Abstract
In general, in most organ systems, intracellular protein homeostasis is the sum of many factors, including chromosomal state, protein synthesis, post-translational processing and transport, folding, assembly and disassembly into macromolecular complexes, protein stability and clearance. In the heart, there has been a focus on the gene programs that are activated during pathogenic processes, but the removal of damaged proteins and organelles has been underappreciated as playing an important role in the pathogenesis of heart disease. Proteotoxicity refers to the adverse effects of damaged or misfolded proteins and even organelles on the cell. At the cellular level, the ultimate outcome of uncontrolled or severe proteotoxicity is cell death; hence, the pathogenic impact of proteotoxicity is maximally manifested in organs with no or very poor regenerative capability such as the brain and the heart. Evidence for increased cardiac proteotoxicity is rapidly mounting for a large subset of congenital and acquired human heart disease. Studies carried out in animal models and in cell culture have begun to establish both sufficiency and, in some cases, the necessity of proteotoxicity as a major pathogenic factor in the heart. This dictates rigorous testing for the efficacy of proteotoxic attenuation as a new strategy to treat heart disease. This review article highlights some recent advances in our understanding of how misfolded proteins can injure and are handled in the cell, examining the emerging evidence for targeting proteotoxicity as a new therapeutic strategy for heart disease.
1. Introduction
Our interest in this review topic is driven by the hypothesis that both increased production and diminished removal of damaged/misfolded proteins in cardiomyocytes can serve as either a primary or secondary driver for the progression of cardiac remodeling and failure from a multitude of primary etiologies. Defining how the protein complement of the heart is regulated remains fundamental to our ability to productively identify the processes and proteins that underlie normal and abnormal cardiac function. As heart disease remains the most common cause of death and significant disability in the developed world [1], it is imperative that we understand the mechanistic bases that are responsible for controlling the normal and abnormal protein complements of the different cell types present in the cardiac compartment.
The concept of proteotoxicity is inextricably linked to protein homeostasis in the cell in general and in the heart in particular. Protein homeostasis is the sum total of protein synthesis (translation), post-translational processing and transport, folding, assembly and disassembly into macromolecular complexes, stability and clearance [2]. If any one of these processes is perturbed by internal or external stimuli, the cell must respond to either regulate that specific process or compensate by regulating some other function, which may not necessarily be closely linked mechanistically to the particular system (eg, up- or down-regulation of RNA metabolism). If a protein contains a mutation that predisposes it to incorrect folding or aggregation, or some other ancillary process is disturbed such that protein misfolding/aggregation occurs, the potential for proteotoxicity arises [3, 4]. Thus one early working definition of proteotoxicity is the process by which a protein or proteins mis-fold and form homo- or heteromeric oligomers such that soluble or insoluble aggregates become stable and exert a toxic effect on cellular metabolism [5]. This can occur as a result of either the protein-centric, autonomous effect of a detrimental mutation, or a defect or inability to compensate on the part of a protein or proteins linked to the other protein homeostasis processes referred to above. That is, the amino acid sequence of the protein itself may be normal and its overall synthetic rate within normal limits. But, if it is unable, for example, to fold correctly because of a loss-of-function of a critical chaperone, the potential arises for it to become proteotoxic and form aggregates [1, 6–8]. Alternatively, if a protein serves out its function over its normal lifetime, but quality control, degradation and/or clearance functions are or become abnormal, accumulations of the now aged and damaged protein may occur and also lead to toxic accumulations of aggregated species [7, 9, 10]. Two major pathways of protein degradation function in the cell: the ubiquitin-proteasome system (UPS) and autophagy. While able to operate independently, they can certainly “talk” to one another through specific signaling molecules that interact with components of both [11]. Protein degradation through the UPS is highlighted elsewhere in this volume (Wang and Robbins). This review will highlight the processes associated with autophagy and how they might function in cardiac disease.
2. Proteotoxicity
2.1. Protein homeostasis and aggregation in the post-mitotic cell: a need for clearance
There are numerous proteins that prevent protein misfolding and aggregation. These chaperones have been intensively studied in both lower and higher organisms although their mechanisms have been most comprehensively studied in systems whose genetics and cell fates are most easily catalogued and manipulated, such as yeast [12], flies [13], and worms [14]. In the heart, some chaperones are abundant and ubiquitously expressed [15, 16]. However, in the cardiomyocyte it is largely unknown whether critical chaperones remain fully engaged with their multiple or specific substrates, or whether significant concentrations are stockpiled to be used when the inevitable stresses occur. Both noninducible and inducible chaperones exist in the heart and the class of inducible chaperones and small heat shock proteins (HSPs), whose synthesis is induced as part of the general stress-inducible response, play important roles modulating protein homeostasis [8, 15, 17]. Some molecular chaperones, such as the small heat shock protein αB-crystallin (CryAB), are present at high concentrations in the cardiomyocyte under basal conditions [18, 19]. These chaperones and HSPs constitute a cell’s first line of defense against proteotoxicity, ensuring the correct folding of nascent proteins during their synthesis and maintaining protein intermediates in a folding pathway such that the equilibrium favors correct folding and assembly [3, 7].
Protein homeostasis is important to the overall health of any cell type, but the post-mitotic cell is particularly challenged if homeostasis is perturbed to the extent that misfolded proteins accumulate and aggregates begin to form. One would therefore expect that, in an organ whose essential cells are largely post-mitotic, proteotoxic processes would have a particularly significant impact on normal function. If toxic concentrations of proteotoxic proteins or aggregates are reached, the cell cannot divide and effectively, after reaching the size characteristic for its type, decrease the concentration of toxic proteins or aggregates. Compounding the sensitivity of the organ, if proteotoxicity leads to cell death, the tissue has no facile way of replacing the damaged or dead cells. This implies that the mechanisms dealing with protein homeostasis and quality control must be highly effective and regulated in cell types such as the neuron and cardiomyocyte and when these systems are perturbed, there are significant pathogenic consequences. In fact, the brain, in which critical cell types such as the neuron are largely post-mitotic, suffers from numerous proteinopathies [20]. For example, many neurodegenerative diseases, including Huntington’s disease [21], Parkinson’s disease [22, 23], prion disease [24, 25], and amyotrophic lateral sclerosis [26] are characterized by the accumulation of significant protein aggregates and a number of pathogenic consequences can be traced to malfunctions in either the chaperones themselves or in the clearance machinery that functions to remove misfolded or damaged proteins [3]. In the heart, as outlined in detail below, this is also true and the expression of ectopic polypeptides capable of exerting proteotoxic effects can, by themselves, cause cardiac disease and heart failure, even when present in only small amounts [27].
