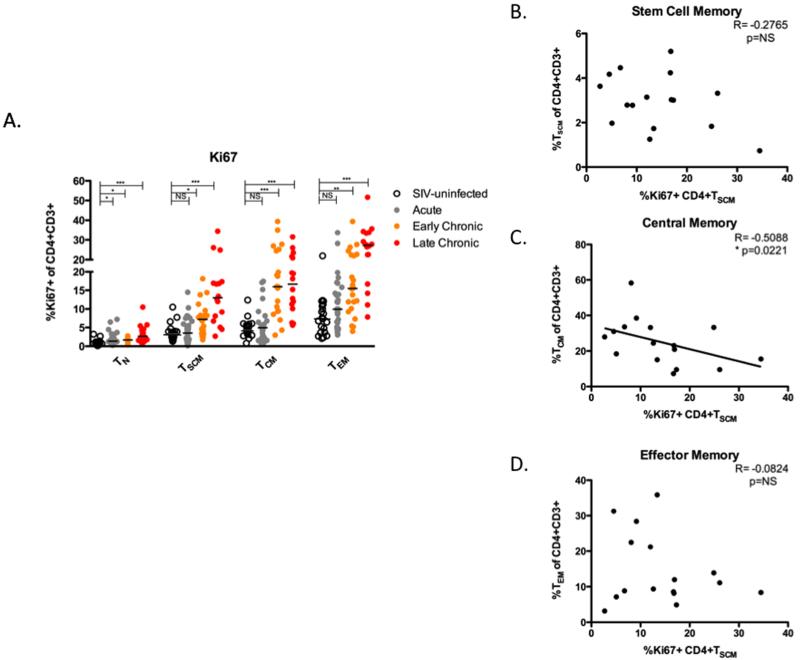
Figure 4. Pathogenic SIV infection of RM is associated with increased proliferation of CD4+ TSCM that inversely correlates with the level of CD4+ T CM.
(A) Frequency of Ki67+ TN, TSCM, TCM, TEM in PBMC during pathogenic SIV infection. Data represents the following RM: 22 SIV-uninfected, 29 acutely infected (day 7-14), 22 early chronic infection (day 65-84), and 18 late chronic infection (day 128-365). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, NS = not significant (Kruskal-Wallis test, compared to SIV-uninfected). Bars are drawn at the median. (B-D) Correlations of the fraction of Ki67+CD4+ TSCM and fraction of circulating CD4+ TSCM, CD4+ TCM, and CD4+ TEM during late chronic infection. R and p values were determined by Spearman correlation.
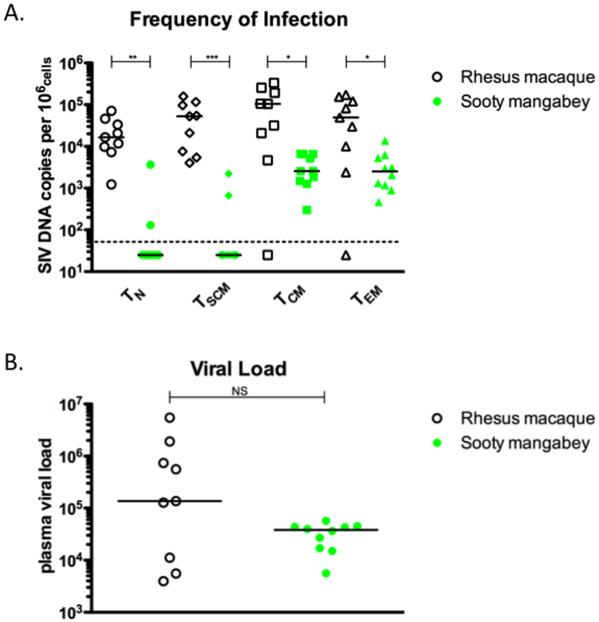
Figure 6. Robust levels of CD4+ TSCM infection in vivo are observed in SIV-infected RM but not in SIV-infected SM.
