Abstract
The pathogenesis of complex regional pain syndrome (CRPS) is unresolved, but TNF-α and IL-6 are elevated in experimental skin blister fluid from CRPS affected limbs, as is tryptase, a marker for mast cells. In the rat fracture model of CRPS exaggerated sensory and sympathetic neural signaling stimulate keratinocyte and mast cell proliferation, causing the local production of high levels of inflammatory cytokines leading to pain behavior. The current investigation used CRPS patient skin biopsies to determine whether keratinocyte and mast cell proliferation occur in CRPS skin and to identify the cellular source of the up-regulated TNF-α, IL-6, and tryptase observed in CRPS experimental skin blister fluid. Skin biopsies were collected from the affected skin and the contralateral mirror site in 55 CRPS patients and the biopsy sections were immunostained for keratinocyte, cell proliferation, mast cell markers, TNF-α, and IL-6. In early CRPS keratinocytes were activated in the affected skin, resulting in proliferation, epidermal thickening, and up-regulated TNF-α and IL-6 expression. In chronic CRPS there was reduced keratinocyte proliferation with epidermal thinning in the affected skin. Acute CRPS patients also had increased mast cell accumulation in the affected skin, but there was no increase in mast cell numbers in chronic CRPS.
Keywords: complex regional pain syndrome, pain, immunology, keratinocytes, mast cells
Introduction
Complex regional pain syndrome (CRPS) is a painful, disabling and often chronic condition with an estimated 50,000 new cases in the US each year.7 Evidence from CRPS patients and human volunteers suggest that both sensory primary afferent c-fibers and sympathetic neurons function aberrantly in CRPS, resulting in vascular symptoms, trophic changes and pain.4, 5, 30 Additional studies measuring cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) have demonstrated that these mediators are elevated in the experimental skin blister fluid of CRPS patients,11, 17, 19, 31 as is tryptase, a marker for mast cells capable of releasing a host of nociceptive and vasoactive mediators.21 Furthermore, TNF-α levels are increased in CRPS skin biopsies when measured by enzyme immunoassay and in CRPS affected hands scintigraphically imaged with radiolabeled anti-TNF-α antibody.1, 24
Population based studies indicate that distal limb fracture is the most common cause of CRPS7, 37 and we have developed a distal tibia fracture model in rats and mice that closely parallels the regional changes observed in early CRPS, including hindlimb allodynia, unweighting, increased spinal Fos-immunoreactivity, increased skin temperature, edema, increased spontaneous protein extravasation, and periarticular bone loss.12, 13, 29, 34, 35 Using the rat fracture model of CRPS, we have shown that facilitated substance P (SP) and calcitonin gene-related peptide (CGRP) signaling from sensory c-fibers,44 as well as norepinephrine released from sympathetic nerve terminals, induce inflammation and pain sensitization by the activation of neuropeptide and adrenergic receptors on the surface of keratinocytes, causing keratinocytes to proliferate and secrete high levels of inflammatory cytokines and nerve growth factor (NGF), inflammatory mediators that contribute to pain behavior in this CRPS model.14, 26, 27, 29, 34, 35, 39, 45 The release of SP from c-fibers also caused mast cell accumulation and degranulation, further contributing to pain behavior in the rat fracture model.28 Based on these data, we postulate that post-traumatic up-regulated neuropeptide and sympathetic adrenergic signaling induces keratinocyte and mast cell activation, proliferation, and inflammatory mediator expression in CRPS skin. This investigation examined patient skin biopsies to determine whether keratinocyte and mast cell proliferation occurs in CRPS skin and to identify the cellular sources of the up-regulated TNF-α, IL-6, and tryptase previously observed in CRPS experimental skin blister fluid and skin biopsies.11, 19, 21, 31
Methods
Subjects and clinical data collection
The study protocols were approved by the respective local institutional review boards at Murdoch University and at the University of Mainz. Thirty-seven women (67%) and 18 men (33%) with CRPS were enrolled after giving written informed consent. The subjects’ average age was 49.8 ± 1.8 years with a range of 20 to 72 years. Inclusion criteria included; 1) meeting the new IASP clinical diagnostic criteria for CRPS at the time of biopsy,16 2) unilateral symptoms in the hand or foot, allowing use of the contralateral limb for the control biopsy, and 3) no recent glucocorticoid or bisphosphonate treatments (as these drugs potentially inhibit cytokine expression). Patient demographics and clinical data were recorded, including age, gender, CRPS duration and etiology, involved limb, pain medications, numerical 11 point pain score, allodynia, and limb temperature. Allodynia was tested by applying 3–4 light strokes with a small brush to the affected skin and asking patients if this evoked a normal or abnormal sensation. If the sensation was described as abnormal the patient was asked to give a qualitative description of the sensation. Descriptions of the brushing as uncomfortable or painful were regarded as allodynia. Patients were further tested by applying 3–4 light strokes to the skin with the tip of a paper clip and if the patient described that stimulation as uncomfortable or painful the response was also categorized as allodynia. Limb temperature was also measured over 5 separate sites in the affected skin and over the mirror-image area of the contralateral healthy limb, using an infrared skin thermometer (Tempett IR Thermometer, Somedic or Fluke IR Thermometer No 561, Fluke), and the difference between the averaged values in the CRPS affected limb– the contralateral limb was calculated.
Tissue processing and microscopy
Under local anesthesia full thickness 3mm skin punch biopsies were obtained from the bilateral hands or feet of 55 CRPS patients. The biopsies were collected from the same location bilaterally in each patient, so that the normal (unaffected) side control biopsy was the mirror image of the affected side skin biopsy. Biopsies were fixed in Zamboni’s fixative (FD NeuroTechnologies) for 4 hours at 4°C, then rinsed with 0.1M PB (pH 7.4) and 50% ethanol followed by embedding in TissurePrep2 paraffin (Fisher Scientific). Following embedding 8–10μM slices were cut, mounted onto slides (Tru Scientific), deparaffinized in xylene, hydrated through graded alcohols to distilled water. Paraffin sections were antigen-retrieved by IHC-Tek Epitope Retrieval Solution (IHC World) steaming for 40 minutes, then cooled to room temperature. Sections were permeabilized and blocked with PBS containing 10% donkey serum (Jackson ImmunoResearch Laboratories) and 0.3% Triton X-100, followed by exposure to primary antibodies overnight at 4°C in PBS containing 2% serum. Upon detection of the first antigen, primary antibody from a different species against the second antigen was applied to the sections and visualized using an alternative fluorophore-conjugated secondary antibody. Sections were then rinsed in PBS and incubated with fluorophore-conjugated secondary antibodies against the immunoglobulin of the species from which the primary antibody was generated. After three washes, the sections were counterstained with DAPI (Thermo Fisher Scientific) solution (0.25μg/ml) to identify nuclei and mounted with anti-fade mounting medium (Invitrogen Molecular Probes). Images were obtained using confocal microscopy (Zeiss LSM/510 Upright 2 photon; Carl Zeiss) and stored on digital media. With regard to primary antibodies, rabbit anti human IL-6, 1:600 (Abcam), mouse monoclonal to human mast cell tryptase Ab-2, clone AA1, 1:1000 (Thermo Fisher Scientific), mouse monoclonal antibody to Pan keratin, 1:75 (Thermo Fisher Scientific), and rabbit monoclonal antibody to Ki-67, clone SP6, 1:1000 (Thermo Fisher Scientific) were used. Double labeling immunofluorescence was performed with donkey anti-mouse IgG (1:500) conjugated with Dylight 549, donkey anti-rabbit IgG (1:500) conjugated with Dylight 488 secondary antibodies (Jackson ImmunoResearch Laboratories), incubated with respective primary antibodies. Control experiments included incubation of slices in primary and secondary antibody-free solutions both of which led to low intensity non-specific staining patterns in preliminary experiments (data not shown).