3. The Proteasome and autophagy in protein homeostasis
3.1. The ubiquitin-proteasome system
Cells in general and cardiac cells in particular have multiple mechanisms for preventing the formation, and/or removing proteotoxic proteins and damaged organelles. The ubiquitin-proteasome system (UPS) and autophagy-lysosome process are the major proteolytic systems of the cell and they play a critical role for the removal of proteins, aggregates and organelles. The UPS has been studied intensively and its modulation and control clearly play an important role in both normal cardiac function and in different disease processes [11, 28–30]. In the UPS, proteins are targeted for degradation by the 26S proteasome through covalent attachment of a linear chain of ubiquitin molecules. This multistep pathway involves first the activation of the small ubiquitin protein by an ATP-dependent activating enzyme or E1, which then transfers the highly reactive ubiquitin to one of the ubiquitin-conjugating enzymes or E2s. Then, an ubiquitin protein ligase, or E3, binds the protein substrate and the ubiquitin-E2 thio-ester and catalyzes the formation of a ubiquitin chain on the protein. This reaction is the rate-limiting step in the ubiquitination process and therefore affects the kinetics of degradation. In fact, once the protein is ubiquitinated it is docked to the proteasome for degradation, unless the polyubiquitin chain is removed by the de-ubiquitinating enzymes [31, 32]. Different E2–E3 pairs function in the degradation of different proteins, and the specificity of the E3s for specific groups of proteins provides exquisite selectivity to the degradation process [33]. These processes can be significantly affected during the course of human heart disease and cardiac failure [34, 35].
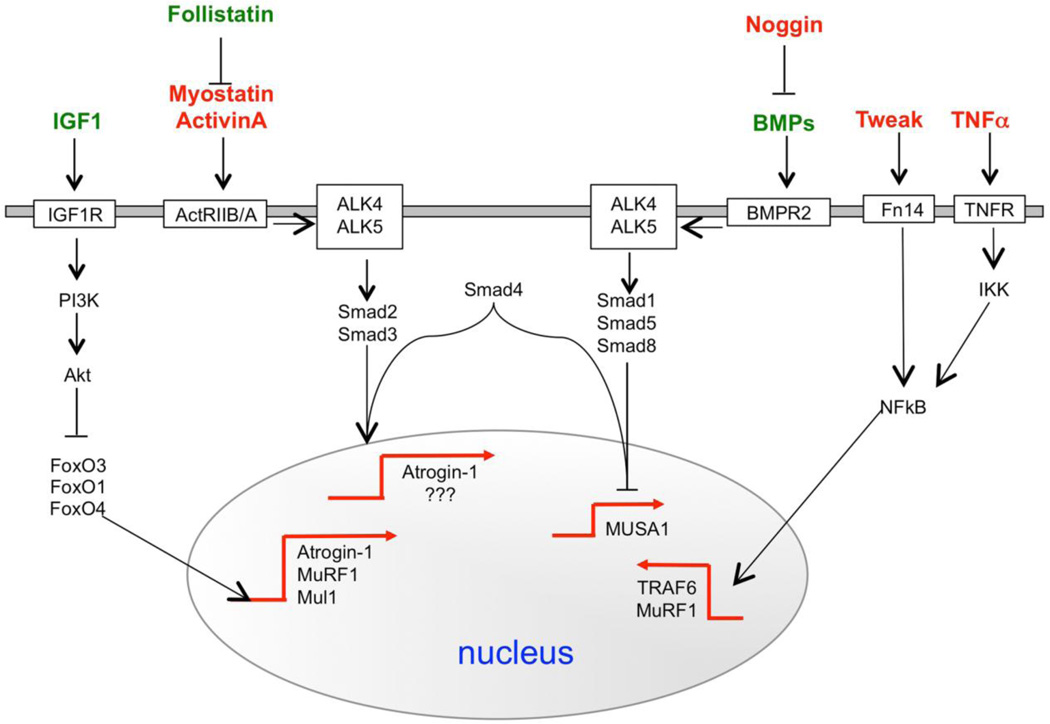
The human genome contains over 650 ubiquitin ligases, which are involved in the precise regulation of different cell processes, and in recent years, dramatic progress has been made in elucidating the roles of different E3s in regulating metabolism, transcription, cell cycle, oncogenesis and cell size [36]. A major contribution in identifying the ubiquitin-ligases involved in atrophy process was provided by the pioneering studies on gene expression profiling [37–39]. The idea to compare gene expression in different models of muscle atrophy led to the identification of a subset of genes that are commonly up- or down-regulated in atrophying muscle. Since all the diseases used for the microarray experiments (i.e. diabetes, cancer cachexia, chronic renal failure, fasting and denervation) have muscle atrophy in common, the commonly up- or down-regulated genes are believed to regulate the atrophic process and are called atrophy-related genes or atrogenes [40]. The two most induced genes are two muscle-specific ubiquitin ligases, namely atrogin-1/MAFbx and MuRF1. Mice lacking these two enzymes are resistant to muscle atrophy induced by denervation [37]. The upregulation of these E3s and in general of proteasome-dependent protein breakdown is blocked by Insulin/Akt pathway through negative regulation of FoxO transcription factors. This family of transcription factors is critical for the expression of these atrophy-related ubiquitin-ligases. Further studies identified IKK/NFkB and Myostatin-TGFβ/ALK2-3/Smad2-3 pathways as additional player in the regulation of the atrophy process [41–44] (Figure 1). Their function is more context dependent and indeed both these signaling pathways are mainly induced during catabolic conditions characterized by high levels of inflammatory cytokines. Interestingly, a new set of ubiquitin ligases, including MUSA1, TRAF6, Nedd4, Mul1, Fbxo40 have been recently found to control protein breakdown or anabolic pathways in striated muscles [43, 45–48]. Their involvement in atrophy is of interest and currently under study (Figure 1).
Figure 1.
Signalling pathways that control the expression of the ubiquitin ligases in striated muscles. In red are depicted the hormones/cytokines that have a catabolic action while in green are underlined the anabolic ones.