(A) Fraction of SIV-infected CD4+ TN, TSCM, TCM, and TEM cells, as determined by quantitative PCR for the number of SIV gag (RM) or SIV utr (SM) DNA copies per cell in 9 SIVmac251-infected RM, 6 experimentally SIVsmm-infected SM, and 4 naturally SIVsmm-infected SM. Cell number was determined using simultaneous PCR for albumin gene copy number. (B) Plasma viral load of RM and SM shown in (A) as determined by RT-PCR. * p < 0.05, ** p < 0.01, *** p < 0.001, NS = not significant (Mann-Whitney test). Bars are drawn at the median.
Results
Identification of CD4+ TSCM in healthy rhesus macaques and sooty mangabeys
CD4+ and CD8+ TSCM have been phenotypically identified in humans as CD45RA+CD45RO−CD62L+CCR7+CD27+CD28+CD127+CD95+CD122+, and in RM and pigtailed macaques are defined as CD45RA+CCR7+CD27+CD28+CD127+CD95+ (13, 15). We have first confirmed this immunophenotypic definition in healthy RM (see Figure 1A for gating strategy) and SM (data not shown). As expected, CD4+ TSCM isolated from both RM and SM expressed intermediate levels of CXCR3 and LFA-1 that were between those of naive and central memory CD4+ T cells (Supplementary Figure 1). We also confirmed in healthy RM that CD4+ TSCM can be readily identified in the blood, lymph nodes, bone marrow, and spleen, but are present at a lower frequency in the intestinal mucosa (data not shown). We next compared the levels of circulating CD4+ TSCM in healthy SIV-uninfected RM and SM as percentage of total CD4+ T cells. As shown in Figure 1B, the percentage of CD4+ TSCM ranged between 1-8% in RM and 0.5-3% in SM, with the levels observed in RM being significantly higher (p = 0.0004). Interestingly, CD4+ TSCM from SIV-uninfected SM show higher levels of proliferation (measured as expression of Ki67) as compared to RM (p = 0.0034, Figure 1C), perhaps suggesting that a relatively smaller pool of CD4+ TSCM maintains CD4+ memory T cell homeostasis through higher baseline rates of proliferation in SM. We also found that CD4+ TSCM from RM also express slightly higher levels of the inhibitory marker PD-1 as compared to CD4+ TSCM from SM, though this trend was not statistically significant (Figure 1D).
Several previous studies have shown that CD4+ T cells of both SIV-infected and uninfected SM express lower levels of the SIV coreceptor CCR5 than CD4+ T cells of humans and RM, and that this difference is particularly evident for CD4+ TCM (3, 19). We next examined the levels of CCR5 expression on CD4+ TSCM from healthy RM and SM and, consistent with previous findings, we found significantly higher percentages of CCR5+CD4+ TSCM from RM compared to SM (p = 0.0009, Figure 1E). Three representative examples of CCR5 staining on CD4+ TSCM are shown in Figure 1F, which emphasize the almost complete absence of CCR5 on CD4+ TSCM of SM.
