Abstract
Background
Cervical screening consumes substantial resources, but little is known about utilization in the United States or compliance with guideline recommendations.
Methods
To describe population screening coverage, utilization and outcomes and examine time trends from 2008 to 2011, cervical cytology reports from women residing in New Mexico (981,063 tests from 511,381 women) were evaluated.
Results
From 2008–2011 cervical screening utilization decreased at all ages, but especially in younger women, with a two-thirds reduction at ages 15–20 years. 94% of women aged 25–29 years were screened within 48 months but coverage decreased at older ages, to 69% at 45–49 years and 55% at 60–64 years. Intervals between screening tests were significantly longer in 2011 compared to 2008 (hazard ratio = 1.23, 95% CI = 1.22–1.24) although the commonest rescreening interval was 13 months. In 2011, 91.9% of screening tests for women aged 21–65 years were negative, 6.6% showed minor abnormalities, and 1.0% high grade abnormalities.. High grade abnormality rates were relatively constant over time, but minor abnormalities and atypical cells cannot rule out high-grade (ASC-H) were increasing.
Conclusion
This population-based evaluation of cervical screening shows high coverage under the age of 40 years but lower levels in older women. Screening under age 21 years is becoming less common and screening intervals are lengthening, reflecting updates in national screening guidelines.
Impact
Assessment of cervical screening intervals and population outcomes is essential for accurately estimating the impact and effectiveness of changing recommendations and vaccination against human papillomavirus infections.
Keywords: Cervical screening, utilization, outcome, coverage, New Mexico
Introduction
Although cervical cancer screening by cytology has never been subjected to a randomized clinical trial, there is indisputable evidence from disease trend data in whole populations and case-control studies that it has been highly effective in reducing the incidence of and mortality from cervical cancer in parts of the world where adequate infrastructure and program organization exists. The best outcomes have been achieved by organized programs such as those in Finland, Sweden, the Netherlands and the United Kingdom (1–8), and such programs are also able to regularly monitor performance, not only for cancer reduction, but also in terms of utilization and adherence to guidelines. With this information cost-effectiveness analyses can be performed to evaluate and modify screening recommendations, such as age at starting and stopping screening, screening interval and appropriate surveillance algorithms.
We believe the New Mexico HPV Pap Registry (NMHPVPR) is the first population-based registry in the United States (US) which is monitoring the full spectrum of cervical cancer preventive care. Its remit is to document patterns of cervical cancer screening utilization, outcomes and treatment of precancerous lesions and to facilitate surveillance of the population coverage and effectiveness of prophylactic HPV vaccination. This will enable evaluation of adherence to guidelines and monitoring of changes in disease prevalence as HPV vaccination and HPV-based screening become more widely introduced. In conjunction with the well-established New Mexico Tumor Registry, it will also facilitate cohort and case-control analysis of the impact of screening on cancer incidence and mortality, as has been done elsewhere (2, 8–19).
Cervical cancer screening recommendations in the US have undergone steady evolution as new knowledge becomes available. To enable appropriate interpretations of cervical screening surveillance information, an overall understanding of changes in cervical screening guidelines is necessary as clinical practice changes are gradually adopted and outcomes are potentially impacted. As such we briefly outline those changes that immediately preceded (since the year 2000), were coincident with, or occurred following the population-based evaluations presented in this report.
In 2002, the American Cancer Society (ACS) recommended annual Pap or bi-annual liquid based cytology (LBC), starting at the age 21 years or 3 years after the age of sexual initiation and stopping at age 70 following three negative Pap tests (20). ACS also made a preliminary recommendation for high-risk human papillomavirus (HR-HPV) every 3 years for women 30 and older. In 2003, American College of Obstetricians and Gynecologists (ACOG) and U.S. Preventive Services Task Force (USPSTF) updated their recommendations from the 1990’s. ACOG recommended annual cytology (no distinction between Pap or LBC) and extending screening intervals to 2–3 years in women 30 and older following 3 consecutive negative cytology screenings, with the same starting ages as recommended by the ACS and no specific recommendation to stop screening (21). USPSTF recommend cytology screening at least every 3 years, with the same starting ages as recommended by the ACS and stopping at the age of 65 years (22).
The ACS, the American Society for Colposcopy and Cervical Pathology (ASCCP), and the National Institutes of Health/National Cancer Institute (NIH/NCI), in 2004 and following the first U.S. Food-and-Drug approval of the clinical test for HR-HPV to be used in cervical cancer screening issued an interim guidance (23) for concurrent HR-HPV and cytology testing (“cotesting”) every 3 years, with a 12-month follow-up of women with HR-HPV negative and atypical squamous cells of undetermined significance (ASC-US) (HR-HPV-negative ASC-US) and 6–12 month follow-up of HR-HPV positive and normal/negative cytology (HR-HPV-positive negative cytology). The ASCCP reiterated these recommendations in 2006 and suggested that when HPV16 and HPV18 testing was available, women with HR-HPV-positive negative cytology who tested positive for either or both HPV16 and HPV18 could be referred immediately to colposcopy (24, 25). In 2009, both ASCCP (26) and ACOG (27) recommended that cervical cancer screening uniformly starts at the age of 21 years.
Finally, based on an even greater body of knowledge, following a lengthy process of carefully consideration by many individuals and organizations, new US screening guidelines by the major organizations that provide recommendations on cervical cancer screening were issued in 2012 (28–30). The 2012 revised recommendations are very briefly summarized as follows: screening for all women should begin at age 21 years; for women 21–29 years, cytology alone every 3 years is recommended; for women 30–65 years, co-testing every 5 years is recommended, or cytology alone every 3 years may be continued to age 65 years. Most women can discontinue screening at age 65 years or after hysterectomy with removal of the cervix.
This report examines screening utilization and population coverage and time trends in New Mexico to determine the impact of changes associated with these recommendations (20–27), notably initiating cervical cancer screening at the older age of 21years, extending the interval between screens and stopping screening at age 65 years. Here we document screening utilization and outcome before a high uptake of HPV-vaccination occurs which provides a baseline for determining the impact of the HPV vaccine on screening practices and disease incidence and allows evaluation of the implementation of more recent national cervical screening recommendations in the US (28–30).