3.2. Autophagy
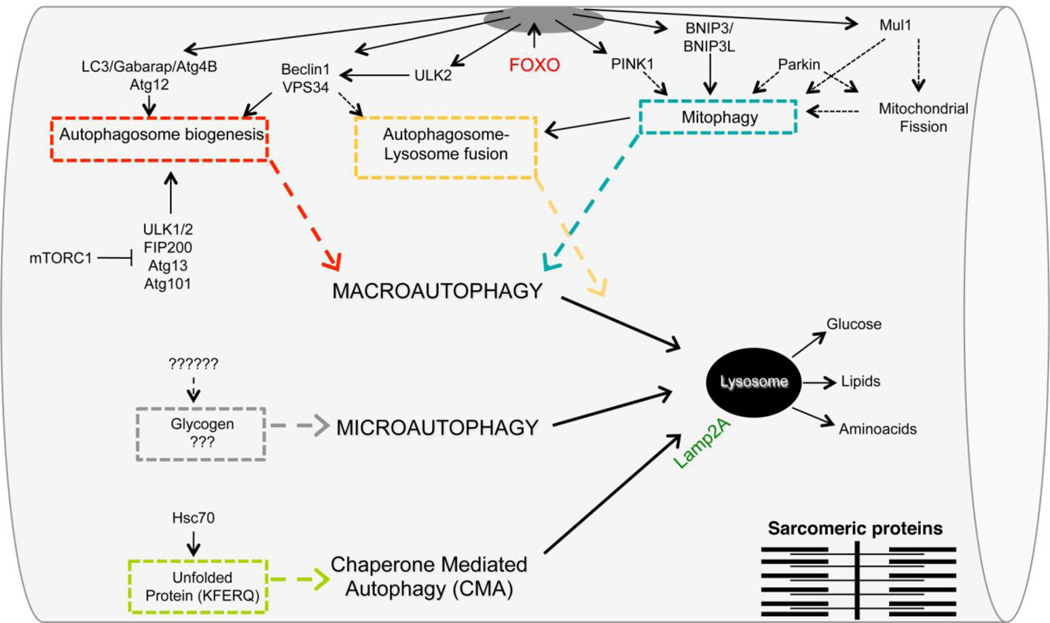
Lysosomes are constituted by membrane-bounded vesicles containing low pH and different acidic hydrolases that can degrade a variety of molecules. The lysosome is the end point of several pathways including endocytosis, pinocytosis, phagocytosis and autophagy. The docking system is crucial for substrate recognition, sequestration and delivery to the lysosome for degradation and its modification can have a major impact on the rate of substrate removal. Three different mechanisms were described in mammals for the delivery of the autophagic cargo to lysosomes: microautophagy, chaperone-mediated autophagy (CMA) and macroautophagy [49– 51] (Figure 2). Microautophagy is a direct engulfment into lysosomes of small portion of cytosol [52, 53]. Although the physiological relevance of microautophagy in mammals is still unclear, electron microscopy observations indicated that microautophagy can participate in glycogen uptake into lysosomes in muscle cells (Figure 2). In CMA, the substrates are soluble proteins that are recognized by a complex which is mainly constituted by heat shock cognate protein of 70 kDa (hsc70), a member of the heat shock protein (hsp)70 family of chaperones [54]. The substrates that are recognized by hsc70 contain the Lys-Phe-Glu-Arg-Gln (KFERQ) pentapeptide sequence (Figure 2). Sequence analysis reveals that 30% of cytosolic proteins carry this motif, thus being potential CMA substrates. The hsc70-substrate complex is rapidly targeted to the lysosomes where it binds to Lamp2a, a lysosomal transmembrane protein. After binding to Lamp2a, the substrate undergoes complete unfolding and translocates into the lumen of lysosome (Figure 2). Once the unfolded protein reaches the lysosome lumen, it is destroyed within minutes.
Figure 2.
Striated muscle regulation of macroautophagy, microautophagy and chaperone-mediated autophagy (CMA). The different colors underline modules describing the critical steps in the three types of autophagy. The different modules are: autophagosome formation, selective autophagic removal of mitochondria (mitophagy), autophagosome docking, direct glycogen uptake from lysosomes and removal of unfolded/misfolded proteins containing a KFERQ sequence. The dotted lines point to mechanisms that are not yet known. FOXO transcription factors are master regulators of several modules in macroautophagy.
Macroautophagy is characterized by membranes that are committed to growth, becoming double-membrane vesicles that surround a portion of cytoplasm, organelles, glycogen and protein aggregates. In macroautophagy, small ubiquitin-like molecules (LC3, GABARAP, GATE 16, ATG12) are transferred from the conjugation systems to membranes for their growth and commitment to become a double-membrane vesicle, called the autophagosome. This reaction requires the recruitment and assembly of different components of the autophagy machinery on phospholipids but only the ubiquitin-like components, LC3, GABARAP and GATE16, are covalently bound to the phospholipid phosphatidylethanolamine. In the cells there are two conjugation systems that work in parallel and are composed by the Autophagy Genes (ATG) which are highly conserved between species and act in a hierarchical manner [55]. The coordinated action of these conjugation complexes allows the commitment of the membrane to become autophagosome, the elongation of the phospholipid bilayer and fusion to form a mature double membrane vesicle that is finally docked to the lysosomes for degradation of the cargo, that is, the material that was initially engulfed by the autophagosome. The fusion of the outer membrane of the autophagosome with the lysosomal membrane also determines the degradation of the inner membrane and of the proteins that are associated with it [56]. Because of the transient nature of the autophagosomes, the lifetime of LC3 and its homologs is rather short. Thus, the main difference between the two conjugation systems is related to the fate of the ubiquitin-like proteins. In fact the autophagy system progressively loses the ubiquitin-like proteins LC3/GABARAP/GATE16, forcing the cell to replenish them in order to maintain autophagic flux. Therefore, a transcriptional-dependent program is important to sustain autophagosome biogenesis, protein breakdown and the atrophy program. In fact, among the atrophy-related genes there are several autophagy-related genes such as LC3, GABARAP, p62 and Bnip3. Therefore, the quick activation of autophagy involves post-translational modifications of the regulatory components of autophagy. In contrast, prolonged autophagic induction, such as during atrophic conditions, requires transcriptional control in order to replenish critical proteins that are destroyed during the fusion of autophagosomes with lysosomes.
3.2.1. Signaling and specificity
The FOXO family of transcription factors is critical to autophagy [57, 58]. The family’s four members (FOXO1, 3, 4 and 6), lie downstream of the insulin pathway and are negatively regulated by PI3K-AKT/PKB signaling [31, 59, 60]. FOXOs are well conserved and have a critical role in many cellular processes including apoptosis, cell cycle regulation, DNA repair, glucose metabolism and the anti-oxidant response. Interestingly, the FOXO family has been shown to regulate autophagy ranging from flies to mammals. Besides the FOXO-dependent regulation of the atrophy-related ubiquitin ligases atrogin-1 and MuRF1, FOXOs regulate expression of LC3, GABARAP, ATG12, VPS34, beclin1, ULK2, Bnip3, PINK1, Mul1 and other positive regulators of autophagy (Figure 2). Moreover FoxOs negatively regulate the mTORC1 complex, which is an important regulator of autophagosome formation [57, 58, 61, 62]. Indeed, mTORC1 inhibits autophagy by phosphorylating ULK1 and blocking the formation of the active ULK1 complex with ATG13, and FIP200 [63] (Figure 2).