For the detection of TNF-α expression in the epidermal keratinocytes, a mouse monoclonal antibody to human TNF-α, clone 2C8 (Meridian Life Sciences, Inc.) was labeled with Alexa Fluor 555 microscale protein labeling kit (Invitrogen Molecular Probes) under optimum conditions according to the manufacture's protocol, and quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (Thermo Fisher Scientific). The concentration of purified protein conjugate was 0.31 mg/ml. Then an equal volume of glycerol (ACS grade) was added for a final concentration of 50%, and stored at −20°C as a liquid. Upon detection of the keratin as described above, 250 μl of a 1:350 dilution of the conjugate was used for the TNF-α immunostaining.
Quantitative studies and statistical analysis
To assess the differences in epidermal skin thickness between the healthy (unaffected) and affected sides in CRPS patients, a pan-keratinocyte marker recognizing both the acidic and basic (Type 1 and II) subfamilies of cytokeratins was used to label keratinocytes and fluorescent immunohistochemistry and confocal microscopy were performed as previously described.27 Epidermal thickness measurements were made using LSM Image Browser from Zeiss LSM Data Server. A blinded observer selected 4 to 8 sections from each skin biopsy and 4 to 8 thickness measurements were made in each section to derive a mean score for that biopsy. The individual mean scores were then used to calculate the mean thickness and S.E.M. for the healthy limbs and the affected limbs for all patients. To evaluate keratinocyte proliferation the nuclear proliferation marker protein Ki-67, which is expressed in cells undergoing the S/G2/M transition, was immunostained in skin sections previously labeled for keratin.10, 38, 41 All Ki-67 positive epidermal keratinocytes were counted in each section under a LEICA DM 2000 microscope by a blinded observer, examining 4–8 transverse sections of each biopsy. The average number of Ki-67 immunostained epidermal cells per section was then calculated for each biopsy. To determine the number of mast cells in skin biopsies obtained from CRPS patients, tryptase was used as a cellular marker for skin mast cells.40, 42 A blinded observer counted the total number of tryptase positive mast cells per high-power field (400X) in the dermis. The average number of tryptase positive cells per field was based on averaging four or more replicates per biopsy.
A paired Student t test was used to compare the values obtained from the CRPS affected limb to the contralateral normal side. Differences in epidermal thickness, Ki-67 labeled cell numbers, tryptase labeled cell numbers, and skin temperature were calculated by subtracting the values from the healthy contralateral limb from the values obtained in the CRPS affected limb. Relationships between interval variables (epidermal thickness differences, Ki-67 labeled cell number differences, tryptase labeled cell number differences, numerical pain scores, skin temperature differences, patient age, and duration of CRPS) were tested by Spearman rank-order correlation due to skewed distributions. Relationships between interval variables and dichotomous variables (allodynia, fracture, CRPS I and II, and gender) were tested by point biserial correlation analysis. All data are presented as the mean ± standard error of the mean, and differences are considered significant at a P value less than 0.05 (Prism 6, GraphPad Software).
Results
Patient demographics and clinical presentation
Table 1 presents the demographics and clinical presentation of the patients evaluated in this study. The majority (67%) of the subjects were female, the average age was 49.4 ± 1.8 years, and the median duration of CRPS was 16 (3–920) weeks. The average numerical pain score on an 11-point scale was 6.4 ± 0.3 at the time of biopsy and allodynia was present in 48% of patients. The average skin temperature difference between the affected limb and the contralateral healthy limb was 0 ± 0.2°. Biopsies were obtained bilaterally from the hands in 45 patients and from the feet in 10 patients. Distal limb fracture was the most common etiology of CRPS, accounting for 38% of patients. CRPS type I accounted for 80% of patients and type II for 20%.
Table 1.
Demographic and clinical data from CRPS patients
| Subject ID | CRPS duration (weeks) | Age (years) | Sex | Limb | Skin temp (Δ°C) | NPS | Allo-dynia | FX | CRPS II | Etiology |
|---|---|---|---|---|---|---|---|---|---|---|
| G9 | 3 | 67 | female | arm | −0.4 | 8 | + | + | − | FX-phalangeal |
| G11 | 3 | 61 | male | arm | − | − | + | Surgery-hand | ||
| G5 | 4 | 53 | male | arm | 1.5 | 8 | + | − | − | Surgery-metacarpalphalangeal |
| G17 | 4 | 48 | male | arm | 2.0 | 3 | − | + | − | FX-navicular |
| G24 | 4 | 60 | female | arm | 2.2 | 6 | + | + | − | FX-radius |
| G12 | 5 | 68 | female | arm | 6 | − | + | + | Nerve injury-radial, FX-humerus | |
| G22 | 5 | 56 | female | arm | 0.1 | 9 | + | + | − | FX-radius |
| G6 | 7 | 65 | male | arm | 0.4 | 3 | + | + | + | Nerve injury-ulnar, FX-humerus |
| G23 | 7 | 63 | female | arm | 1.0 | 10 | + | + | − | FX-radius |
| G27 | 7 | 56 | female | arm | 3.8 | 5 | + | + | − | FX-radius |
| A923 | 8 | 20 | male | leg | −1.3 | 4 | + | − | − | Soft tissue injury-ankle |
| A900 | 8 | 20 | female | arm | 0.6 | 1 | + | − | − | IV cannulation-wrist |
| G13 | 8 | 50 | male | arm | 0.4 | 6 | − | + | − | FX-metacarpal |
| G25 | 8 | 43 | male | arm | 1.5 | 10 | − | − | − | Soft tissue injury |
| G28 | 8 | 61 | female | arm | 1.8 | 4 | − | + | − | FX-radius |
| G31 | 8 | 62 | female | arm | + | − | FX-radius | |||
| G35 | 8 | 55 | female | arm | −0.7 | 7 | − | − | − | Soft tissue injury-tendon |
| G19 | 11 | 53 | male | arm | 0.2 | 7 | + | − | + | Surgery-partial amputation DIII |
| G26 | 11 | 57 | male | arm | 0.2 | 4 | − | − | − | Surgery-wrist subluxation |