Materials and Methods
Design and Overview
The New Mexico HPV Pap Registry
The NM HPV Pap Registry (NMHPVPR) is located at the University of NM (Albuquerque, NM, USA) and acts as a designee of the New Mexico Department of Health (NMDOH). The NMHPVPR operates under NMAC 7.4.3, which specifies the list of Notifiable Diseases and Conditions for the state of NM. In 2006, NMAC 7.4.3 specified that laboratories must report to the NMHPVPR all cytology tests and results, cervical pathology, and HPV tests performed on women residing in NM. NMAC 7.4.3 was updated in 2009 to also include vulvar and vaginal pathology (31). Ongoing evaluations of cervical screening, diagnosis and treatment by the NMHPVPR have been reviewed and approved under exempt status by the University of New Mexico Human Research Review Committee.
Data on cervical cytology were obtained for the period 1 Jan 2007 to 1 Jan 2012 from 9 laboratories in NM and 9 out-of-state laboratories (corporate entities operating multiple facilities were counted as one unit). All Hospitals and clinical practices in NM report through these laboratories. Probabilistic matching and linking of different tests to the same woman was performed using Registry Plus Link Plus (32) and augmented by manual reviews where linkage (non-linkage) was uncertain. Manual checking involved checking addresses and near matches for personal identifiers.
Setting and Participants
Population and Cytologic Classification
A total of 981 063 cervical cytology tests performed on women residing in New Mexico were obtained from 2008–2011. These were linked to 511 381distinct women. Here we focus on women aged 21–65 years (863 608 tests and 451 255 women), but for completeness provide results for the broader age band 15–84 years in the Supplementary material (978 369 tests and 509 488 women, Supplemental Figure 1),. We defined a ‘screening test’ as a cytology test which was at least 300 days after any previous cytology test (33) in order to exclude tests resulting from short term surveillance following an abnormal result, but to include an ‘annual’ cytology test that may have occurred up to 2 months early. Annual cytology test utilization and screening test rates by year and age group were calculated using US Census data for 2010, intercensal population estimates for years 2008–2009 and postcensal estimates for 2011(34). The estimated NM female population aged 21–65 years was 591 413 in 2008, 605 344 in 2010 and 608 943 in 2011.
Outcomes and Follow-up
Age-specific screening coverage rates were calculated for 18, 36, and 48 months prior to the landmark date of 1 January 2012 (using the 2011 census estimate to provide a denominator for that time point) for comparison with 3-year screening rates based on guideline recommendations (20–27). and to parallel estimates of 1 and 3 year coverage reported by the US Centers for Disease Control and Prevention (CDC) (35). For the 1 year estimate we included aa 6month ‘grace period’ by showing coverage at 18 months and for the 3 year estimate we included a 12 month ‘grace period” by showing coverage at 48 months. This approach incorporates additional time to accommodate for short delays in annual or triennial screening and provides the most favorable estimate of coverage likely achieved.. Screening intensity was defined as the number of screening tests per woman in the four years from 2008 to 2011. The number of unscreened women was calculated as the difference between the 2008 census population estimate and women with a recorded test during 2008–2011. Data collection and handling methods were unchanged throughout the entire evaluation period. All cervical cytology results were included in this study, including co-testing or cytology alone.
Cytologic results were classified according to the 2001 Bethesda System (36) as high-grade squamous intraepithelial lesion (HSIL), atypical squamous cells cannot rule out HSIL (ASC-H), atypical glandular cells (AGC), low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells of undetermined significance (ASC-US), and negative for intraepithelial lesion or malignancy. Tests reported as LSIL cannot rule out HSIL (LSIL-H) were categorized as ASC-H, CIN1 as LSIL, and CIN2, CIN2-3, CIN3, carcinoma in situ (CIS), or possible carcinoma as HSIL as previously described.23
Statistical Methods
We primarily have used tabular and graphical methods for proportions and changes in proportions with 95% confidence intervals based on binomial statistics and a normal approximation where appropriate. Kaplan-Meier analyses and proportional hazard models were used in reverse time to examine intervals between screening tests. Reverse-time intervals were censored on 31 December 2006 or on the 15th birthday if it was later. Log-linear binomial regression was used to assess trends in cervical screening coverage and abnormality rates over time. The association between age and screening intensity was assessed using a Cochran-Armitage trend test. SAS v9.3 (SAS Institute Inc., Carey, NC) was used to perform analyses.
Results
Cytology Utilization, Screening Intervals and Coverage
In 2011, 219 610 cytology tests were recorded from 210 273 women aged 15–84 years (Supplemental Figure 1). Of these 200 159 tests (91.1%) were considered ‘screening tests’. Screening utilization from 2008–2011 by age is shown in Figure 1 and Supplemental Table 1, indicating a significant decrease in the percent of women screened for all age groups (P < .001). The decrease was greatest in the 15–20 year age group, with a 61% reduction from 22.4% in 2008 to 8.7%, in 2011. Reductions in all other age groups during this period were smaller, ranging between 3–6% for absolute reductions and 12–27% in relative terms.
Figure 1.
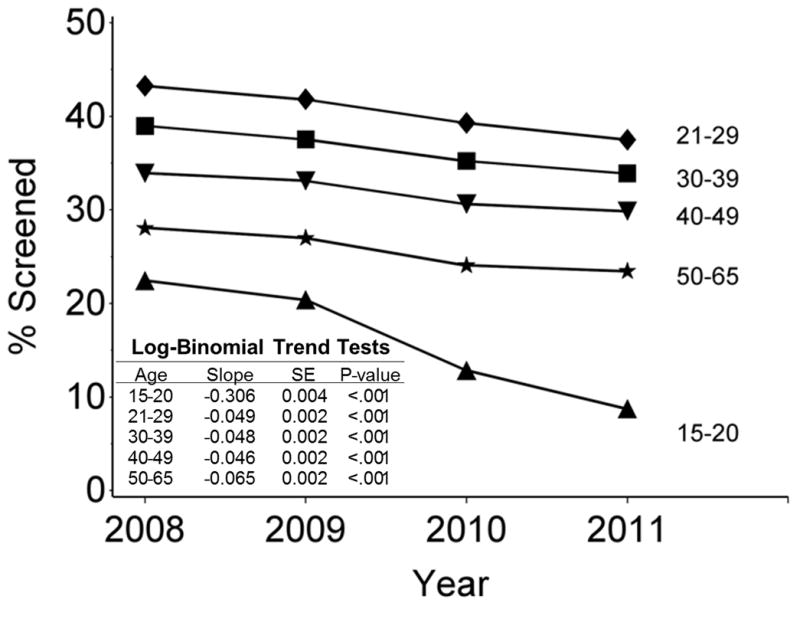
Percent of New Mexico female population with a screening cytology test (Pap test) during a specific year by age group.