Although autophagy was initially considered a non-selective degradation pathway, the significance of more selective forms of autophagy is becoming increasingly evident. Rendering selectivity to this process are a growing number of specific cargo receptors: p62, NBR1, Nix (BNIP3L), Bnip3 and Optineurin [64]. These adaptor proteins are equipped with both a cargo binding domain, with the capability to recognize and attach directly to molecular tags on the organelles and, at the same time, a LIR domain (LC3 interacting region) that is able to recruit and directly bind to essential autophagosome membrane proteins [65, 66]. The adaptor proteins are able to recognize specific flag molecules or specific post-translational modifications, such as ubiquitin chains that are presented on the surface of the cargo [67].
Through adaptor proteins, autophagy can trigger the selective removal of specific organelles such as mitochondria using a process named mitophagy [68]. In mammals, parkin, PINK1, Mul1, Bnip3 and Bnip3L have been shown to regulate mitophagy, and inactivation of the genes coding for these proteins leads to mitochondrial abnormalities [31] (Figure 2). PINK1 is normally absent in healthy mitochondria because it is constitutively degraded by mitochondrial proteases. However, once mitochondria are damaged, PINK1 is no longer degraded and accumulates. PINK1 induces parkin recruitment to mitochondria, promoting mitophagy through ubiquitination of outer mitochondrial membrane proteins that are recognized by p62, which then brings autophagic vesicles to ubiquitinated mitochondrial proteins [69, 70]. Recently a new ubiquitin-ligase, named Mul1, has been found to be under FoxO regulation [45]. Mul1 ubiquitinates mitochondrial proteins, leading to organelle removal via a p62-dependent mechanism (Figure 2). Bnip3 and Bnip3L are BH3-only proteins localized at the outer membrane of the mitochondria after cellular stress, and reportedly bind directly to LC3, thereby recruiting the autophagosome to damaged mitochondria [71, 72] [71, 72]. Selective removal of damaged organelles can be critical and is one of the mechanisms that maintains cell viability during catabolic conditions.
4. Understanding the role of autophagy in cardiac disease: animal models
4.1. Establishing causality
Limited space in this mini-review precludes a comprehensive discussion of all the animal models that have been prepared to study the role of autophagy in cardiac disease and heart failure. A few examples will suffice to illustrate current approaches. Early attempts at understanding the role that autophagy might play in cardiac disease focused on ischemia/reperfusion injury [73] and pressure overload [74], as these conditions can be rather straightforwardly produced in mice and rats and are an important and ever-growing issue in cardiac disease and heart failure in developed countries. The importance of basal autophagy in normal cardiac function could be inferred from systemic and targeted gene ablation experiments carried out on some of the genes thought to be essential for autophagy such as atg7 deletion in hepatocytes [75] and atg5 in neurons [76] or cardiomyocytes [77]. In each case, the phenotypes were severe, with systemic ablation causing fetal death and cell-type specific ablation causing significant cell or organ disease, although, in the heart, the immediacy of onset and severity was developmental stage-dependent [77, 78]. Finally and most conclusively, the genetic cause of Danon cardiomyopathy has been traced to a deficiency of the lysosomal protein LAMP2. This leads to a compromised ability of the autophagosome to fuse effectively and efficiently to the lysosome, disrupting the final steps of autophagy [79]. Although clinical presentation can vary, usually cardiomyopathy, skeletal myopathy and developmental delays are present and the cytoplasm contains massive glycogen accumulations: the disease is characterized by a buildup of glycogen, morphologically abnormal lysosomes and accumulations of autophagic vacuoles [80–82]. Cardiomyopathy may not present until second or third decade and transplant is the only known effective treatment.
4.2. Autophagy and heart failure
Heart failure represents the convergent disease process from a wide variety of congenital and non-congenital cardiac and systemic deficits. In the developed countries, the chronic exposure of the heart to increased hemodynamic load as a result, for example of vascular disease, represents an important and increasing factor in deaths due to cardiac failure. Although changes in cardiomyocyte size can be beneficial, such as occurs in an athlete’s heart [83], sustained, increased load due to conditions such as hypertension, results in a transition from a compensated hypertrophy to decompensated heart failure, with cardiomyocyte hypertrophy a hallmark of the process. Many other processes can lead to cardiac remodeling but protein homeostasis is clearly affected in many of these processes [74]. It has been known for a number of years that lysosomal activity is increased in diseased and failing human hearts [84] and the involvement of lysosomal pathways in the disease processes was implied by those data, making autophagy an attractive process for further study. The process of decompensation, which is characterized by dramatic cardiac remodeling, a transition from hypertrophy to dilation as a result of significant cardiomyocyte cell death, necessarily involves an imbalance between protein synthesis and degradation and modulation of autophagy during these processes has been attempted.
An elegant study carried out by Hill and colleagues shed light on the potential role that autophagy might play during load induced development of cardiac disease. Well-described models of pressure overload induced hypertrophy by aortic banding have been described in mice and these authors used a novel, engineered “autophagy-reporter” animal to look at autophagic activity subsequent to banding [85]. Pressure overload significantly increased autophagic activity and when they attempted to disrupt the autophagic pathway by placing the model into a heterozygote for a null allele of Beclin1, which is essential for autophagy, they found decreased autophagic activity and decreased pathological remodeling. Conversely, a gain-of-function for Beclin activity, as a result of trangenically-driven Beclin 1 expression specifically in cardiomyocytes, resulted in increased autophagy with increased pathogenesis, leading these investigators to conclude that, in the context of hemodynamic overload, autophagy could be maladaptive [74].
However, the role that autophagy plays in these processes can be subtle and the specifics of the model being used appear to be critical to the data obtained and the conclusions drawn. In a study alluded to above, Nakai and colleagues ablated another gene critical for autophagy, atg5. Using cardiomyocyte gene ablation early in development, the authors saw that while early development appeared overtly normal, the hearts rapidly decompensated subsequent to pressure overload induced hypertrophy while delay of the gene ablation prior to maturity resulted in significant hypertrophy after ablation and early cardiac failure [77]. As noted above, these data both point to the essential nature of autophagy in basal cardiac function but also imply that upregulation of autophagy is a beneficial adaptive response to hemodynamic overload. The differences in systems, methodologies of genetic activation and gain of function as well as the particular genes being targeted undoubtedly led to these conflicting conclusions and additional models will need to be constructed to sort out exactly when and under what pathological conditions autophagy might be maladaptive or beneficial. Other potential confounding factors have also been discussed [74, 86].