Numeric preservation of CD4+ TSCM during pathogenic SIV infection of RM
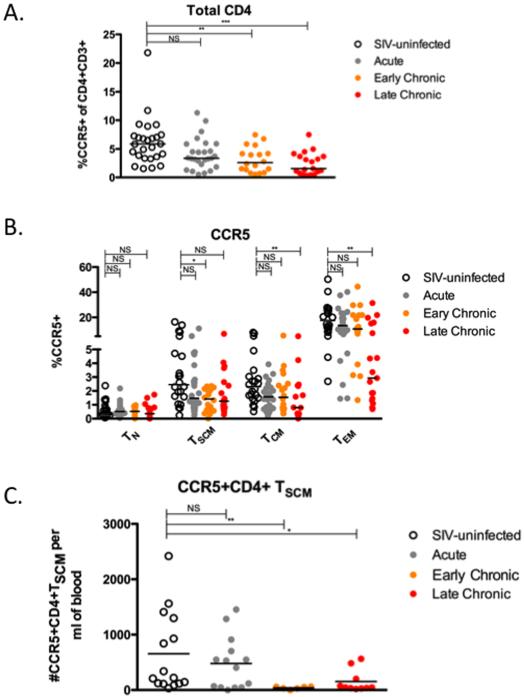
Pathogenic SIV infection of RM is characterized by a progressive depletion of CD4+ T cells from blood and mucosal tissues, which is typically associated with the loss of CD4+ TCM homeostasis (4). To examine the dynamics of CD4+ TSCM during pathogenic SIV infection of RM, we examined a total of 51 RM, including healthy SIV-uninfected animals and SIV-infected animals at different stages of infection. Consistent with many previous studies (20-23), the animals studied here exhibited the well-characterized progressive depletion of circulating CD4+ T cells associated with SIV infection, measured as the fraction of CD3+ T lymphocytes (Figure 2A) or absolute number of cells per micro liter of blood (Figure 2B). We next examined, in the same animals, the relative distribution of four key CD4+ T cell subsets, i.e., naive (CD28+CD95−); CD4+ TSCM (CD45RA+CCR7+CD27+CD28+CD95+CD122+); CD4+ TCM (CD28+CD95+CCR7+); and CD4+ TEM (CD95+CCR7−). As expected, levels of both CD4+ TCM and TEM (primary targets for SIV infection) were altered by SIV infection, with a significant decline of the fraction and absolute number of CD4+ TCM in both early and late chronic SIV infection (p < 0.01 and p < 0.001, respectively, Figure 2C-D), and a significant decline of the absolute number of CD4+ TEM in late chronic SIV infection (p < 0.001, Figure 2D). Interestingly, neither the fraction nor the absolute number of CD4+ TSCM was decreased in either acute or chronic SIV infection of RM (Figure 2C-D). As such, these data indicate that pathogenic SIV infection of RM is not associated with a significant numerical decline of circulating CD4+ TSCM.
Pathogenic SIV infection of RM is associated with a significant depletion of CCR5+CD4+ TSCM
CCR5 is the main coreceptor for both HIV and SIV, and depletion of CD4+CCR5+ T cells, particularly in mucosal tissues, is a well-known hallmark of pathogenic HIV and SIV infections (21, 24, 25). In this study, we first confirmed the depletion of circulating CD4+CCR5+ T cells that begins in acute infection and continues during early and late chronic SIV infection of RM (Figure 3A). We next measured, in our four groups of animals, the levels of CCR5 expression on the four studied subsets of CD4+ T cells (TN, TSCM, TCM, and TEM). As shown in Figure 3B, we found that the fraction of CCR5+ cells is significantly decreased during late chronic SIV infection of RM in both CD4+ TCM and TEM (p < 0.05 and p < 0.01, respectively). Interestingly, we observed that the fraction of CCR5+CD4+ TSCM was significantly decreased in SIV-infected RM examined during early chronic SIV infection compared to uninfected animals (p < 0.05). Although the median level of CCR5+CD4+TSCM was also decreased in late chronic SIV infection, this difference was not statistically significant when compared to SIV-uninfected animals, likely due to a wide range of values. Importantly however, we found a significant depletion of the absolute number of CCR5+CD4+TSCM (Figure 3C) during both early and late chronic SIV infection. Together with the data shown in Figure 2, these results indicate that pathogenic SIV infection of RM is associated with a depletion of CCR5+CD4+ TSCM that occurs in the context of an overall preservation of the CD4+ TSCM pool.
Figure 3. Pathogenic SIV infection of RM is associated with significant depletion of CCR5+CD4+ TSCM.
(A) Frequency of total CCR5+CD4+ T cells as a frequency of CD3+ lymphocytes during pathogenic SIV infection of RM. (B) Frequency of CCR5+ cells found in each of the four subsets (TN, TSCM, TCM, and TEM). Data in (A) and (B) represent the following RM: 26 SIV-uninfected, 26 acutely infected (day 7-14), 18 early chronic infection (day 65-84), and 19 late chronic infection (day 128-D365). (C) Absolute number of CCR5+CD4+ TSCM during pathogenic SIV infection of RM per ml of peripheral blood. Data in (C) represent the following RM: 15 SIV-uninfected, 15 acutely infected, 6 early chronic SIV infection, and 10 late chronic SIV infection. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, NS = not significant (Kruskal-Wallis, compared to SIV-uninfected). Bars are drawn at the median.