The time since the previous screening test by year in which the index test was collected is shown in Figure 2A for women aged 21–65 years. It was significantly longer in 2011 compared to 2008 (hazard ratio = 1.23, 95% CI = 1.22, 1.24). Longer screening intervals imply lower annual screening rates, and this decrease appeared to be largely due to fewer women being screened within 2 years. Coverage within five years was around 80%. Figure 2B shows the median time to last screening test by age. For women aged 21–65 years it increased from 1.50 years in 2008 to 1.87 years in 2011.
Figure 2.
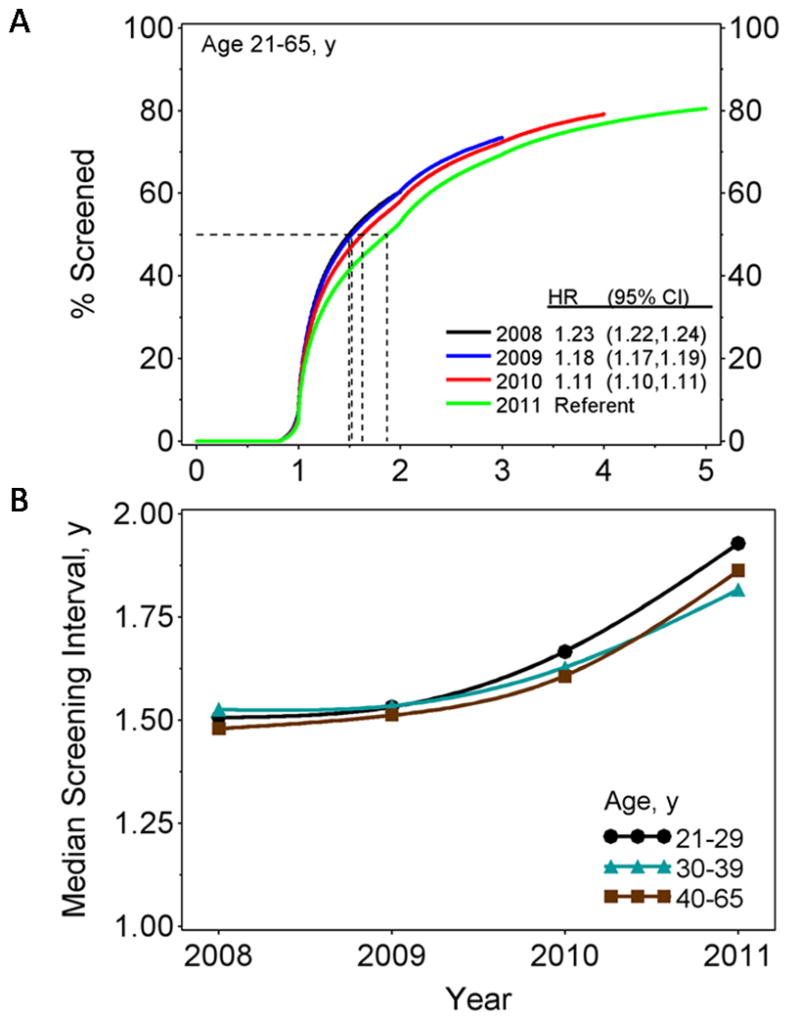
(A) Kaplan-Meier estimates for time since previous cervical screening test by year index test was performed with hazard ratios (HR) and 95% confidence intervals from Cox model regression analyses and (B) median time to last screening test by age group in the given year.
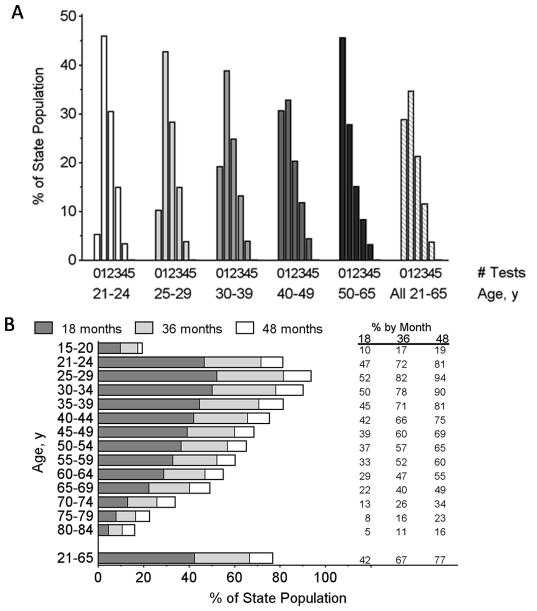
Figure 3A shows a histogram of number of screening tests or intensity of screening over the 4-year period of 2008–2011 by age on 1 January 2008, where those with no tests are inferred from the state population. Overall, 28.9% of women aged 21–65 had no screening tests and this increased with age from 5.3% among women aged 21–24, to 45.6% for age 50–65. Only 15.2% of women aged 21–65 were regularly screening on an annual basis as demonstrated by 3 or more screening tests within this 4-year period. This rate decreased significantly with age (P < .001). Regular annual screening was practiced by 18.3% of women aged 21–24, 18.7% at age 25–29, 17.1% at age 30–39, 16.2% at age 40–49 and 11.6% of women aged 50–65.
Figure 3.
(A) Percent of women with different numbers of cervical screening tests by age (on 1 Jan 2008) between years 2008 – 2011. (B) Percent of women with at least one cervical screening test in the previous 18, 36 and 48 months before 1 January 2012 by age on 1 January 2012.