| A817 | 12 | 38 | female | leg | −3.8 | 3 | + | − | − | Soft tissue injury-ankle |
| G3 | 12 | 82 | female | arm | 0.1 | 8 | + | + | − | FX-radius |
| G16 | 12 | 71 | female | arm | 2.4 | 7 | − | + | − | FX-radius |
| G18 | 12 | 56 | female | arm | 0.8 | 10 | − | + | − | FX-radius |
| G21 | 12 | 60 | female | arm | 0.1 | 8 | − | − | − | Soft tissue injury-shoulder |
| G34 | 12 | 25 | female | arm | −1.7 | 9 | − | − | − | Soft tissue injury-wrist |
| G14 | 16 | 46 | female | arm | −2.0 | 7 | − | − | + | Surgery-carpal tunnel syndrome |
| A622 | 16 | 42 | male | leg | −1.3 | 8 | + | + | − | FX-tibia |
| G14 | 16 | 46 | female | arm | −2.0 | 7 | − | − | + | Surgery-carpal tunnel syndrome |
| G30 | 16 | 41 | female | arm | 0.0 | 10 | − | − | − | Soft tissue injury-shoulder |
| A1296 | 22 | 53 | female | leg | 0.5 | 3 | + | − | − | Soft tissue injury-ankle |
| A486 | 24 | 23 | male | arm | 0.0 | 6 | + | − | + | Nerve injury-ulnar |
| A789 | 24 | 44 | male | leg | 0.2 | 5 | − | + | + | Nerve injury-sural, FX-tibia |
| G4 | 24 | 33 | male | arm | 0.8 | 8 | + | + | − | FX-radius |
| G33 | 24 | 72 | female | arm | −1.8 | 7 | − | + | − | FX-phalangeal |
| G32 | 32 | 54 | female | arm | −1.1 | 5 | + | − | + | Nerve injury-radial, Surgery-MCP |
| A158 | 40 | 35 | female | arm | 0.4 | 2 | + | − | − | Thrombosis-superior vena cava |
| G2 | 40 | 43 | female | leg | −1.6 | 9 | − | − | − | Soft tissue injury-ankle |
| A355 | 44 | 36 | female | leg | −3.0 | 5 | + | − | − | Surgery-knee |
| A835 | 48 | 58 | female | arm | 0.5 | 5 | + | − | − | Electrocution-hand |
| G29 | 56 | 57 | female | arm | −1.1 | 7 | − | + | − | FX-radius |
| A320 | 76 | 44 | male | arm | 2 | − | − | − | Surgery-shoulder | |
| A260 | 80 | 68 | male | leg | 0.6 | 8 | − | − | + | Nerve injury-L5/S1 |
| A1236CD | 92 | 48 | male | arm | −0.4 | 8 | + | − | + | Nerve injury-ulnar |
| A386 | 104 | 44 | female | arm | −0.4 | 6 | + | + | − | FX-hand |
| A636 | 108 | 54 | female | arm | 0.0 | 7 | − | − | − | Soft tissue injury-shoulder |
| A1059 | 108 | 35 | female | arm | 0.4 | 7 | − | − | − | Soft tissue injury-wrist |
| A707 | 144 | 38 | female | arm | 1.4 | 9 | + | − | − | Spontaneous onset |
| A485 | 184 | 45 | female | arm | 1.6 | 6 | − | − | + | Surgery-carpal tunnel syndrome |
| A1030 | 186 | 50 | female | leg | −1.1 | 2 | − | + | − | FX-ankle |
| A569AB | 228 | 38 | female | arm | 0.0 | 8 | + | − | − | Soft tissue injury |
| A1177 | 288 | 27 | female | leg | −1.5 | 8 | − | − | − | Spontaneous onset |
| A249 | 472 | 41 | female | arm | 0.8 | 8 | − | − | + | Nerve injury-brachial plexus |
| A1232 | 500 | 51 | female | leg | −0.3 | 7 | − | + | − | FX-ankle |
| A569CD | 516 | 51 | male | arm | 0.5 | 8 | + | − | − | Soft tissue injury-shoulder |
| A1236AB | 920 | 50 | male | arm | −1.1 | 5 | + | + | − | FX-wrist |
The median duration of CRPS was 16 (3–920) weeks, the average age was 49.4±1.8 years, and the majority of subjects were female (67%). The majority of biopsies were obtained in the hands (80%), the average skin temperature difference between hands was 0±0.2°C, and the average numerical pain score (NPS) was 6.4±0.3 at the time of biopsy. Allodynia was present in 48% of subjects, 42% of subjects had suffered a fracture, and 24% of subjects had evidence of nerve injury (CRPS II). Temp (Δ°C), temperature difference between the CRPS affected limb and the contralateral side in degrees centigrade; FX, fracture
Based on our prior analysis of 145 CRPS patients demonstrating increased edema and warmth in acute CRPS versus chronic CRPS,3 and the inverse correlation between CRPS duration and epidermal thickness, number of Ki-67 labeled keratinocytes, and number of tryptase labeled mast cells (Table 2), we separated the patients into 2 cohorts, acute (< 3 months CRPS duration, n = 26) and chronic (> 3 months CRPS duration, n = 29) CRPS. No differences were observed between the acute and chronic groups for any demographic or clinical presentation variable except for age. The average age for the acute group was 53.7 ± 2.9 years and for the chronic group it was 45.6 ± 2.0 years (p < 0.05).
Table 2.
Spearman rank-order correlations of outcome variables in CRPS patients
| Epidermal thickness | Ki-67 labeled cells | Tryptase labeled cells | CRPS duration | NPS | Temp | Age | |
|---|---|---|---|---|---|---|---|
| Epidermal thickness | 0.72*** | −0.003 | −0.68*** | −0.13 | −0.02 | 0.36* | |
| Ki-67 labeled cells | 0.72*** | −0.24 | −0.49** | 0.04 | −0.12 | 0.38* | |
| Tryptase labeled cells | −0.002 | −0.24 | −0.45* | 0.28 | 0.47* | −0.02 | |
| CRPS duration | −0.68*** | −0.49** | −0.45* | 0.04 | −0.27 | −0.38** | |
| NPS | −0.12 | 0.04 | 0.28 | 0.04 | 0.01 | 0.04 | |
| Temp | −0.02 | −0.11 | 0.47* | −0.27 | 0.01 | 0.26 | |
| Age | 0.36* | 0.38* | −0.02 | −0.38* | 0.04 | 0.26 |
Epidermal thickness, Ki-67 labeled cell number, tryptase labeled cell number, and temperature values represent the differences between the CRPS affected limb and the contralateral healthy limb. NPS, numerical pain score; Temp, temperature;
P < 0.05,
P < 0.01,
P < 0.001
The relationships between the interval variables (epidermal thickness differences, Ki-67 labeled cell number differences, tryptase labeled cell number differences, skin temperature differences, age, CRPS duration, and numerical pain scores) and the dichotomous variables (gender, limb, and the presence of allodynia, fracture, CRPS I, and CRPS II) were tested by point biserial correlation analysis. The only significant correlations between dichotomous and interval variables were for fracture and Ki-67 labeled cell number differences (49 ± 27 cells in fracture vs 8 ± 11 cells in no fracture, r = 0.37, p = 0.05), fracture and skin temperature differences (0.6 ± 0.3° in fracture vs −0.3 ± 0.2° in no fracture, r = 0.32, p < 0.05), and fracture and age (57.3 ± 2.6 years in fracture vs 43.9 ± 2.1 years in no fracture, r = 0.33, p < 0.05).