Pathogenic SIV infection of RM is associated with increased proliferation of CD4+ TSCM that correlates inversely with circulating level of CD4+ TCM
To further investigate whether and to what extent pathogenic SIV infection of RM perturbs the homeostasis of CD4+ TSCM we next measured, in our four groups of animals, the expression of the proliferation marker Ki67 in the studied subsets of CD4+ T cells (TN, TSCM, TCM, and TEM). Consistent with previous studies (4), we observed that early and late chronic SIV infection is associated with increased proliferation of both TCM and TEM (Figure 4A). Interestingly, the percentage of Ki67+CD4+ TSCM was also significantly increased in SIV-infected RM examined during both early (p < 0.05) and late (p < 0.001) stages of infection as compared to healthy uninfected animals (Figure 4A). The observed increase in the fraction of cycling CD4+ TSCM could be the result of homeostatic proliferation in response to the overall depletion of memory CD4+ T cells, due to chronic immune activation, or both. In an attempt to assess whether homeostatic proliferation may be responsible for the increased proliferation of CD4+ TSCM in SIV-infected RM, we investigated the relationship between the fraction of Ki67+CD4+ TSCM and the levels of the four studied CD4+ T cell subsets. As shown in Figure 4B-D, we found a significant inverse correlation between the percentage of Ki67+CD4+ TSCM and the percentage of circulating CD4+ TCM (p = 0.02), but not with the level of any of the other memory CD4+ T cell subsets. These data suggest that the increased proliferation of CD4+ TSCM observed in SIV-infected RM represents, at least in part, a compensatory response to the CD4+ TCM depletion induced by SIV infection.
Dynamics of CD4+ TSCM during nonpathogenic SIV infection of SM
Previous studies have shown that nonpathogenic SIV infection of SM is typically associated with preserved CD4+ T cell counts and low levels of immune activation (16, 26-28). Here we examined, for the first time, how SIV infection impacts the levels of CD4+ TSCM in SM. As shown in Figure 5A, we found no difference in the levels of any of the four studied subsets of CD4+ T cells (TN, TSCM, TCM, and TEM) in SIV-infected SM as compared to uninfected animals, in contrast to the depletion of TCM and TEM seen in chronic SIV infection of RM (Figure 2C). Similarly, no differences were found between SIV-infected and uninfected SM with respect to the absolute number of CD4+ TSCM per micro liter of blood (Figure 5B). We next investigated whether SIV infection of SM is associated with a selective depletion of CD4+CCR5+ TSCM, and unlike the loss of these cells in SIV-infected RM (Figure 3B), we found similar levels of CD4+CCR5+ TSCM in SIV-infected and uninfected SM (Figure 5C). To determine whether SIV infection of SM is associated with increased proliferation of CD4+ TSCM, we measured the fraction of these cells expressing Ki67. As shown in Figure 5D, we found no difference in the fraction of CD4+ TSCM expressing Ki67 among SIV-infected and uninfected SM. Taken together, these data indicate the that nonpathogenic SIV infection of SM is characterized by overall preservation of the CD4+ TSCM compartment, involving both CD4+CCR5− and CD4+CCR5+ cells, and does not result in increased turnover of this memory cell subset, consistent with the previously described maintenance of peripheral CD4+ T cell homeostasis in SIV-infected SM (3).