4.3. Transgenic models: gain of function
In a series of studies, our group elected to study the role of autophagy in the context of a well characterized proteotoxic disease, Desmin Related Myopathy (DRM), caused by a mutation in the small, heat shock-like protein alpha B crystallin (CryAB). The disease is characterized by moderate to severe muscle weakening and development of restrictive, hypertrophic or dilated cardiomyopathy and can be caused by a CryAB mutation, R120G (CryABR120G) [87]. The reasoning for studying autophagy in this context was that many cardiovascular diseases converge on a pathway where protein accumulations and pre-amyloidogenic but pathogenic oligomers, termed pre-amyloid oligomers can accumulate internally in cardiomyocytes [88, 89]. Using a transgenic mouse that expressed CryABR120G in cardiomyocytes [90] as a background, the effects of manipulating autophagy on the disease processes were determined. Prior to those experiments, basal levels of autophagy were determined in CryABR120G-expressing cardiomyocytes and found to be down regulated. When these effects were rescued by forced expression of Atg7, a critical effector of autophagy such that upregulation of autophagy occurred, the characteristic protein aggregates were significantly decreased [91].
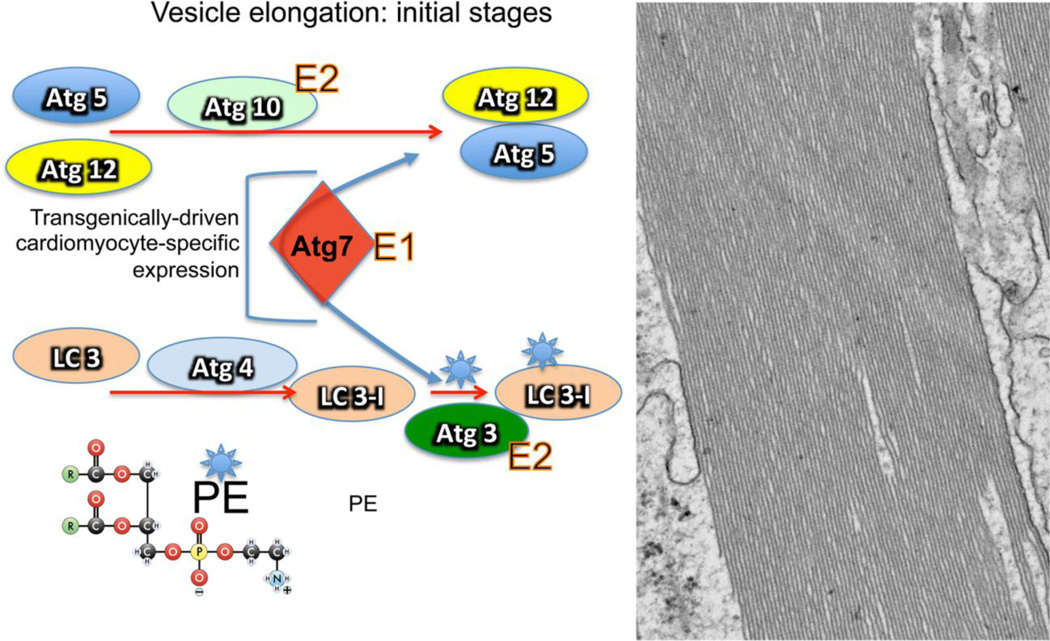
These cell-based experiments thus set the stage for in vivo manipulation of autophagic flux. The target chosen was the protein Atg7, a critical component of autophagy, which functions in elaboration of the membrane responsible for engulfing the cargo [56]. Atg7 is an E1 enzyme, which when bound through a thioester linkage, activates two ubiquitin-like proteins, Atg8 (the mammalian homologue is LC3) and Atg12. Subsequently Atg7 mediates their transfer to a specific E2-like protein, Atg3 and Atg10, respectively. These are then conjugated to either phosphotidylethanolamine (Atg3/LC3-I) or Atg5 (Atg12). These interactions are critical for correct function of the two ubiquitin-like conjugation systems that underlie part of the initial vesicle’s elongation process [92, 93] (Figure 3). The authors hypothesized that Atg7 might be a rate-limiting component for autophagic activity and expressing increased quantities of this particular protein might up-regulate autophagic flux. Indeed this proved to be the case and cardiomyocyte-restricted expression of an atg7 transgene resulted in significantly increased flux through the autophagic pathway [94]. Confirming its role in vesicle elongation, long, linear arrays of membrane were easily visible and distributed throughout the cardiomyocyte’s cytoplasm (Figure 3). Somewhat surprisingly, considering a concern that heightened, chronic autophagy might well be detrimental [95] the hearts (and mice) tolerated these increased levels well, with no cardiac hypertrophy, fibrosis or remodeling apparent over the animals’ lifetimes. These animals were then crossed to mice harboring the CryABR120G transgene as well and the outcome of increasing autophagy in a model where cardiac autophagy is normally down regulated determined. Increasing autophagy in these hearts by Atg7 overexpression both attenuated and/or delayed developing pathologies and the usual compromises in cardiac function that are observed in the CryABR120G hearts. The probability of survival into mid-adulthood was also increased although these animals did suffer premature death. We also observed significant reductions in activation of the gene programs normally associated with developing cardiac pathology such as activation of the fetal gene program seen during the development of pathological hypertrophy [96] and cardiac fibrosis. These experiments indicate that under basal conditions, the hearts are able to tolerate significantly increased autophagic flux and restoring autophagy to normal levels in proteotoxic hearts suffering from decreased autophagy can be therapeutically beneficial.
Figure 3.
The initial stages of vesicle elongation in macroautophagy. A. Only the initial stages are shown, focusing on the seminal role played by Atg7 in the process, where it intersects with the two possible pathways. The first pathway (top) occurs through the direct interaction of Atg12 with Atg5. This interaction requires the E1-like activity of Atg7. The second pathway involves the addition of phosphotidylethanolamine (PE, shown lower left) to LC3-I, the mammalian homologue of the yeast protein, Atg8. This process is mediated by a protease, Atg4 followed by Atg7’s E1-like enzymatic activity to add PE to LC3-I, coupled with the E2-like activity of Atg3. B. Electron micrograph of a cardiomyocyte derived from the heart of mouse that overexpresses (approximately 10-fold) Atg7. Immediately apparent are elaborated, linear membranes. These structures do not affect cardiac function during the animal’s lifetime [94].