Keratinocyte proliferation in CRPS
To test the hypothesis that keratinocytes are activated and proliferating in CRPS affected skin, we performed immunostaining on sections from skin punch biopsies obtained from CRPS affected limbs, comparing the results to sections from biopsies obtained in the non-affected contralateral limb skin. A pan-keratinocyte marker recognizing the acidic and basic (Type 1 and II) subfamilies of cytokeratins was used to label epidermal keratinocytes (Fig.1) and sections were co-stained for Ki-67 to determine keratinocyte proliferation (Fig. 2). As expected, there was a strong direct correlation between epidermal thickness and the number of keratinocytes co-labeled with the cellular proliferation marker Ki-67 (r = 0.72, p < 0.001, n = 34, Table 2). There was also a moderate inverse correlation between the duration of CRPS and epidermal thickness (r = −0.68, p < 0.001, n = 46), and a moderate direct correlation between the patient’s age and epidermal thickness (r = 0.36, p < 0.05, n = 46). Similarly, there was a moderate inverse correlation between CRPS duration and the number of Ki-67 labeled keratinocytes (r = −0.49, p < .01, n = 40), and a moderate direct correlation between the patient’s age and the number of Ki-67 labeled keratinocytes (r = 0.38, p < 0.05, n = 40). The correlations between epidermal thickness and Ki-67 labeled keratinocyte numbers with age may be attributable to an inverse correlation between age and the duration of CRPS (r = −0.38, p < 0.05, n = 55). Neither epidermal thickness nor Ki-67 labeled keratinocyte numbers correlated with tryptase labeled dermal cell numbers, numerical pain scales, or skin temperature differences.
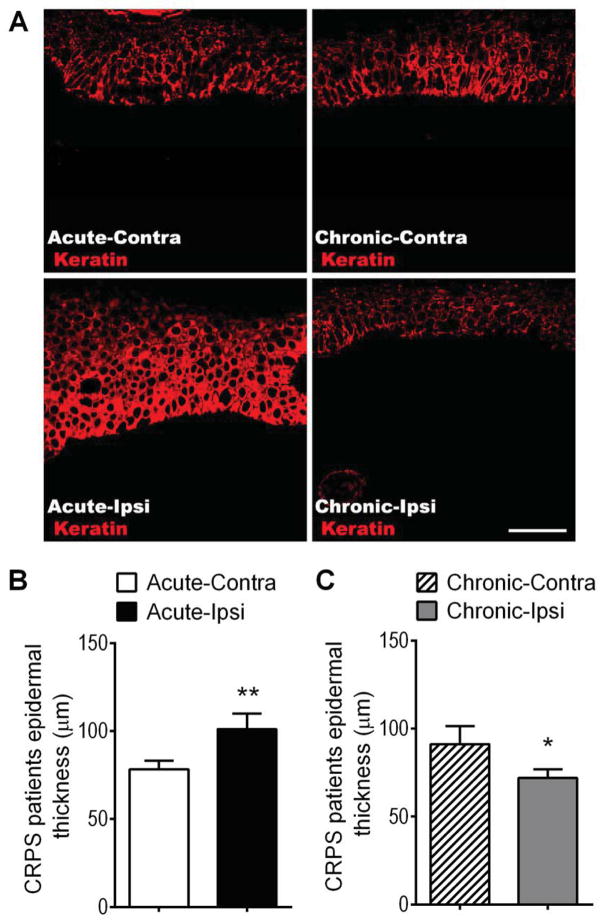
Figure 1.
Epidermal thickness changes in the affected skin of patients suffering from acute and chronic CRPS. (A) Immunostaining for keratin protein (red, a keratinocyte marker) in skin sections obtained from patients with acute (< 3 months duration, left panels) and chronic (> 3 months duration, right panels) CRPS. (A) Panels exhibit confocal images from an acute CRPS patient’s contralateral hand (Acute-Contra, upper left panel), the same patient’s ipsilateral CRPS affected hand (Acute-Ipsi, lower left panel), a chronic CRPS patient’s contralateral hand (Chronic-Contra, upper right panel), and the same patient’s ipsilateral CRPS affected hand (Chronic-Ipsi, lower right panel). Scale bar = 50 μm. (B) There was a 29% increase in epidermal thickness in the affected skin of patients with acute CRPS (101 ± 9 μm, n = 20), as compared to the contralateral healthy limb (78 ± 5 μm). (C) In chronic CRPS patients the inverse was true, there was a 21% decrease in epidermal thickness in the affected skin (72 ± 5 μm, n = 26), as compared to the contralateral healthy limb (91 ± 10 μm). Although the epidermal thickness in the contralateral healthy skin of the acute and chronic CRPS patient cohorts differed, this did not reach significance and may be a reflection of the different average ages between the acute (53.7 ± 2.9) and chronic (45.6 ± 2.0) cohorts (B,C). *P < 0.05, ***P < 0.001 for ipsi vs. contra values. Contra: contralateral, Ipsi: ipsilateral.
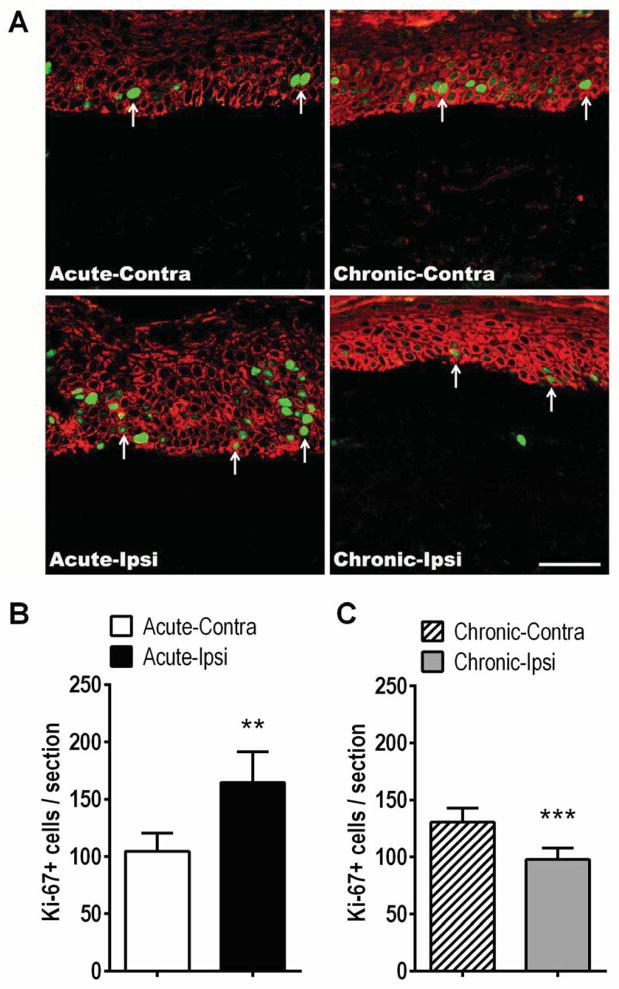
Figure 2.