Robust levels of CD4+ TSCM infection in vivo are observed in SIV-infected RM but not in SIV-infected SM
In two prior studies we have shown that pathogenic SIV infection of RM is associated with higher levels of SIV DNA in both circulating CD4+ TCM as well as lymph node-based CD4+ T cells as compared to SIV-infected SM (3, 5). These results led us to hypothesize that preservation of the CD4+ TCM compartment is a key determinant of the nonpathogenic nature of SIV infection of SM. To expand upon these observations, here we measured the levels of SIV DNA in flow-cytometrically sorted samples of the four studied subsets of CD4+ T cells (TN, TSCM, TCM, and TEM) in nine SIVmac251-infected RM and ten SIVsmm-infected SM. Please note that for sorting we have phenotypically defined TCM as CD45RA−CD28+CD95+CCR7+ and TEM as CD45RA−CD95+CCR7−, which differs slightly from the markers used previously in Paiardini et al, and Brenchley et al (3,5). The reason for this choice was to incorporate the definition of TSCM in nonhuman primates established by Lugli et al (15). As shown in Figure 6A, we observed a robust frequency of infection (i.e., greater than 1/1000 cells) in CD4+ TSCM isolated from nine out of nine SIV-infected RM. In contrast, SIV DNA levels in CD4+ TSCM were undetectable in eight out of ten SIV-infected SM. As previously reported, (3,5), the level of SIV DNA was higher in CD4+ TN and CD4+ TCM of RM as compared to SM. Shown in Figure 6B, plasma viral loads of SIV-infected RM tended to be higher than those of SM, although this trend was not statistically significant. Overall, these results indicate that in vivo infection of CD4+ TSCM is frequent during pathogenic SIV infection of RM, but is absent or rare during nonpathogenic SIV infection of SM.
Discussion
In the past several years, the mechanisms responsible for AIDS pathogenesis have been extensively investigated in the pathogenic model of SIV infection of RM and the nonpathogenic model of SIV infection of SM (2, 29). These comparative studies led to the definition of a model in which chronic immune activation and disrupted homeostasis of central memory CD4+ T cells are the key mechanisms responsible for the lentivirus-associated immunodeficiency (3, 4, 6, 30). More recently, a novel subset of memory CD4+ and CD8+ T cells has been identified, and named “memory stem cell” (TSCM) based on their unique ability to generate all other memory T cell subsets de novo (13, 15). In this study, we sought to determine how CD4+ TSCM are affected by pathogenic and nonpathogenic SIV infections of RM and SM, respectively. To the best of our knowledge this study represents the first systematic investigation of the dynamics of CD4+ TSCM during SIV infection.
The main results of this study are that: (i) CD4+ TSCM are numerically preserved during both pathogenic and nonpathogenic SIV infections, with SIV-infected RM showing a selective depletion of CD4+CCR5+ TSCM; (ii) CD4+ TSCM show significantly higher levels of proliferation that correlate inversely with the percentage of CD4+ TCM in SIV-infected RM, but not SM; (iii) robust levels of direct virus infection of CD4+ TSCM are found only in SIV-infected RM, with the majority of SIV-infected SM showing no evidence of CD4+ TSCM infection. The observation that CD4+ TSCM of healthy, SIV-uninfected RM express higher levels of CCR5 as compared to CD4+ TSCM of healthy SM is consistent with previous findings in TCM and a potential inherent resistance to direct infection at the virus entry level in SM (3).
Taken together, these data allow us to delineate a model for the role of CD4+ TSCM in SIV pathogenesis. In SIV-infected RM, the we observe significant perturbation of the homeostasis of CD4+ TSCM in three ways, as these cells can be directly infected by the virus, are depleted in the percentage of cells expressing CCR5, and manifest increased proliferation. In contrast, none of these perturbations in the TSCM pool are present in SIV-infected SM. The significant inverse correlation between CD4+ TSCM proliferation and CD4+ TCM depletion we observed in SIV-infected RM suggests that CD4+ TSCM proliferate at least in part to compensate for the progressive loss of CD4+ TCM. While the overall numeric homeostasis of CD4+ TSCM is not altered in SIV-infected RM, it is possible that this cellular compartment loses, in time, the ability to effectively support the maintenance of CD4+ TCM. Whether and to what extent the deficit of CD4+ TCM that is associated with progression to AIDS is related to a functional exhaustion of CD4+ TSCM as opposed to the direct depleting effects of virus infection and/or bystander apoptosis remains to be determined.