Screening at intervals of less than 300 days following a negative result (designated ‘over screening’ below) has been decreasing. For women aged 21–65 years, those who had a screening test in 2010 with a negative outcome, the proportion who had their next test within 300 days was 3.6% (95% CI, 3.5–3.7) compared to 4.2% (4.2–4.3) in 2008. Over screening also decreased with age (P<0.001), being 6.9% (95% CI, 6.7–7.1) among women aged 21–29 years, 4.3% (4.1–4.4) for women aged 30–39 years and 1.7% (1.7–1.8) for women aged 40–65 years screened in 2010.
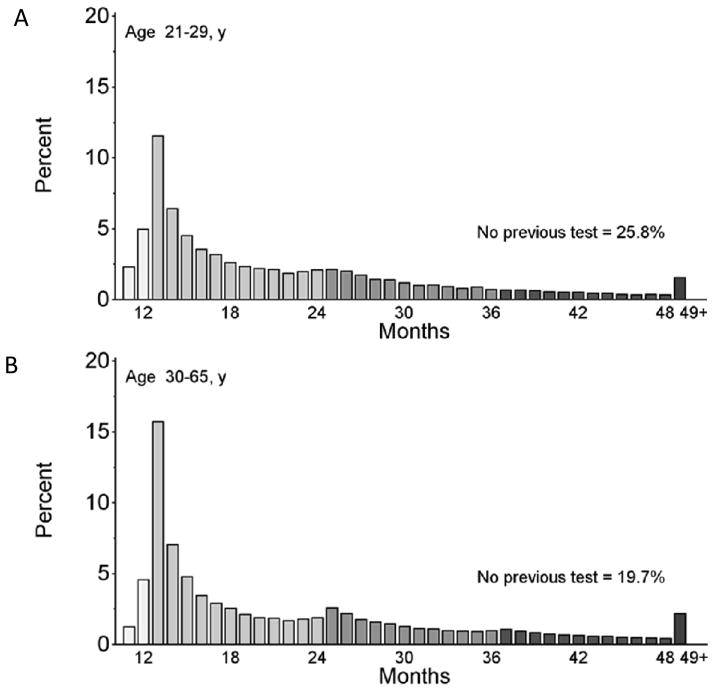
As of 1 Jan 2012, 76.7% of the population aged 21–65 years had a screening test within the prior 48 months, 64.6% within 36 months, and 42.4% within 18 months (Figure 3B). Forty-eight month coverage peaked at 93.6% for women aged 25–29 years and then decreased with age. For women who had a screening test in 2011 and at least one previous test, the most common return time was 13 months for women aged 21–29 years and women aged 30–65 years (Figure 4 A and B).
Figure 4.
Screening interval distributions (months between cytology tests) among women with a screening test in 2011 and aged 21–29 (A) and 30–65 (B) years. The >48 months category includes women with no previous screening test.
Screening outcomes
Cytology results in 2011 are shown for different ages in Table 1. Overall 91.9% of screening tests were negative, 6.6% were minor cytologic abnormalities (ASCUS, LSIL) and 0.9% exhibited higher grade abnormalities (ASC-H, HSIL, AGC, adenocarcinoma in situ, adenocarcinoma squamous cell carcinoma or unspecified cancer (CA)). Over a quarter of adenocarcinoma (ADCA) or 10 of 38 cases were diagnosed among women beyond the age for which screening is still recommended. Most abnormalities were more common in younger women; ASCUS results were reported in 8.2% of tests for women aged 15–20 years, but in only 2.8% of women aged 50–65 years. Similarly LSIL dropped from 8.4% to 0.6% for these age groups and ASC-H from 0.6% to 0.2%. Full details of outcome by year are shown in Supplemental Table 2. HSIL was highest in the 21–29 year age group (0.5%) while AGC peaked in the 40–49 year age group (0.4%). ASCUS, LSIL, and ASC-H rates increased from 2008–2011 (Table 2), with annual increases of 5.0% for ASCUS 9.1% for LSIL, and 6.8% for ASC-H
Table 1.
Cervical cytology results from screening tests in 2011 overall and by age.
| Test Resulta | Age Group | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15–20 | 21–29 | 30–39 | 40–49 | 50–65 | 66–84 | 21–65 | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Negative | 6,099 | 81.9 | 41,162 | 87.1 | 39,021 | 91.8 | 37,225 | 93.5 | 50,113 | 95.3 | 9,370 | 95.4 | 167,521 | 91.9 |
| ASC-US | 612 | 8.2 | 3,314 | 7.0 | 1,983 | 4.7 | 1,676 | 4.2 | 1,496 | 2.8 | 194 | 2.0 | 8,469 | 4.6 |
| LSIL | 628 | 8.4 | 2,018 | 4.3 | 850 | 2.0 | 415 | 1.0 | 323 | 0.6 | 44 | 0.4 | 3,606 | 2.0 |
| ASC-H | 42 | 0.6 | 295 | 0.6 | 177 | 0.4 | 72 | 0.2 | 100 | 0.2 | 17 | 0.2 | 644 | 0.4 |
| HSIL | 24 | 0.3 | 247 | 0.5 | 147 | 0.3 | 67 | 0.2 | 43 | 0.1 | 12 | 0.1 | 504 | 0.3 |
| SCC | 0 | 0 | 1 | 0.0 | 4 | 0.0 | 2 | 0.0 | 4 | 0.0 | 2 | 0.0 | 11 | 0.0 |
| AGC | 3 | 0.0 | 80 | 0.2 | 126 | 0.3 | 148 | 0.4 | 160 | 0.3 | 28 | 0.3 | 514 | 0.3 |
| AIS | 0 | 0 | 2 | 0.0 | 3 | 0.0 | 1 | 0.0 | 0 | 0 | 0 | 0 | 6 | 0.0 |
| ADCA | 0 | 0 | 2 | 0.0 | 2 | 0.0 | 4 | 0.0 | 20 | 0.0 | 10 | 0.1 | 28 | 0.0 |
| CA | 0 | 0 | 1 | 0.0 | 0 | 0 | 1 | 0.0 | 2 | 0.0 | 2 | 0.0 | 4 | 0.0 |
| Insufficient/No Dx | 43 | 0.6 | 157 | 0.3 | 184 | 0.4 | 219 | 0.5 | 347 | 0.7 | 143 | 1.5 | 907 | 0.5 |
| All | 7,451 | 100.0 | 47,279 | 100.0 | 42,497 | 100.0 | 39,830 | 100.0 | 52,608 | 100.0 | 9,822 | 100.0 | 182,214 | 100.0 |
| ASC-US + LSIL | 1,240 | 16.6 | 5,332 | 11.3 | 2,833 | 6.7 | 2,091 | 5.2 | 1,819 | 3.5 | 238 | 2.4 | 12,075 | 6.6 |
| ASC-H & HSIL+ | 69 | 0.9 | 628 | 1.3 | 459 | 1.1 | 295 | 0.7 | 329 | 0.6 | 71 | 0.7 | 1,711 | 0.9 |
Screening tests were performed at least 300 days after any previous cytology test.