Keratinocyte proliferation changes in the affected skin of patients suffering from acute and chronic CRPS. (A) Immunostaining for keratin protein (red, a keratinocyte marker) and Ki-67 (green, a marker of DNA synthesis) in skin sections obtained from patients with acute (< 3 months duration, left panels) and chronic (> 3 months duration, right panels) CRPS. (A) Left panels are representative confocal microscopy images from an acute CRPS patient’s contralateral hand (Acute-Contra, upper left panel) and the same patient’s ipsilateral CRPS affected hand (Acute-Ipsi, lower left panel). The right panels are representative images of a chronic CRPS patient’s contralateral hand (Chronic-Contra, upper right panel), and the same patient’s ipsilateral CRPS affected hand (Chronic-Ipsi, lower right panel). Arrows: Ki-67 positive keratinocytes. Scale bar = 50 μm. (B) There was a 57% increase in Ki-67 positive keratinocyte numbers in the affected skin of patients with acute CRPS (165 ± 27), as compared to the contralateral healthy limb (105 ± 16, n = 18). (C) In chronic CRPS patients the inverse was true, there was a 25% decrease in Ki-67 labeled keratinocytes in the affected skin (98 ± 10), as compared to the contralateral healthy limb (131 ± 12, n = 25). Although the contralateral healthy skin Ki-67 labeled keratinocyte counts in the acute patients (105 ± 16) and the chronic patients (131 ± 12) differed, this did not reach significance and may be a reflection of the different average ages between the acute (53.1 ± 2.7) and chronic (45.7 ± 1.9) cohorts (Fig. 2B,C) **P < 0.01 for ipsi vs. contra values. Contra: contralateral, Ipsi: ipsilateral.
Figure 1 illustrates that when the patients were separated into acute (< 3 months CRPS duration) and chronic (> 3months CRPS duration) groups there was a significant increase in epidermal thickness in the CRPS affected skin of the acute patient population and a decrease in epidermal thickness in the chronic patients. As shown in Figure 2, in acute CRPS keratinocyte expression of Ki-67 was increased in CRPS affected skin, as compared to healthy skin from the contralateral limb, demonstrating keratinocyte hyperplasia. Conversely, in chronic CRPS keratinocyte Ki-67 expression was decreased in affected skin, compared to the contralateral side.
Keratinocyte expression of TNF-α and IL-6 in CRPS
Figure 3 presents representative photomicrographs illustrating up-regulation of TNF-α in the epidermal keratinocytes of the CRPS affected skin, compared to the contralateral side. Co-labeling with DAPI demonstrated that TNF-α immunoreactivity is localized primarily in the keratinocyte cytoplasm. Blinded subjective analysis suggested that in acute patients TNF-α immunostaining in CRPS affected skin was increased in 42%, decreased in 4%, and unchanged in 54% of patients, compared to the contralateral side. In chronic CRPS patients, TNF-α immunostaining in CRPS affected skin appeared increased in 25%, decreased in 14%, and unchanged in 61% of patients, compared to the contralateral side.
Figure 3.
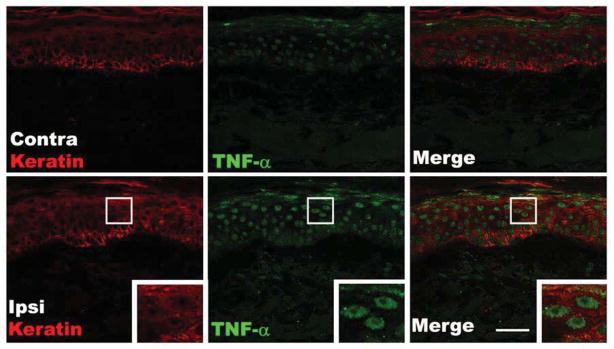
Fluorescence photomicrographs showing co-localization of keratin immunostaining in keratinocytes (red) with immunostaining for TNF-α (green) in skin biopsy sections from a patient with acute CRPS. Compared with the contralateral healthy skin (upper panels), TNF-α expression was up-regulated in the cytoplasm of epidermal keratinocytes of the ipsilateral CRPS affected skin (lower panels). The insets are enlargements of the boxed regions. Scale bar = 40μm. Contra: contralateral, Ipsi: ipsilateral.
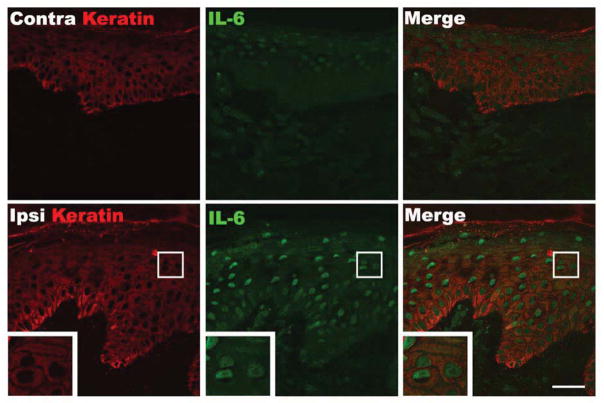
Figure 4 presents representative photomicrographs illustrating increased IL-6 expression in the epidermal keratinocytes of the CRPS affected skin, compared to the contralateral side. Co-labeling with DAPI demonstrated that IL-6 immunoreactivity is localized in the keratinocyte cytoplasm. Blinded subjective assessment suggested that in acute patients IL-6 immunostaining in CRPS affected skin was increased in 39%, decreased in 0%, and unchanged in 61% of patients, compared to the contralateral side. In chronic CRPS patients, IL-6 immunostaining in CRPS affected skin appeared increased in 23%, decreased in 12%, and unchanged in 65% of patients, compared to the contralateral side.
Figure 4.
Fluorescence photomicrographs showing co-localization of keratin immunostaining in keratinocytes (red) with immunostaining for IL-6 (green) in skin biopsy sections from a patient with acute CRPS. Compared with the contralateral healthy skin (upper panels), IL-6 expression was up-regulated in the cytoplasm of epidermal keratinocytes in the ipsilateral CRPS affected skin. The insets are enlargements of the boxed regions. Scale bar = 40μm. Contra: contralateral, Ipsi: ipsilateral.
Mast cell accumulation in CRPS affected skin
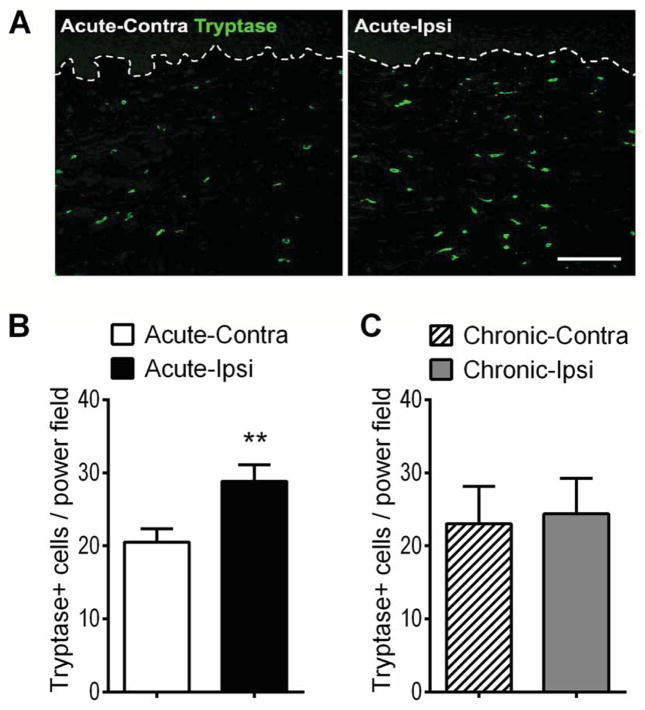
Figure 5 shows photomicrographs illustrating increased numbers of tryptase immunostained dermal mast cells in CRPS affected skin. There was a moderate inverse correlation between the duration of CRPS and the number of tryptase labeled cells (r = −0.45, p < 0.05, n = 27), and a moderate direct correlation between tryptase labeled cell numbers and skin temperature asymmetry (r = 0.47, p < 0.05, n = 24) (Table 2). When patients were separated into acute and chronic cohorts, there was a significant increase in tryptase positive dermal mast cell numbers in the CRPS affected skin of the acute patient group and no changes in tryptase positive mast cell numbers in the CRPS affected skin of the chronic patients (Fig. 5).