A striking difference between SIV-infected RM and SIV-infected SM is the level of virus infection of these cells as measured by fraction of SIV DNA positive cells. While all SIV-infected RM showed a calculated percentage of CD4+ TSCM infection between 0.3 and 10%, eight out of ten SIV-infected SM show undetectable levels of CD4+ TSCM infection (i.e., less than 0.005%). Given the lower levels of CCR5 expression on CD4+ TSCM of SM as compared to RM, one possibility is that these cells are resistant to virus infection at the entry level, analogous to what has been observed for CD4+ TCM (3). It should be noted, however, that other co-receptors, in addition to CCR5 (31, 32), as well as post-entry factors may be involved in determining the different levels of SIV infection in CD4+ TSCM of RM and SM. Unfortunately, due to the relatively small number of CD4+ TSCM that can be isolated from SM, we were not able to directly confirm in vitro that these cells are intrinsically more resistant to in vitro SIV infection then CD4+ TSCM of RM.
A recent compelling study indicates that CD4+ TSCM represent an increasingly important component of the persistent reservoir of latently infected cells in HIV-infected individuals treated with antiretroviral therapy (ART) (34). The observation that CD4+ TSCM are infected at high levels during pathogenic SIV infection of RM is consistent with the possibility that, once virus replication is suppressed by ART, a subset of these cells remain latently infected and may seed the previously described persistent reservoir in TCM (11). Under these circumstances, the contribution of CD4+ TSCM to the persistent reservoir may increase over time simply as a consequence of their enhanced proliferative ability. An intriguing corollary of this hypothesis is that, in SIV-infected SM, the absence of virus infection in CD4+ TSCM may result in an inability to maintain a persistent reservoir of latently infected CD4+ T cells when virus replication is suppressed by ART. An experiment in which SIV-infected SM are treated with ART for increasing periods of time is currently ongoing in our laboratory. We hope that the results of these studies will further elucidate the role of direct CD4+ TSCM infection as an obstacle to a functional cure for HIV infection.
In conclusion, this study provides evidence that pathogenic SIV infection of RM, but not nonpathogenic SIV infection of SM, is associated with significant infection and homeostatic perturbation of CD4+ TSCM. We therefore propose that CD4+ TSCM play an important role both in the pathogenesis of disease progression as well as the persistence of infection under ART.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grants R37 AI66998 to G.S., NICHD Child Health Research Career Development Award to A.C., RR000165/OD011132 to the Yerkes National Primate Research Center, and P30 AI050409 to the Emory Center for AIDS Research. We would like to thank the animal care and veterinary staff at the Yerkes National Primate Research Center.
REFERENCES
- 1.Brenchley JM, Silvestri G, Douek DC. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity. 2010;32:737–742. doi: 10.1016/j.immuni.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV hosts: showing AIDS the door. Science. 2012;335:1188–1193. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, Vinton C, Gordon SN, Bosinger SE, Francella N, Hallberg PL, Cramer E, Schlub T, Chan ML, Riddick NE, Collman RG, Apetrei C, Pandrea I, Else J, Munch J, Kirchhoff F, Davenport MP, Brenchley JM, Silvestri G. Low levels of SIV infection in sooty mangabey central memory CD(4)(+) T cells are associated with limited CCR5 expression. Nat Med. 2011;17:830–836. doi: 10.1038/nm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, Sylwester AW, Piatak M, Jr., Lifson JD, Maino VC, Sodora DL, Douek DC, Axthelm MK, Grossman Z, Picker LJ. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, Lifson JD, Silvestri G, Estes JD. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120:4172–4181. doi: 10.1182/blood-2012-06-437608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Descours B, Avettand-Fenoel V, Blanc C, Samri A, Melard A, Supervie V, Theodorou I, Carcelain G, Rouzioux C, Autran B. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin Inf Dis. 2012;54:1495–1503. doi: 10.1093/cid/cis188. [DOI] [PubMed] [Google Scholar]