5. Exploring therapeutic windows
Manipulation of autophagy in treating various cancers is now established [97–100]. BH3 mimetics [101], small molecules targeting mTOR activity [102] and the use of cannabinoids [103] are all being actively explored. Chloroquine, a potent autophagy inhibitor, is currently in use as an anti-cancer drug but can have serious side effects [100]. Current therapies directed at the heart follow, to some extent, these established paradigms, depending upon approved substances and/or drugs [104] that affect either the upstream events that might trigger autophagy or the downstream signaling pathways. For example, Morrell et al noted that chloroquine ameliorated development of pulmonary hypertension induced by administering monocrotaline in Sprague-Dawley rats, partially through the inhibition of autophagy [105]. Hill and colleagues have extensively explored small molecule inhibitors of the histone deacetylases (HDACs), which are beneficial in ameliorating a pathogenic hypertrophic response. Strikingly, their anti-hypertrophic effect is more pronounced in the Beclin 1 transgenic mice in which autophagy is augmented. Thus, the fact that inhibition of HDAC activity decreased the incremental growth seen in the Beclin 1 overexpressors implied that HDACs might impact negatively on autophagic activity and this might partially explain their effectiveness in blunting pathogenic hypertrophy [106, 107]. Resveratrol, a natural phenol produced by several plants, has been reported to effectively enhance autophagy by activating the AMP-activated protein kinase (often referred to as an “energy sensor”) [108] and silent information regulator-1, and thereby impacting on mTOR activity [109]. Other investigators have proposed treating more specialized aspects of autophagy, such as mitophagy, as potential targets, either in the general aging process or in the context of cardiovascular disease [110]. Sala-Mercado et al tested the efficacy of chloramphenicol succinate (CAPS), which can upregulate autophagy in the context of a porcine model in which the coronary artery was occluded for 45 minutes and then reperfused for 3 hours. Accompanying the upregulation of protein markers for autophagy, including Beclin-1 and LC3-II was reduced infarct size. Although these are preliminary data and actual autophagic flux was not determined, the data are consistent with the hypothesis that autophagy can be cardioprotective during ischemic-reperfusion injury [111].
This concept is consistent with the presence of high levels of aggregates containing toxic pre-amyloid oligomers that have been observed in heart derived from human heart failure patients [88, 89, 112]. However, progress in understanding the role of autophagy in heart disease has not yet been translatable. Of the 34 active clinical trials currently underway in the NIH registry using the keyword “autophagy” (clinicaltrial.gov), 31 of them are directed towards cancer. There is one trial looking at the effects of a low protein diet on Collagen VI Related Myopathies, one looking at TGFβ signaling and a third directed at cardiac allograft remodeling. Thus although there is now intense interest in the potential therapeutic value of impacting on autophagy with some 20 different general targets being studied (reviewed in [113]), their translation to the heart is in its infancy. Additionally, we are using rather blunt instruments: when one systemically disrupts a ubiquitous and intrinsic metabolic process such as autophagy, either by its up- or down-regulation, non-specific effects in diverse cell types and organ systems are to be expected. Therapies targeted specifically to the heart will need to be developed and may rely on the preparation of either small molecules that can precisely target a defined step(s) in autophagy or can be targeted to a specific cell type through either its delivery through stents or by coupling to specific ligands that will find their cardiac-specific receptors. With these classes of tools, it would then become possible to more precisely manipulate an essential metabolic pathway but restrict that manipulation to the target organ or even cell type.
Highlights.
Proteotoxicity is a pathology that develops due to damaged or misfolded protein accumulations
The removal of damaged proteins and organelles from the heart is essential.
Deficits in autophagy can occur during development of heart disease
Altering autophagic flux may be a potential therapy in heart disease and failure.
Acknowledgements
This work was supported by NIH grants P01HL077101, P01HL049058, R01HL105924 to J.R.) and The Transatlantic Network of Excellence Program grant from Le Fondation Leducq (to M.S and J.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have nothing to declare
References
- 1.Calamini B, Morimoto RI. Protein homeostasis as a therapeutic target for diseases of protein conformation. Curr Top Med Chem. 2012;12:2623–2640. doi: 10.2174/1568026611212220014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers ET, Balch WE. Diversity in the origins of proteostasis networks--a driver for protein function in evolution. Nat Rev Mol Cell Biol. 2013;14:237–248. doi: 10.1038/nrm3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction--Alzheimer's disease of the heart? N Engl J Med. 2013;368:455–464. doi: 10.1056/NEJMra1106180. [DOI] [PubMed] [Google Scholar]
- 5.Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 6.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morimoto RI. The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harb Symp Quant Biol. 2011;76:91–99. doi: 10.1101/sqb.2012.76.010637. [DOI] [PubMed] [Google Scholar]
- 8.Portbury AL, Willis MS, Patterson C. Tearin' up my heart: proteolysis in the cardiac sarcomere. J Biol Chem. 2011;286:9929–9934. doi: 10.1074/jbc.R110.170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastle M, Grune T. Interactions of the proteasomal system with chaperones: protein triage and protein quality control. Prog Mol Biol Transl Sci. 2012;109:113–160. doi: 10.1016/B978-0-12-397863-9.00004-3. [DOI] [PubMed] [Google Scholar]
- 10.Latonen L. Nucleolar aggresomes as counterparts of cytoplasmic aggresomes in proteotoxic stress. Proteasome inhibitors induce nuclear ribonucleoprotein inclusions that accumulate several key factors of neurodegenerative diseases and cancer. Bioessays. 2011;33:386–395. doi: 10.1002/bies.201100008. [DOI] [PubMed] [Google Scholar]
- 11.Su H, Wang X. The ubiquitin-proteasome system in cardiac proteinopathy: a quality control perspective. Cardiovasc Res. 2010;85:253–262. doi: 10.1093/cvr/cvp287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verghese J, Abrams J, Wang Y, Morano KA. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol Mol Biol Rev. 2012;76:115–158. doi: 10.1128/MMBR.05018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira PA, Orry A. From Drosophila to humans: reflections on the roles of the prolyl isomerases and chaperones, cyclophilins, in cell function and disease. J Neurogenet. 2012;26:132–143. doi: 10.3109/01677063.2011.647143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaiser AM, Kaiser CJ, Haslbeck V, Richter K. Downregulation of the Hsp90 system causes defects in muscle cells of Caenorhabditis elegans. PLoS One. 2011;6:e25485. doi: 10.1371/journal.pone.0025485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein HF, Benian GM. Paradigm shifts in cardiovascular research from Caenorhabditis elegans muscle. Trends Cardiovasc Med. 2012;22:201–209. doi: 10.1016/j.tcm.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Xu Q, Metzler B, Jahangiri M, Mandal K. Molecular chaperones and heat shock proteins in atherosclerosis. Am J Physiol Heart Circ Physiol. 2012;302:H506–H514. doi: 10.1152/ajpheart.00646.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christians ES, Ishiwata T, Benjamin IJ. Small heat shock proteins in redox metabolism: implications for cardiovascular diseases. Int J Biochem Cell Biol. 2012;44:1632–1645. doi: 10.1016/j.biocel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwaki T, Kume-Iwaki A, Liem RK, Goldman JE. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989;57:71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- 19.Bennardini F, Wrzosek A, Chiesi M. Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circ Res. 1992;71:288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- 20.He C, Klionsky DJ. Autophagy and neurodegeneration. ACS Chem Biol. 2006;1:211–213. doi: 10.1021/cb600182h. [DOI] [PubMed] [Google Scholar]