Figure 5.
Mast cell accumulation changes in the affected skin of patients suffering from acute and chronic CRPS. (A) Immunostaining for tryptase positive cells (green, mast cell marker) in the dermis of the skin sections obtained from a patient with acute CRPS. The dotted line denotes the basement membrane at the dermal-epidermal border. Scale bar = 200μm. (B) There was a 41% increase in tryptase positive mast cell numbers at the affected skin (29 ± 2) of patients with acute CRPS (< 3 months duration), compared to the contralateral healthy limb (21 ± 2, n=20). (C) In chronic CRPS (> 3 months duration) patients no differences were observed between the tryptase positive mast cell numbers in the affected skin (24 ± 5) and the contralateral healthy skin (23 ± 5, n=10). **P < 0.01 for ipsi vs. contra values. Contra: contralateral, Ipsi: ipsilateral.
Discussion
Previously we observed that 4 weeks after a distal tibia fracture in rats there was an increase in SP signaling in the injured limb, inducing keratinocyte activation and proliferation with a dramatic increase in hindpaw epidermal thickness.27, 43, 44 The distal tibia fracture model in rats induces chronic vascular, inflammatory, nociceptive and boney changes in the injured hindlimb that closely parallel the clinical presentation of acute CRPS,12, 13, 29, 34, 35 therefore, we hypothesized that skin keratinocyte proliferation and epidermal thickness would be increased in patients with acute CRPS. In the current study there was a direct correlation between epidermal thickness and the number of Ki-67 labeled proliferating keratinocytes in the CRPS affected skin (Table 2). There was also an inverse correlation between the duration of CRPS and the epidermal thickness and the number of Ki-67 labeled proliferating keratinocytes in CRPS affected skin, indicating that epidermal thickening and increased keratinocyte proliferation were more prevalent early in the disease. When patients were separated into acute and chronic cohorts, epidermal thickness (Fig. 1) and keratinocyte proliferation (Fig. 2) were increased in the acute phase and decreased in the chronic phase. The changes observed in early CRPS patient skin confirm our previous observations of increased epidermal thickness and keratinocyte proliferation in the hindpaw skin at 4 weeks after tibia fracture in the rat. These results support the hypothesis that the rat fracture model might be utilized to predict novel pathophysiology in CRPS patients. We have not determined whether epidermal thickness or keratinocyte proliferation in the fracture rat model decline over time, but intend to pursue these questions in a future study.
Increased levels of secreted TNF-α and IL-6 cytokines have been observed in experimental suction blister fluid obtained from CRPS skin11, 17, 19, 21, 46, 47 and TNF-α levels are increased in CRPS skin biopsies when measured by EIA.24 It has been proposed that potential cellular sources for these inflammatory mediators in CRPS skin include macrophages, monocytes, fibroblasts, and mast cells.17 Previously we observed that TNF-α and IL-6 gene expression and protein levels are elevated in rat hindpaw skin at 4 weeks post-fracture and that keratinocytes are the primary source for the TNF-α and IL-6 observed in the fractured limb skin.27, 29, 34 Based on these translational studies we predicted that the keratinocytes would be the primary source of up-regulated TNF-α and IL-6 expression in CRPS skin and the current study suggests that this is indeed the case (Figs. 3,4). Subjectively we observed increases in TNF-α and IL-6 immunostaining more frequently in acute vs chronic CRPS patient skin sections (Figs 3, 4), and this impression corresponds with the results of a prior study looking at changes in CRPS skin blister fluid cytokine levels over time. Over a 6 year period there was a gradual resolution of elevated TNF-α and IL-6 levels in CRPS skin blister fluid, despite persistent pain symptoms, and the authors postulated that peripheral cytokines may be important for the initiation and maintenance of pain in early or semi-acute CRPS, but pain in more chronic CRPS patients is primarily due to central sensitization.46 Rats treated in the 4 weeks post-fracture with the IL-6 receptor antagonist TB-2-081, TNF-α inhibitor etanercept, or the global cytokine inhibitor pentoxifylline had reduced allodynia and hindlimb unweighting, suggesting an important role for cytokine and growth factor signaling in the development of trauma induced chronic pain.14, 26, 34, 45 We postulated that TNF-α and IL-6 pronociceptive effects in this CRPS model occurred in the periphery, perhaps by sensitizing primary sensory afferents (nociceptors).6, 22, 33
Additional support for the hypothesis that cytokine signaling contributes to CRPS development derives from several case reports of pain reduction in CRPS patients after TNF-α inhibitor treatment.2, 20 Interestingly, each successfully treated patient had a warm affected limb. Eisenberg et al recently published a CRPS case series observing dramatic pain reduction in 3 out of 10 CRPS patients receiving a 6 week course of anti-TNF treatment.9 The authors observed that anti-TNF treatment was more likely to be effective in early CRPS patients and in patients with warm affected extremities. Drickx et al recently published preliminary data from a discontinued randomized controlled trial that enrolled 6 CRPS patients treated with anti-TNF and 7 placebo treated controls.8 The data presentation in that paper did not allow the breakdown of results by duration of CRPS or limb warmth, but it appeared that at least one anti-TNF treated patient went from severe to moderate pain and none of the 7 placebo treated CRPS patients changed their categorical pain ratings. Collectively, the preliminary results from these 4 reports suggest that some CRPS patients may have a dramatic response to anti-TNF treatment, particularly the subset of early CRPS patients with warm limbs.
Previously we observed that mast cell accumulation, activation, and degranulation increased in the hindpaw skin of tibia fracture rats at 4 weeks post-fracture and that mast cell degranulation contributes to pain sensitization in this CRPS model.28 Based on these observations we predicted that there would be an increase in mast cell numbers in CRPS skin. In the current study there was an inverse correlation between the duration of CRPS and the number of tryptase labeled dermal mast cells in CRPS affected skin (Table 2), indicating that increased mast cell numbers were more likely to be observed early in the disease. When patients were separated into acute and chronic cohorts, there was an increase in tryptase labeled mast cells in CRPS skin in the first 3 months of the disease and no change in mast cell numbers in chronic CRPS skin (Fig. 5). These results suggest that increased dermal mast cell accumulation contributes to the elevated tryptase levels previously observed in experimental suction blister fluid from CRPS skin.21 Those authors noted a significant correlation between blister fluid tryptase levels and the patient’s pain intensity, suggesting that mast cell degranulation contributes to CRPS pain.21 Degranulation of mast cells releases numerous inflammatory mediators, including tryptase, proteases, histamine, cytokines, and eicosanoids, all potentially capable of inducing inflammation and sensitization. In the current study we observed a direct correlation between mast cell numbers and skin temperature in the CRPS affected skin, consistent with histamine induced vasodilation (Table 2).