- 8.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS pathogens. 2013:e1003211. doi: 10.1371/journal.ppat.1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 10.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2013;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E, Price DA, Waldmann TA, Restifo NP, Franchini G, Roederer M. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123:594–599. doi: 10.1172/JCI66327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, Kaur A, Lackner AA, Veazey RS, Silvestri G. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letvin NL, Daniel MD, Sehgal PK, Desrosiers RC, Hunt RD, Waldron LM, MacKey JJ, Schmidt DK, Chalifoux LV, King NW. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 18.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 19.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr., Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 21.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 24.Silvestri G, Fedanov A, Germon S, Kozyr N, Kaiser WJ, Garber DA, McClure H, Feinberg MB, Staprans SI. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J Virol. 2005;79:4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumpter B, Dunham R, Gordon S, Engram J, Hennessy M, Kinter A, Paiardini M, Cervasi B, Klatt N, McClure H, Milush JM, Staprans S, Sodora DL, Silvestri G. Correlates of preserved CD4(+) T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J Immunol. 2007;178:1680–1691. doi: 10.4049/jimmunol.178.3.1680. [DOI] [PubMed] [Google Scholar]
- 26.Taaffe J, Chahroudi A, Engram J, Sumpter B, Meeker T, Ratcliffe S, Paiardini M, Else J, Silvestri G. A five-year longitudinal analysis of sooty mangabeys naturally infected with simian immunodeficiency virus reveals a slow but progressive decline in CD4+ T-cell count whose magnitude is not predicted by viral load or immune activation. J Virol. 2010;84:5476–5484. doi: 10.1128/JVI.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenchley JM, Paiardini M. Immunodeficiency lentiviral infections in natural and non-natural hosts. Blood. 2011;118:847–854. doi: 10.1182/blood-2010-12-325936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhoeven D, Sankaran S, Dandekar S. Simian immunodeficiency virus infection induces severe loss of intestinal central memory T cells which impairs CD4+ T-cell restoration during antiretroviral therapy. J Med Primatol. 2007;36:219–227. doi: 10.1111/j.1600-0684.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 29.Elliott ST, Riddick NE, Francella N, Paiardini M, Vanderford TH, Li B, Apetrei C, Sodora DL, Derdeyn CA, Silvestri G, Collman RG. Cloning and analysis of sooty mangabey alternative coreceptors that support simian immunodeficiency virus SIVsmm entry independently of CCR5. J Virol. 2012;86:898–908. doi: 10.1128/JVI.06415-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riddick NE, Hermann EA, Loftin LM, Elliott ST, Wey WC, Cervasi B, Taaffe J, Engram JC, Li B, Else JG, Li Y, Hahn BH, Derdeyn CA, Sodora DL, Apetrei C, Paiardini M, Silvestri G, Collman RG. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog. 2010:6. doi: 10.1371/journal.ppat.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meythaler M, Martinot A, Wang Z, Pryputniewicz S, Kasheta M, Ling B, Marx PA, O’Neil S, Kaur A. Differential CD4+ T-lymphocyte apoptosis and bystander T-cell activation in rhesus macaques and sooty mangabeys during acute simian immunodeficiency virus infection. J Virol. 2009;83:572–583. doi: 10.1128/JVI.01715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klatt NR, Shudo E, Ortiz AM, Engram JC, Paiardini M, Lawson B, Miller MD, Else J, Pandrea I, Estes JD, Apetrei C, Schmitz JE, Ribeiro RM, Perelson AS, Silvestri G. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS pathog. 2010;6:e1000747. doi: 10.1371/journal.ppat.1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanderford TH, Slichter C, Rogers KA, Lawson BO, Obaede R, Else J, Villinger F, Bosinger SE, Silvestri G. Treatment of SIV-infected sooty mangabeys with a type-I IFN agonist results in decreased virus replication without inducing hyperimmune activation. Blood. 2012;119:5750–5757. doi: 10.1182/blood-2012-02-411496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014 doi: 10.1038/nm.3445. E pub doi:10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.