- 21.Roze E, Bonnet C, Betuing S, Caboche J. Huntington's disease. Adv Exp Med Biol. 2010;685:45–63. [PubMed] [Google Scholar]
- 22.Ebrahimi-Fakhari D, Wahlster L, McLean PJ. Protein degradation pathways in Parkinson's disease: curse or blessing. Acta Neuropathol. 2012;124:153–172. doi: 10.1007/s00401-012-1004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Double KL. Neuronal vulnerability in Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S52–S54. doi: 10.1016/S1353-8020(11)70018-9. [DOI] [PubMed] [Google Scholar]
- 24.Safar JG. Molecular pathogenesis of sporadic prion diseases in man. Prion. 2012;6:108–115. doi: 10.4161/pri.18666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guest WC, Silverman JM, Pokrishevsky E, O'Neill MA, Grad LI, Cashman NR. Generalization of the prion hypothesis to other neurodegenerative diseases: an imperfect fit. J Toxicol Environ Health A. 2011;74:1433–1459. doi: 10.1080/15287394.2011.618967. [DOI] [PubMed] [Google Scholar]
- 26.Crippa V, Sau D, Rusmini P, Boncoraglio A, Onesto E, Bolzoni E, et al. The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS) Hum Mol Genet. 2010;19:3440–3456. doi: 10.1093/hmg/ddq257. [DOI] [PubMed] [Google Scholar]
- 27.Pattison JS, Sanbe A, Maloyan A, Osinska H, Klevitsky R, Robbins J. Cardiomyocyte expression of a polyglutamine preamyloid oligomer causes heart failure. Circulation. 2008;117:2743–2751. doi: 10.1161/CIRCULATIONAHA.107.750232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Su H, Ranek MJ. Protein quality control and degradation in cardiomyocytes. J Mol Cell Cardiol. 2008;45:11–27. doi: 10.1016/j.yjmcc.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mearini G, Schlossarek S, Willis MS, Carrier L. The ubiquitin-proteasome system in cardiac dysfunction. Biochim Biophys Acta. 2008;1782:749–763. doi: 10.1016/j.bbadis.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C. Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res. 2010;106:463–478. doi: 10.1161/CIRCRESAHA.109.208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45:2121–2129. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandri M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013 doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pagan J, Seto T, Pagano M, Cittadini A. Role of the ubiquitin proteasome system in the heart. Circ Res. 2013;112:1046–1058. doi: 10.1161/CIRCRESAHA.112.300521. [DOI] [PubMed] [Google Scholar]
- 34.Calise J, Powell SR. The ubiquitin proteasome system and myocardial ischemia. Am J Physiol Heart Circ Physiol. 2013;304:H337–H349. doi: 10.1152/ajpheart.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day SM. The ubiquitin proteasome system in human cardiomyopathies and heart failure. Am J Physiol Heart Circ Physiol. 2013;304:H1283–H1293. doi: 10.1152/ajpheart.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D, Goldberg A. Atrogin1/MAFbx: what atrophy, hypertrophy, and cardiac failure have in common. Circ Res. 2011;109:123–126. doi: 10.1161/CIRCRESAHA.111.248872. [DOI] [PubMed] [Google Scholar]
- 37.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 38.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 40.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 41.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 42.Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, et al. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol. 2009;296:C1248–C1257. doi: 10.1152/ajpcell.00104.2009. [DOI] [PubMed] [Google Scholar]
- 43.Sartori R, Schirwis E, Blaauw B, Bortolanza S, Zhao J, Enzo E, et al. BMP signaling controls muscle mass. Nat Genet. 2013 doi: 10.1038/ng.2772. [DOI] [PubMed] [Google Scholar]
- 44.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 45.Lokireddy S, Wijesoma IW, Teng S, Bonala S, Gluckman PD, McFarlane C, et al. The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metab. 2012;16:613–624. doi: 10.1016/j.cmet.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Nagpal P, Plant PJ, Correa J, Bain A, Takeda M, Kawabe H, et al. The ubiquitin ligase Nedd4-1 participates in denervation-induced skeletal muscle atrophy in mice. PLoS One. 2012;7:e46427. doi: 10.1371/journal.pone.0046427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul PK, Gupta SK, Bhatnagar S, Panguluri SK, Darnay BG, Choi Y, et al. Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J Cell Biol. 2010;191:1395–1411. doi: 10.1083/jcb.201006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi J, Luo L, Eash J, Ibebunjo C, Glass DJ. The SCF-Fbxo40 complex induces IRS1 ubiquitination in skeletal muscle, limiting IGF1 signaling. Dev Cell. 2011;21:835–847. doi: 10.1016/j.devcel.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Kaushik S, Cuervo AM. Chaperones in autophagy. Pharmacol Res. 2012;66:484–493. doi: 10.1016/j.phrs.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaushik S, Singh R, Cuervo AM. Autophagic pathways and metabolic stress. Diabetes Obes Metab. 2010;12(Suppl 2):4–14. doi: 10.1111/j.1463-1326.2010.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rotter D, Rothermel BA. Targets, trafficking, and timing of cardiac autophagy. Pharmacol Res. 2012;66:494–504. doi: 10.1016/j.phrs.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7:673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 53.Tolkovsky AM. Mitophagy. Biochim Biophys Acta. 2009;1793:1508–1515. doi: 10.1016/j.bbamcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol. 2011;23:184–189. doi: 10.1016/j.ceb.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB., 3rd Autophagy: Regulation and role in development. Autophagy. 2013;9:951–972. doi: 10.4161/auto.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci. 2011;124:161–170. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–526. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- 60.Sandri M. FOXOphagy path to inducing stress resistance and cell survival. Nat Cell Biol. 2012;14:786–788. doi: 10.1038/ncb2550. [DOI] [PubMed] [Google Scholar]
- 61.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dunlop EA, Hunt DK, Acosta-Jaquez HA, Fingar DC, Tee AR. ULK1 inhibits mTORC1 signaling, promotes multisite Raptor phosphorylation and hinders substrate binding. Autophagy. 2011;7:737–747. doi: 10.4161/auto.7.7.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaid S, Brandts CH, Serve H, Dikic I. Ubiquitination and selective autophagy. Cell Death Differ. 2013;20:21–30. doi: 10.1038/cdd.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirkin V, Lamark T, Sou YS, Bjorkoy G, Nunn JL, Bruun JA, et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell. 2009;33:505–516. doi: 10.1016/j.molcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 66.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 67.McEwan DG, Dikic I. The Three Musketeers of Autophagy: phosphorylation, ubiquitylation and acetylation. Trends Cell Biol. 2011;21:195–201. doi: 10.1016/j.tcb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu H, Li G, Liu L, Feng L, Wang X, Jin H. Regulation and function of mitophagy in development and cancer. Autophagy. 2013;9 doi: 10.4161/auto.26550. [DOI] [PubMed] [Google Scholar]