ASC-US, atypical squamous cells of undetermined significance
ASC-H, atypical squamous cells cannot rule out HSIL
LSIL, low-grade grade squamous intraepithelial lesion
HSIL, high-grade squamous intraepithelial lesion
SCC, squamous cell carcinoma
AGC, atypical glandular cells
AIS, adenocarcinoma in situ
ADCA, adenocarcinoma
CA, carcinoma not otherwise specified
Insufficient/No Dx, insufficient sample for diagnostic test or no diagnosis provided
Table 2.
Annual change (percent) in cervical screening test outcome by age (and overall) from 2008–2011.
| Test Resulta | Age Group | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15–20 | 21–29 | 30–39 | 40–49 | 60–65 | 66–84 | 21–65 | ||||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Negative | −0.9 | (−1.3, −0.6) | −0.6 | (−0.7, −0.4) | −0.5 | (−0.6, −0.4) | −0.3 | (−0.4, −0.1) | −0.1 | (−0.1,0.0) | −0.1 | (−0.3,0.1) | −0.3 | (−0.4, −0.3) |
| ASC-US | 2.9 | (0.2,5.7) | 4.7 | (3.2,6.3) | 5.7 | (3.7,7.8) | 5.4 | (3.2,7.6) | 4.1 | (1.7,6.5) | 3.5 | (−2.4,9.8) | 5.0 | (4.0,6.0) |
| LSIL | 8.7 | (5.6,12) | 6.2 | (4.1,8.3) | 14 | (10,18) | 10 | (5.3,15) | 13 | (7.0,19) | 14 | (−1.0,30) | 9.1 | (7.5,11) |
| ASC-H | 4.0 | (−6.9,16) | 7.5 | (2.1,13) | 6.9 | (0.0,14) | −1.1 | (−9.9,8.6) | 11 | (1.2,22) | 4.9 | (−15,29) | 6.8 | (3.1,11) |
| HSIL | −7.0 | (−19,6.2) | 0.5 | (−4.8,6.1) | 3.9 | (−3.2,12) | 12 | (0.1,25) | 2.5 | (−10,17) | 15 | (−8.3,45) | 3.2 | (−0.7,7.3) |
| AGC | −8.3 | (−32,24) | 0.5 | (−8.9,11) | 6.6 | (−1.7,15) | −1.8 | (−8.4,5.4) | 2.5 | (−4.6,10) | 0.1 | (−14,17) | 1.6 | (−2.3,5.7) |
| AIS | --b | 4.2 | (−61,177) | 81 | (−42,461) | 6.8 | (−73,325) | -- | 0.0 | (0.0,0.0) | 32 | (−32,159) | ||
| CA | -- | 0.0 | (0.0,0.0) | −47 | (−95,481) | 6.1 | (−57,163) | −3.4 | (−42,62) | 20 | (−36,123) | 16 | (−26,79) | |
| SCC | -- | 0.0 | (0.0,0.0) | 16 | (−32,97) | 0.4 | (−48,95) | −17 | (−41,17) | 16 | (−30,92) | −4.2 | (−26,24) | |
| ADCA | -- | 31 | (−41,191) | 4.5 | (−61,178) | 18 | (−24,82) | 24 | (−4.6,61) | 14 | (−14,53) | 25 | (1.1,55) | |
| Insufficient/No Dx | −4.4 | (−14,5.8) | −12 | (−17, −6.6) | −11 | (−16, −5.8) | −8.2 | (−13, −3.4) | −13 | (−17, −10) | −5.5 | (−11,0.7) | −11 | (−14, −9.3) |
| ASC-US & LSIL | 5.5 | (3.6,7.5) | 5.2 | (4.0,6.5) | 7.9 | (6.1,9.7) | 6.2 | (4.2,8.2) | 5.4 | (3.3,7.6) | 5.1 | (−0.4,11) | 6.1 | (5.3,7.0) |
| ASC-H & HSIL+ | −1.3 | (−9.1,7.1) | 3.9 | (0.3,7.6) | 6.1 | (1.8,11) | 1.5 | (−3.4,6.6) | 5.3 | (0.1,11) | 6.8 | (−3.2,18) | 4.3 | (2.1,6.6) |
Annual changes (percent and 95% confidence interval) are based on rate ratios for a log-linear model with binomial errors.
ASC-US, atypical squamous cells of undetermined significance
ASC-H, atypical squamous cells cannot rule out HSIL
LSIL, low-grade grade squamous intraepithelial lesions
HSIL, high-grade squamous intraepithelial lesions
SCC, squamous cell carcinoma
AGC, atypical glandular cell
AIS, endocervical adenocarcinoma in situ
ADCA, endocervical adenocarcinoma not otherwise specified
CA, carcinoma not otherwise specified
Insufficient/No Dx, insufficient sample for diagnostic test or no diagnosis
– Not analyzed due to insufficient sample numerator
Discussion
The NMHPVPR is the first state wide registry in the US, and here we provide the first population-based snapshot of cervical screening utilization, coverage, and outcomes across the spectrum of clinical practice care delivery, funding structures, organizational systems, facilities, and practice and provider levels. Not only does the NMHPVPR provide critical basic information, but it also creates an opportunity to evaluate the effectiveness of cervical screening in reducing cancer incidence and to understand in detail the natural history of HPV-related cervical disease. Some countries currently use similar systems more actively to invite women for screening, improve follow up of abnormal screening results and monitor outcomes on an individual basis (37). Over time data collected from this surveillance system have the potential to pinpoint reasons for cancer development including lack of attendance for screening, failure to follow up abnormal tests, missed abnormalities present in the sample, inadequate sample, inadequate treatment of abnormal findings and an apparently adequate sample but negative test result despite disease being present. This information can identify opportunities for improving the delivery of the service under standard care practices, and also monitor the impact of new guidelines such as those related to the age at starting and stopping screening, the introduction of HPV testing - either as an adjunct to cytology or as the sole screening test and extending screening intervals. It is also important to have a baseline measure of screening parameters before HPV vaccination has an impact on women aged 21 or more. In a 2010 national US survey, very few women aged >26years had been vaccinated and an estimated 17.5% of those aged 21–26 years had at least one dose with <10% having had 3 or more doses (38). In New Mexico, preliminary data indicate women aged 21–26 may have even lower rates of HPV vaccination so this will have had a minimal impact on our current findings (data not shown). As more of the population becomes vaccinated against human papillomavirus infections, the primary cause of cervical cancer, reductions in disease prevalence will require attendant adjustments in screening policy.