There are several limitations of the current study. Bilateral skin biopsies were collected in patients with one CRPS affected limb, thus the contralateral limb biopsy was used as a control. It is unknown where the clinically uninvolved extremity may have exhibited keratinocyte or inflammatory changes. Contralateral mirror limb changes in nociceptive processing and neurogenic inflammation have been observed in animal models after unilateral nerve and tissue injuries 15, 23 and increased substance P induced extravasation responses have been observed in the contralateral limb of CRPS patients.25 Another concern is that the skin biopsies were obtained from CRPS patients recruited in Australia and Germany and inflammatory responses can differ between races.18, 32 Ethnicities of the CRPS patients were not determined in the current study, thus potential differences in the Australian and German patient populations are a concern. Another limitation is there was no post-fracture (without CRPS) control group to look at the effects of trauma alone. In addition, there was a modest inverse correlation between the patient’s age and the duration of CRPS, indicating a selection bias (Table 2). This patient selection bias for age and CRPS duration explains the modest direct correlation between patient age and epidermal thickness/keratinocyte proliferation, as CRPS duration directly correlated to epidermal thickness and keratinocyte proliferation (Table 2). In normal subjects there is no correlation between age and epidermal thickness.36
The strengths of the current study are; 1) the large number of bilateral CRPS patient biopsies obtained (n = 55), 2) the range of CRPS duration in these 55 patients allowed us to examine large numbers of both acute (< 3 month duration) and chronic (>3 month duration) patients, thus demonstrating changes in side-to-side differences in epidermal thickness, keratinocyte proliferation, and mast cell numbers over time, and 3) the use of bilateral skin biopsies in patients with a single CRPS affected extremity allowed the use of the contralateral “normal” limb as a control, thus compensating for age, gender, race, medications, and other medical co-morbidities.
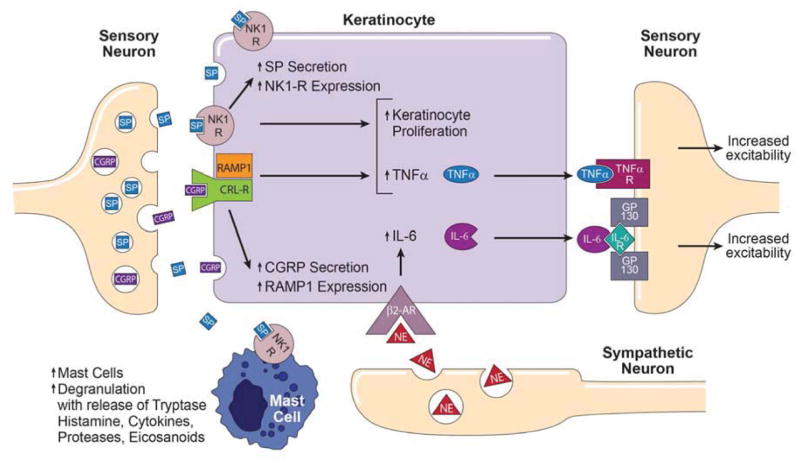
Collectively, these results indicate that in early CRPS epidermal keratinocytes are activated in the affected skin, resulting in proliferation, epidermal thickening, and up-regulated TNF-α and IL-6 protein expression. In chronic CRPS patients keratinocyte proliferation is reduced with epidermal thinning. Acute CRPS patients also exhibit increased mast cell accumulation in the affected skin, but in chronic CRPS skin mast cells numbers are unchanged. These results are consistent with prior clinical and translational studies supporting the hypothesis illustrated in Figure 6 that early CRPS involves activation of the cutaneous innate immune system, with exaggerated sensory and sympathetic signaling, keratinocyte and mast cell activation and proliferation, inflammatory mediator release, and pain.
Figure 6.
Proposed model for the integration of primary afferent and sympathetic nervous system signaling with mast cell and keratinocyte innate immune responses in supporting CRPS-related nociception. Cutaneous primary sensory afferents release SP and CGRP in the epidermis. These neuropeptides then diffuse through the interstitial space to bind and activate their cognate receptors on the keratinocyte cell surface, the SP NK1-R and the CGRP receptor dimer complex of CRLR and RAMP1. Keratinocyte NK1-R activation stimulates cellular proliferation, SP expression and secretion, NK1-R expression, and stimulates TNFα expression and secretion. SP also binds to NK1-Rs on the mast cell surface, resulting in mast cell accumulation, activation, degranulation, and release of tryptase, histamine, cytokines, proteases and eicosanoids, all of which might sensitize nociceptive neurons in the skin. Similarly, activation of the keratinocyte CGRP receptor dimer complex stimulates keratinocyte proliferation, CGRP expression and secretion, RAMP1 receptor expression, and up-regulates TNFα expression and secretion. Sympathetic neurons release NE into the interstitial space, which binds and activates β2-ARs on the keratinocyte cell surface, resulting in IL-6 expression and secretion. Keratinocyte secreted inflammatory cytokines can directly activate their cognate receptors expressed on cutaneous sensory afferent neurons, with subsequent pain sensitization. CGRP, calcitonin gene-related peptide; SP, substance P; NK1-R, neurokinin 1 receptor; CRL-R, calcitonin receptor-like receptor; RAMP1 receptor activity-modifying protein; NE, norepinephrine; β2-AR, β2 adrenergic receptor; IL-6, interleukin-6; IL-6R, IL-6 receptor; TNF-α, tumor necrosis factor α; TNF-α R, TNF-α receptor.
Perspective.
The results of this study support the hypotheses that CRPS involves activation of the innate immune system, with keratinocyte and mast cell activation and proliferation, inflammatory mediator release, and pain.
Acknowledgments
National Institutes of Health grant NS072168, Department of Veterans Affairs Rehabilitation Research and Development Merit grant F7137R, German Research Foundation Bi579/8, EU FP7, Foundation Rhineland-Palatinate Project 936, the Hopp Stiftung, National Health and Medicine Research Council of Australia grants 437205 and APP1030379, and Australian and New Zealand College of Anaesthetics grant 10/21. This study contains essential parts of the MD thesis of N. Albrecht, which will be submitted to the Faculty of Medicine, University Medical Center of the Johannes Gutenberg-University, Mainz, Germany. We would also like to acknowledge the contributions of Dr Lone Knudsen who performed the initial patient evaluations in Australia.