- 69.Narendra DP, Youle RJ. Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid Redox Signal. 2011;14:1929–1938. doi: 10.1089/ars.2010.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson AB. Microtubule-Associated Protein 1 Light Chain 3 (LC3) Interacts with Bnip3 to Selectively Remove Endoplasmic Reticulum and Mitochondria via Autophagy. J Biol Chem. 2012 doi: 10.1074/jbc.M111.322933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ Res. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 77.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 78.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 79.Cheng Z, Fang Q. Danon disease: focusing on heart. J Hum Genet. 2012;57:407–410. doi: 10.1038/jhg.2012.72. [DOI] [PubMed] [Google Scholar]
- 80.Malicdan MC, Nishino I. Autophagy in lysosomal myopathies. Brain Pathol. 2012;22:82–88. doi: 10.1111/j.1750-3639.2011.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malicdan MC, Noguchi S, Nonaka I, Saftig P, Nishino I. Lysosomal myopathies: an excessive build-up in autophagosomes is too much to handle. Neuromuscul Disord. 2008;18:521–529. doi: 10.1016/j.nmd.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 82.Ruivo R, Anne C, Sagne C, Gasnier B. Molecular and cellular basis of lysosomal transmembrane protein dysfunction. Biochim Biophys Acta. 2009;1793:636–649. doi: 10.1016/j.bbamcr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 83.George K, Whyte GP, Green DJ, Oxborough D, Shave RE, Gaze D, et al. The endurance athletes heart: acute stress and chronic adaptation. Br J Sports Med. 2012;46(Suppl 1):i29–i36. doi: 10.1136/bjsports-2012-091141. [DOI] [PubMed] [Google Scholar]
- 84.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 85.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Przyklenk K, Dong Y, Undyala VV, Whittaker P. Autophagy as a therapeutic target for ischaemia /reperfusion injury? Concepts, controversies, and challenges. Cardiovasc Res. 2012;94:197–205. doi: 10.1093/cvr/cvr358. [DOI] [PubMed] [Google Scholar]
- 87.Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 88.Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, et al. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci U S A. 2004;101:10132–10136. doi: 10.1073/pnas.0401900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanbe A, Osinska H, Villa C, Gulick J, Klevitsky R, Glabe CG, et al. Reversal of amyloid-induced heart disease in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2005;102:13592–13597. doi: 10.1073/pnas.0503324102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, et al. Expression of R120G-alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 91.Pattison JS, Osinska H, Robbins J. Atg7 induces basal autophagy and rescues autophagic deficiency in CryABR120G cardiomyocytes. Circ Res. 2011;109:151–460. doi: 10.1161/CIRCRESAHA.110.237339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 93.Noda NN, Satoo K, Fujioka Y, Kumeta H, Ogura K, Nakatogawa H, et al. Structural basis of Atg8 activation by a homodimeric E1, Atg7. Mol Cell. 2011;44:462–475. doi: 10.1016/j.molcel.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 94.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, et al. Induced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013 doi: 10.1172/JCI70877. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang EY, Biala AK, Gordon JW, Kirshenbaum LA. Autophagy in the heart: too much of a good thing? J Cardiovasc Pharmacol. 2012;60:110–117. doi: 10.1097/FJC.0b013e31824cc427. [DOI] [PubMed] [Google Scholar]
- 96.Vikstrom KL, Bohlmeyer T, Factor SM, Leinwand LA. Hypertrophy, pathology, and molecular markers of cardiac pathogenesis. Circ Res. 1998;82:773–778. doi: 10.1161/01.res.82.7.773. [DOI] [PubMed] [Google Scholar]
- 97.Baek KH, Park J, Shin I. Autophagy-regulating small molecules and their therapeutic applications. Chem Soc Rev. 2012;41:3245–3263. doi: 10.1039/c2cs15328a. [DOI] [PubMed] [Google Scholar]
- 98.Bao XH, Naomoto Y, Hao HF, Watanabe N, Sakurama K, Noma K, et al. Autophagy: Can it become a potential therapeutic target? Int J Mol Med. 2010;25:493–503. doi: 10.3892/ijmm_00000369. [DOI] [PubMed] [Google Scholar]
- 99.Dickey JS, Rao VA. Current and proposed biomarkers of anthracycline cardiotoxicity in cancer: emerging opportunities in oxidative damage and autophagy. Curr Mol Med. 2012;12:763–771. doi: 10.2174/156652412800792561. [DOI] [PubMed] [Google Scholar]
- 100.Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res. 2013;73:3–7. doi: 10.1158/0008-5472.CAN-12-2464. [DOI] [PubMed] [Google Scholar]
- 101.Yu L, Liu S. Autophagy contributes to modulating the cytotoxicities of Bcl-2 homology domain-3 mimetics. Semin Cancer Biol. 2013 doi: 10.1016/j.semcancer.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 102.Santulli G, Totary-Jain H. Tailoring mTOR-based therapy: molecular evidence and clinical challenges. Pharmacogenomics. 2013;14:1517–1526. doi: 10.2217/pgs.13.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cridge BJ, Rosengren RJ. Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag Res. 2013;5:301–313. doi: 10.2147/CMAR.S36105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dimitrakis P, Romay-Ogando MI, Timolati F, Suter TM, Zuppinger C. Effects of doxorubicin cancer therapy on autophagy and the ubiquitin-proteasome system in longterm cultured adult rat cardiomyocytes. Cell Tissue Res. 2012;350:361–372. doi: 10.1007/s00441-012-1475-8. [DOI] [PubMed] [Google Scholar]
- 105.Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res. 2013;112:1159–1170. doi: 10.1161/CIRCRESAHA.111.300483. [DOI] [PubMed] [Google Scholar]
- 106.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, et al. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci U S A. 2011;108:4123–4128. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–2588. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Catoire H, Pasco MY, Abu-Baker A, Holbert S, Tourette C, Brais B, et al. Sirtuin inhibition protects from the polyalanine muscular dystrophy protein PABPN1. Hum Mol Genet. 2008;17:2108–2117. doi: 10.1093/hmg/ddn109. [DOI] [PubMed] [Google Scholar]
- 109.Kimball SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc. 2006;38:1958–1964. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- 110.Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol. 2013;305:H459–H476. doi: 10.1152/ajpheart.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sala-Mercado JA, Wider J, Undyala VV, Jahania S, Yoo W, Mentzer RM, Jr, et al. Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation. 2010;122:S179–S184. doi: 10.1161/CIRCULATIONAHA.109.928242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gianni D, Li A, Tesco G, McKay KM, Moore J, Raygor K, et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation. 2010;121:1216–1226. doi: 10.1161/CIRCULATIONAHA.109.879510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nemchenko A, Chiong M, Turer A, Lavandero S, Hill JA. Autophagy as a therapeutic target in cardiovascular disease. J Mol Cell Cardiol. 2011;51:584–593. doi: 10.1016/j.yjmcc.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]