Our analyses demonstrate a decrease in the proportion of women attending for cervical screening across all age groups, extending a report from CDC’s Behavioral Risk Factor Surveillance System (BRFSS) for women aged 18–30 years (39). Part of this decrease is almost certainly due to earlier changes in US guidelines (20–27), which recommended a later age at initiating screening and a longer interval between screening episodes, especially when co-testing with HPV is used. However this may also reflect a general decrease in attendance for preventive health services as a result of the ongoing economic recession. Overall 28.9% of women 21–65 had no reported tests during the study period. Our results indicate that screening coverage is still high among women aged 21–40 years, but drops substantially above that age. This is a real concern, as the median age for invasive cervical cancer diagnosis in New Mexico (and the US overall) is 48 years (40, 41). At this stage it is not possible to assess the extent to which this is due to more women not attending at all for screening or if it is just a small increase in intervals between screens in a larger fraction of the population. The situation will become clearer as the NMHPVPR database matures so we can look at trends in proportions screened with the last 3 years and last 5 years. This will also help to assess the impact of the more recent guideline changes on extended intervals and to monitor the extent to which screening under age 21 will continue to fall.
A general concern with the BRFSS survey is that coverage estimates were based on landline telephone interviews, and did not include clinical verification (35, 42). For New Mexico the 2010 BRFSS reported at least one cytology test in the previous 3 years as follows: 89.4% for women aged 25–34 years, 88.6% at age 35–44 years, 84.3% at age 45–54 years, and 82.2% for women aged 55–64 years. This is substantially higher than our record-based estimates, especially for older women. Previous studies have suggested that self-reported cancer screening can result in inaccurate estimates of time since last screen (43, 44) and these data highlight the importance of laboratory-verified population-based surveillance to accurately measure the true utilization of screening. Among older women, most women with a hysterectomy no longer need screening. We do not currently have this data, but hope to be able to add it in the future as an adjustment to our estimates of population coverage. Adjustments for hysterectomy may have implications for the population based screening rates reported here, especially for older women
Although high-grade cytologic abnormality rates were fairly constant over the five years of this survey, minor cytologic abnormalities and ASC-H were increasing. These changes could not be explained simply by cumulative effects of longer average intervals between screens, but could be due to a number of other reasons, including changes in clinical diagnostic practices or changes in sexual behavior or other factors affecting HPV prevalence. This will pose problems for assessing the impact of HPV vaccination on ASC-US and LSIL cytology, one of the earliest indicators of vaccine effectiveness, and it will be important to monitor and adjust for differences in screening intervals when studying this endpoint.
An issue regarding the validity of these data is the accuracy of population ascertainment, including issues of undercount and in- and out-migration during the survey period. In the 2010 census undercount for age 20–64 was less than 0.5% based on estimates made for the NMDOH (http://ibis.health.state.nm.us/), and net population immigration was 0.4% between 2010 and 2011 for women aged 20–64 (bber.unm.edu/bber_research_demPop.html). These effects are likely to have a minimal impact on our population estimates. However we do not have information regarding previous screening for women moving into New Mexico, which would lead to under-estimates of coverage. Conversely for women emigrating from the state, their prior screens would be included in our database, but they would not be included in the population denominator, leading to overestimates of coverage. Assuming net in-migration exceeded out-migration by 0.4% per annum at all ages and screening intensity was similar in these women and uniform over time, this could lead to our 3 year coverage estimates being as much as 0.6% too low in relative terms.
Another issue relates to the completeness of the data on screening visits. We estimate a very small percentage (less than 1%) of cytology tests performed on women residing in New Mexico may not be documented in the NMHPVPR.
A further issue is the completeness of linkage of separate cytology reports to individual women. Failure to link records from the same woman would lead to higher estimated overall coverage levels, and longer screening intervals than is truly the case. Extensive efforts indicate that linkage is very complete. For example in other analyses, biopsy reports could be linked to a recent cytology report in 99% of the cases. However only 1.1% of tests had partial matches that were not taken to be linked, but which had an appreciable probability of linkage to a known record had full information been available.
There is evidence from other countries (1–8) that a centralized registry can improve the overall management of a screening program. Our report provides the first detailed population-based evidence of the extent to which guidelines related to increasing the age at initiating screening and extending the interval between screens are being adhered to. Although cervical screening practices in the New Mexico population may not reflect the situation in other parts of the United States, the methodology developed here is likely to have wider use. The activities of the NMHPVPR program require the collation of information from a wide range of healthcare organizational structures, and the strategies developed here could be used to support not only cervical cancer prevention programs, but also serve as a model for monitoring screening activity at other cancer sites such as the breast, bowel, prostate and lung. They may also be helpful for programs targeting other sexually transmitted infections (STI) (e.g. chlamydia, gonorrhea, trichomonas), since opportunistic screening for non-HPV STIs commonly has occurred at the time of cervical screening, especially for young women (45;46). Linkage of the NMHPVPR screening data with laboratory-based reporting systems can help to study changes in STIs reporting and associated outcomes potentially related to changes in cervical screening services. Under the US Affordable Care Act implementation, this will become even more important due to the anticipated expansion of health care coverage, including clinical preventive services (47).
As the NMHPVPR program matures, future analyses will include case-control evaluations of the effectiveness of screening and pinpoint deficiencies. Furthermore, it will provide data necessary for making rational choices about screening intervals for women who have received an HPV vaccine. However even at this early stage, the data have highlighted issues related to poor coverage in older women. We have also identified a continuing proportion of ‘screening tests’ at intervals of less than one year and in women aged < 21 years and >65 years, counter to current screening guidelines. Corrective actions and continued monitoring are needed to minimize these deficiencies.