Footnotes
Disclosures: The authors do not have financial or other relationships that might lead to conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernateck M, Karst M, Gratz KF, Meyer GJ, Fischer MJ, Knapp WH, Koppert W, Brunkhorst T. The first scintigraphic detection of tumor necrosis factor-alpha in patients with complex regional pain syndrome type 1. Anesthesia and analgesia. 2010;110:211–215. doi: 10.1213/ANE.0b013e3181c4bab7. [DOI] [PubMed] [Google Scholar]
- 2.Bernateck M, Rolke R, Birklein F, Treede RD, Fink M, Karst M. Successful intravenous regional block with low-dose tumor necrosis factor-alpha antibody infliximab for treatment of complex regional pain syndrome 1. Anesthesia and analgesia. 2007;105:1148–1151. doi: 10.1213/01.ane.0000278867.24601.a0. [DOI] [PubMed] [Google Scholar]
- 3.Birklein F, Riedl B, Sieweke N, Weber M, Neundorfer B. Neurological findings in complex regional pain syndromes--analysis of 145 cases. Acta Neurol Scand. 2000;101:262–269. doi: 10.1034/j.1600-0404.2000.101004262x./. [DOI] [PubMed] [Google Scholar]
- 4.Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113:713–725. doi: 10.1097/ALN.0b013e3181e3db38. [DOI] [PubMed] [Google Scholar]
- 5.Carroll I, Clark JD, Mackey S. Sympathetic block with botulinum toxin to treat complex regional pain syndrome. Annals of neurology. 2009;65:348–351. doi: 10.1002/ana.21601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesthesia and analgesia. 2003;96:1096–1103. doi: 10.1213/01.ANE.0000055362.56604.78. [DOI] [PubMed] [Google Scholar]
- 7.de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Dirckx M, Groeneweg G, Wesseldijk F, Stronks DL, Huygen FJ. Report of a preliminary discontinued double-blind, randomized, placebo-controlled trial of the anti-TNF-alpha chimeric monoclonal antibody infliximab in complex regional pain syndrome. Pain practice : the official journal of World Institute of Pain. 2013;13:633–640. doi: 10.1111/papr.12078. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg E, Sandler I, Treister R, Suzan E, Haddad M. Anti tumor necrosis factor - alpha adalimumab for complex regional pain syndrome type 1 (CRPS-I): a case series. Pain practice : the official journal of World Institute of Pain. 2013;13:649–656. doi: 10.1111/papr.12070. [DOI] [PubMed] [Google Scholar]
- 10.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. Journal of immunology. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 11.Groeneweg JG, Huygen FJ, Heijmans-Antonissen C, Niehof S, Zijlstra FJ. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC Musculoskelet Disord. 2006;7:91. doi: 10.1186/1471-2474-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2006;121:158–167. doi: 10.1016/j.pain.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Tsujikawa K, Rice KC, Cheng K, Clark DJ, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Mol Pain. 2012;8:85. doi: 10.1186/1744-8069-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansson E. Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta physiologica. 2006;187:321–327. doi: 10.1111/j.1748-1716.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- 16.Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain medicine. 2007;8:326–331. doi: 10.1111/j.1526-4637.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 17.Heijmans-Antonissen C, Wesseldijk F, Munnikes RJ, Huygen FJ, van der Meijden P, Hop WC, Hooijkaas H, Zijlstra FJ. Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediators of inflammation. 2006;2006:28398. doi: 10.1155/MI/2006/28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann SC, Stanley EM, Cox ED, DiMercurio BS, Koziol DE, Harlan DM, Kirk AD, Blair PJ. Ethnicity greatly influences cytokine gene polymorphism distribution. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2002;2:560–567. doi: 10.1034/j.1600-6143.2002.20611.x. [DOI] [PubMed] [Google Scholar]
- 19.Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijistra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators of inflammation. 2002;11:47–51. doi: 10.1080/09629350210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huygen FJ, Niehof S, Zijlstra FJ, van Hagen PM, van Daele PL. Successful treatment of CRPS 1 with anti-TNF. J Pain Symptom Manage. 2004;27:101–103. doi: 10.1016/j.jpainsymman.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during Complex Regional Pain Syndrome type 1. Immunol Lett. 2004;91:147–154. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends in neurosciences. 1999;22:122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- 24.Kramer HH, Eberle T, Uceyler N, Wagner I, Klonschinsky T, Muller LP, Sommer C, Birklein F. TNF-alpha in CRPS and 'normal' trauma--significant differences between tissue and serum. Pain. 2011;152:285–290. doi: 10.1016/j.pain.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Experimental neurology. 2003;183:197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Shi X, Wang L, Guo T, Wei T, Cheng K, Rice KC, Kingery WS, Clark JD. Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain. 2013;154:1224–1236. doi: 10.1016/j.pain.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li WW, Guo TZ, Li XQ, Kingery WS, Clark JD. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain. 2010;151:843–852. doi: 10.1016/j.pain.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li WW, Guo TZ, Liang DY, Sun Y, Kingery WS, Clark JD. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology. 2012;116:882–895. doi: 10.1097/ALN.0b013e31824bb303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 2009;144:303–313. doi: 10.1016/j.pain.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinus J, Moseley GL, Birklein F, Baron R, Maihofner C, Kingery WS, van Hilten JJ. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10:637–648. doi: 10.1016/S1474-4422(11)70106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munnikes RJ, Muis C, Boersma M, Heijmans-Antonissen C, Zijlstra FJ, Huygen FJ. Intermediate stage complex regional pain syndrome type 1 is unrelated to proinflammatory cytokines. Mediators of inflammation. 2005;2005:366–372. doi: 10.1155/MI.2005.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi- ethnic population. Ethnicity & disease. 2011;21:142–149. [PMC free article] [PubMed] [Google Scholar]
- 33.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 34.Sabsovich I, Guo TZ, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 2008;137:507–519. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138:47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandby-Moller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta dermato-venereologica. 2003;83:410–413. doi: 10.1080/00015550310015419. [DOI] [PubMed] [Google Scholar]
- 37.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 38.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. Journal of cellular physiology. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Shi X, Wang L, Clark JD, Kingery WS. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept. 2013 doi: 10.1016/j.regpep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka T, McRae BJ, Cho K, Cook R, Fraki JE, Johnson DA, Powers JC. Mammalian tissue trypsin-like enzymes. Comparative reactivities of human skin tryptase, human lung tryptase, and bovine trypsin with peptide 4-nitroanilide and thioester substrates. The Journal of biological chemistry. 1983;258:13552–13557. [PubMed] [Google Scholar]
- 41.Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2008;56:687–696. doi: 10.1369/jhc.2008.951194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderslice P, Ballinger SM, Tam EK, Goldstein SM, Craik CS, Caughey GH. Human mast cell tryptase: multiple cDNAs and genes reveal a multigene serine protease family. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:3811–3815. doi: 10.1073/pnas.87.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei T, Guo TZ, Li WW, Hou S, Kingery W, Clark JD. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. J Neuroinflammation. 2012;9:181. doi: 10.1186/1742-2094-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144:278–286. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. European journal of pain. 2009;13:253–262. doi: 10.1016/j.ejpain.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Six years follow-up of the levels of TNF-alpha and IL-6 in patients with complex regional pain syndrome type 1. Mediators of inflammation. 2008;2008:469439. doi: 10.1155/2008/469439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Tumor necrosis factor-alpha and interleukin-6 are not correlated with the characteristics of Complex Regional Pain Syndrome type 1 in 66 patients. European journal of pain. 2008;12:716–721. doi: 10.1016/j.ejpain.2007.10.010. [DOI] [PubMed] [Google Scholar]