Supplementary Material
Acknowledgments
All data was reported to the NMHPVPR under NMAC 7.4.3. Jack Cuzick, Orrin Myers and Cosette M. Wheeler prepared the initial manuscript. Orrin Myers performed the statistical analyses. All authors (JC, OM, WCH, NEJ, PEC, VB and CMW) reviewed, provided input and approved the final manuscript submitted for publication. Members of the New Mexico HPV Pap Registry (NMHPVPR) Steering Committee reviewed and gave input to the manuscript and supported the directions of the NMHPVPR including the evaluations presented in this manuscript. The NMHPVPR Steering members participating are as follows: Nancy E. Joste, MD, University of New Mexico Health Sciences Center and Tricore Reference Laboratories, Albuquerque, New Mexico; Walter Kinney, MD, Kaiser Permanente Northern California; Cosette M. Wheeler, PhD, University of New Mexico Health Sciences Center; William C. Hunt, MS, University of New Mexico Health Sciences Center; Deborah Thompson, MD MSPH, New Mexico Department of Health; Susan Baum, MD MPH, New Mexico Department of Health; Linda Gorgos, MD MSc, former Medical director of the Infectious Disease Bureau, New Mexico Department of Health, Alan Waxman, MD MPH, University of New Mexico Health Sciences Center; David Espey MD, US Centers for Disease Control and Prevention; Jane McGrath MD, University of New Mexico Health Sciences Center; Steven Jenison, MD, Community Member; Mark Schiffman, MD MPH, US National Cancer Institute; Philip Castle, PhD MPH, Albert Einstein College of Medicine; Vicki Benard, PhD, US Centers for Disease Control and Prevention; Debbie Saslow, PhD, American Cancer Society; Jane J. Kim PhD, Harvard School of Public Health; Mark H. Stoler MD, University of Virginia; Jack Cuzick, PhD, Wolfson Institute of Preventive Medicine, London; Giovanna Rossi Pressley, MSc, Collective Action Strategies, and RWJF Center for Health Policy at University of New Mexico and Kevin English, RPh MPH, Albuquerque Area Southwest Tribal Epidemiology Center (AASTEC). No compensation was received for contributions to this manuscript by any named authors or by the NMHPVPR Steering Committee members.
Abbreviations
- NM
New Mexico
- US
United States
- NMHPVPR
New Mexico HPV Pap Registry
- NMDOH
NM Department of Health
- NMAC
New Mexico Administrative Code
- CDC
US Centers for Disease Control and Prevention
- SEER
Surveillance Epidemiology and End Results Cancer Registry
- HPV
human papillomavirus
- TBS
the 2001 Bethesda System
- HSIL
high-grade squamous intraepithelial lesion
- LSIL
low-grade grade squamous intraepithelial lesion
- ASC-H
atypical squamous cells cannot rule out HSIL
- AGC
atypical glandular cells
- ASC-US
atypical squamous cells of undetermined significance
- LSIL-H
LSIL cannot rule out HSIL
Footnotes
Disclosures: Information reported in this publication was funded by the US National Institute of Allergy And Infectious Diseases (NIAID) and the US National Cancer Institute (NCI) under cooperative agreementsU19AI084081 and U54CA164336 to C Wheeler. The NIAID and the NCI had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health or the US Centers for Disease Control. The authors had full access to the data and had final responsibility for the decision to submit for publication.
References
- 1.Sasieni P, Cuzick J, Farmery E. Accelerated Decline in Cervical Cancer Mortality in England and Wales. Lancet. 1995;346:1566–7. doi: 10.1016/s0140-6736(95)92099-4. [DOI] [PubMed] [Google Scholar]
- 2.Sasieni P, Cuzick J, Lynch-Farmery Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer. 1996;73:1001–5. doi: 10.1038/bjc.1996.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn M, Babb P, Jones J, Allen E. Effect of screening on incidence of and mortality from cancer of the cervix in England: evaluation based on routinely collected statistics. BMJ. 1999;318:904–8. doi: 10.1136/bmj.318.7188.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laara E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organized screening programmes. Lancet. 1987;1:1247–49. doi: 10.1016/s0140-6736(87)92695-x. [DOI] [PubMed] [Google Scholar]
- 5.IARC Working Group. IARC Handbooks of Cancer Prevention, International Agency for Research on Cancer, World Health Organization. IARC Press; 2005. Cervix cancer screening. [Google Scholar]
- 6.Bulk S, Visser O, Rozendaal L, Verheijen RH, Meijer CJ. Cervical cancer in the Netherlands 1989–1998: Decrease of squamous cell carcinoma in older women, increase of adenocarcinoma in younger women. Int J Cancer. 2000;113:1005–9. doi: 10.1002/ijc.20678. [DOI] [PubMed] [Google Scholar]
- 7.de Kok IM, van der Aa MA, van Ballegooijen M, Siesling S, Karim-Kos HE, van Kemenade FJ, et al. Working Group Output of the Netherlands Cancer Registry. Trends in cervical cancer in the Netherlands until 2007: has the bottom been reached? Int J Cancer. 2011;128:2174–81. doi: 10.1002/ijc.25553. [DOI] [PubMed] [Google Scholar]
- 8.Andrae B, Andersson TM, Lambert PC, Kemetli L, Silfverdal L, Strander B, et al. Screening and cervical cancer cure: population based cohort study. BMJ. 2012;344:e900. doi: 10.1136/bmj.e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macgregor JE, Campbell MK, Mann EM, Swanson KY. Screening for cervical intraepithelial neoplasia in north east Scotland shows fall in incidence and mortality from invasive cancer with concomitant rise in preinvasive disease. BMJ. 1994;308:1407–11. doi: 10.1136/bmj.308.6941.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day NE. The epidemiological basis for evaluating different screening policies. Vol. 76. IARC Sci Publ; 1986. pp. 199–212. [PubMed] [Google Scholar]
- 11.Sawaya GF, Sung HY, Kinney W, Kearney KA, Miller MG, Hiatt RA. Cervical cancer after multiple negative cytologic tests in long-term members of a prepaid health plan. Acta Cytol. 2005:391–7. doi: 10.1159/000326172. [DOI] [PubMed] [Google Scholar]
- 12.Sawaya GF, McConnell KJ, Kulasingam SL, Lawson HW, Kerlikowske K, Melnikow J, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003:3491501–9. doi: 10.1056/NEJMoa035419. [DOI] [PubMed] [Google Scholar]
- 13.Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol. 2003;101:29–37. doi: 10.1016/s0029-7844(02)02454-7. [DOI] [PubMed] [Google Scholar]
- 14.Sung HY, Kearney KA, Miller M, Kinney W, Sawaya GF, Hiatt RA. Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer. 2000;88:2283–9. [PubMed] [Google Scholar]
- 15.Viikki M, Pukkala E, Hakama M. Risk of cervical cancer subsequent to a positive screening cytology: follow-up study in Finland. Acta Obstet Gynecol Scand. 2000;79:576–9. [PubMed] [Google Scholar]
- 16.Viikki M, Pukkala E, Hakama M. Risk of cervical cancer after a negative Pap smear. J Med Screen. 1999;6:103–7. doi: 10.1136/jms.6.2.103. [DOI] [PubMed] [Google Scholar]
- 17.Olesen F. A case-control study of cervical cytology before diagnosis of cervical cancer in Denmark. Int J Epidemiol. 1988;17:501–8. doi: 10.1093/ije/17.3.501. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen NH, Jensen H. Screening for cancer of the uterine cervix in Greenland. APMIS. 1993;101:290–4. doi: 10.1111/j.1699-0463.1993.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 19.Celentano DD, Klassen AC, Weisman CS, Rosenshein NB. Cervical cancer screening practices among older women: results from the Maryland Cervical Cancer Case-Control Study. J Clin Epidemiol. 1988;41:531–41. doi: 10.1016/0895-4356(88)90057-1. [DOI] [PubMed] [Google Scholar]
- 20.Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–62. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 21.ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists. Number 45, August 2003. Cervical cytology screening (replaces committee opinion 152, March 1995) Obstet Gynecol. 2003;102:417–27. doi: 10.1016/s0029-7844(03)00745-2. [DOI] [PubMed] [Google Scholar]
- 22.Screening for cervical cancer: recommendations and rationale. Am Fam Physician. 2003;67:1759–66. [PubMed] [Google Scholar]
- 23. [accessed 4 Nov 2013]; http://www.accessdata.fda.gov/psn/printer-full.cfm?id=20.
- 24.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 25.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11:201–22. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 26.Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis. 2010;14:73–80. doi: 10.1097/lgt.0b013e3181cec411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ACOG Committee on Practice Bulletins--Gynecology. . ACOG Practice Bulletin no109: Cervical cytology screening. Obstet Gynecol. 2009;114:1409–20. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- 28.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–42. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 29.Moyer VA. U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–91. W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 30.ACOG practice bulletin 131: screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222–38. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 31. [accessed on Nov 4 2013]; http://www.nmcpr.state.nm.us/nmac/parts/title07/07.004.0003.htm.
- 32. [accessed on Nov 4, 2013]; http://www.cdc.gov/cancer/npcr/. Version 2, 6/29/2007.
- 33.Wheeler CM, Hunt WC, Cuzick J, Langsfeld E, Pearse A, Montoya GD, et al. A population-based study of human papillomavirus genotype prevalence in the United States: Baseline measures prior to mass human papillomavirus vaccination. Int J Cancer. 2013;132:198–207. doi: 10.1002/ijc.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. [accessed on Sept 26, 2012]; http://www.census.gov.
- 35.Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. [accessed on May 4, 2013]. http://apps.nccd.cdc.gov/BRFSS/age.asp?cat=WH&yr=2010&qkey=4426&state=NM. [Google Scholar]
- 36.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 37.Habbema D, De Kok IM, Brown ML. Cervical cancer screening in the United States and the Netherlands: a tale of two countries. The Milbank quarterly. 2012;90:5–37. doi: 10.1111/j.1468-0009.2011.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams WW, Lu PJ, Saraiya M, Yankey D, Dorell C, Rodriguez JL, Kepka D, Markowitz LE. Factors associated with human papillomavirus vaccination among young adult women in the United States. Vaccine. 2013;31:2937–46. doi: 10.1016/j.vaccine.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CDC. Cervical Cancer Screening Among Women Aged 18–30 Years — United States, 2000–2010. MMWR. 2013 Jan;61(51 & 52):1038–1042. [PubMed] [Google Scholar]
- 40. [accessed on May 4, 2013]; http://seer.cancer.gov/statfacts/html/cervix.html.
- 41.Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2011 Sub, Vintage 2009 Pops (2000–2009) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012, based on the November 2011 submission.
- 42. [accessed on May 4, 2013]; HTTP://WWW.CDC.GOV/BRFSS/ABOUT.HTM.
- 43.Ashok M, Berkowitz Z, Hawkins A, Tangka F, Sariaya M. Recency of Pap testing and future testing plans among women aged 18–64: analysis of the 2007 Health Information National Trends survey. J Women’s Health. 2012;21:705–12. doi: 10.1089/jwh.2012.3562. [DOI] [PubMed] [Google Scholar]
- 44.Howard M, Agarwal G, Lytwyn A. Accuracy of self-reports of Pap and mammography screening compared to medical record: a meta-analysis. Cancer Causes Control. 2009;20:1–13. doi: 10.1007/s10552-008-9228-4. [DOI] [PubMed] [Google Scholar]
- 45.Myers JL. Why do young women get tested for sexually transmitted infections? Evidence from the National Longitudinal Study of Adolescent Health. J Womens Health (Larchmt) 2011 Aug;20(8):1225–31. doi: 10.1089/jwh.2010.2544. [DOI] [PubMed] [Google Scholar]
- 46.Tao G, Hoover KW, Kent CK. Chlamydia testing patterns for commercially insured women, 2008. Am J Prev Med. 2012 Apr;42(4):337–41. doi: 10.1016/j.amepre.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Plescia M, Richardson LC, Joseph D. New roles for public health in cancer screening. CA Cancer J Clin. 2012 Jul;62:217–9. doi: 10.3322/caac.21147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.